ABSTRACT
Cyclodestruction aims to reduce aqueous humor production through the coagulation or destruction of the ciliary body and has been an important treatment choice for glaucoma since the 1930s. The purpose of the current review is to highlight the evidence regarding the safety and efficacy of various cyclodestructive modalities, emphasizing peer-reviewed articles from the last 20 years and the most common variants of these procedures. The review focuses primarily on the two most common variants of transscleral cyclophotocoagulation (TS-CPC), continuous-wave diode cyclophotocoagulation (CW-TSCPC) and MicroPulse diode cyclophotocoagulation (MP-TSCPC) as well as endoscopic cyclophotocoagulation (ECP) and high-intensity focused ultrasound cyclodestruction (HIFU). We believe that the role of cyclodestruction in glaucoma treatment will only continue to expand given the advances in the field, particular with regards to targeted ciliary body destruction and improvement in the safety profile.
INTRODUCTION
Glaucoma is a chronic, progressive, and irreversible optic neuropathy. It is the second most common cause of blindness worldwide, affecting approximately 80 million people.Citation1 Currently, the only modifiable risk factor known to slow the progression of the disease is intraocular pressure (IOP). IOP is the result of the balance of aqueous humor secretion by the ciliary body and its drainage through the trabecular meshwork and uveoscleral outflow pathway.Citation2 Accordingly, glaucoma treatment primarily consists of interventions intended to lower IOP by decreasing aqueous humor secretion or increasing its outflow.
Cyclodestruction is one such intervention, and it has been an important treatment choice since the 1930s. Cyclodestruction aims to reduce aqueous humor production through the coagulation or destruction of the ciliary body. Earlier variants of cyclodestruction included cyclocryotherapy (i.e., freezing injury), cyclodiathermy (i.e., thermal injury), and cyclectomy (i.e., surgical excision) of the ciliary body. However, many complications were commonly associated with these procedures including prolonged uveitis, damage of corneal nerves, eye pain, and phthisis.Citation3,Citation4 Moreover, their clinical efficacy was called into question with a review of 100 cases of cyclodiathermy, for instance, demonstrating that only 5% of cases had a sustainable, clinically-significant reduction in IOP, with phthisis occurring almost as often.Citation5
Limitations in the safety and utility of these early cyclodestructive methods led to the advent of cyclophotocoagulation (CPC)— a more refined, targeted approach to the destruction of the ciliary body.Citation6 In 1972, the use of transscleral cyclophotocoagulation (TS-CPC) using ruby laser was first reported by Beckman and associates.Citation7 Shortly thereafter, the same authors demonstrated that the Neodymium:Yttrium-Aluminum-Garnet (Nd:YAG) laser more effectively ablated the ciliary body.Citation8 Today, CPC can be employed through either a transscleral, endoscopic, or transpupillary approach.
While CPC provides a relatively targeted approach to ciliary body destruction, dissipated laser energy may still cause damage to surrounding tissues resulting in postoperative complications commonly observed with the procedure. Additionally, scattered light energy opposite the treatment location (ciliary body) may cause macular edema, persistent mydriasis, prolonged anterior chamber inflammation, and even neurotrophic keratopathy. However, newer CPC modalities seem to provide better-focused energy, more targeted destruction of the ciliary processes, and more favorable safety profiles. This, in turn, has expanded the role of cyclodestruction in the management of glaucoma to include a broader patient profile and to be used earlier in our treatment algorithms.Citation3
The purpose of the current review is to highlight the evidence regarding the safety and efficacy of various cyclodestructive modalities, emphasizing peer-reviewed articles from the last 20 years and the most common variants of these procedures. It will focus primarily on the two most common variants of transscleral cyclophotocoagulation (TS-CPC), continuous-wave diode cyclophotocoagulation (CW-TSCPC) and MicroPulse diode transscleral laser treatment (MP-TLT). Transpupillary CPC is another cyclodestructive modality that requires a clear visual axis and ciliary processes that can be viewed on gonioscopy, in cases such as aniridia. It is therefore of limited use and will not be discussed further. Endoscopic cyclophotocoagulation (ECP) and high-intensity focused ultrasound cyclodestruction (HIFU) will also be discussed in detail.
Transscleral Cyclophotocoagulation (TS-CPC)
In TS-CPC, laser energy administered through the overlying sclera is absorbed by the melanin in the ciliary processes, resulting in coagulative necrosis of the ciliary body apparatus. Both the Nd:YAG laser (1064 nm) and the semiconductor diode laser (810 nm) can be used for this procedure. The semiconductor diode laser is currently the most popular method of treatment, essentially replacing the Nd:YAG laser due to its ability to provide equivalent efficacy with superior ease of performance and lower incidences of adverse events.Citation9–Citation12
The range of analgesic options used during cyclophotodestructive procedures is wide and depends on a variety of factors. Surgeon and patient preference, and the availability at the location of practice can all impact the type of anesthetic used. The options include general, retrobulbar, peribulbar, sub-Tenon’s or subconjunctival anesthesia. Even heavy sedation with topical anesthesia in the form of topical lidocaine gel without alcohol can be utilized, especially for MP-TLT, which in theory should not be as painful as CW-TSCPC. Of the studies examined for this review, all employed retrobulbar or peribulbar anesthesia, with the occasional use of general anesthesia in pediatric patients or per patient preference.
Continuous Wave Diode Laser (CW–TSCPC)
During CW-TSCPC, continuous-wave laser energy is delivered to the ciliary body via a laser probe placed approximately 1.2 mm from the corneoscleral limbus, with the heel of the probe aligned at the limbus to direct the beam posteriorly toward the ciliary processes.Citation13 Specifically, the G-Probe Device (IRIDEX corp., Mountainview, CA) allows for targeted cyclophotocoagulation of the ciliary body (). A G-probe variant, the G-probe Illuminate, even offers transillumination with a built-in fiber-optic element. Using the G-probe, energy is applied in distinct locations with care to avoid treating the 3 and 9 o’clock positions to minimize the risk of damage to the long posterior vessels and nerves.
The laser power is adjusted just below the level at which “pops” are heard, which signify an intraocular uveal micro-explosion.Citation14 The original recommended settings for the semiconductor diode were based on studies performed by Gaasterland et al. in the 1990s on rabbit eyes and ranges from 1.25 − 1.5 W for 4.0–4.5 second durations.Citation12 Today, settings vary by surgeon preference and patient characteristics. In general, surgeons will start at 2.0 Watts (W) for 2 seconds and titrate the energy down depending on the audible “pop.” Others use an initial power of 1.25 W for a duration of 4 seconds. Overall, a total of 18 − 21 spots are applied with sparing the 3 and 9 o’clock positions where the ciliary nerves lie.
The utility of CW-TSCPC for the management of refractory glaucoma, defined as uncontrolled glaucoma despite prior surgical treatments and/or medical therapy alone, has been well established for over thirty years (). In such cases, it has been reported that between 63 and 89% of patients have achieved a target IOP of less than 22 mmHg after treatment.Citation13,Citation15,Citation16 Of the cohort of studies utilizing refractory glaucoma populations outlined in , IOP reductions range from 10 to 23 mmHg, with an average of approximately 17 mmHg.
Table 1. Selection of Continuous Wave Transscleral Cyclophotocoagulation (CW-TSCPC) Studies from 2001–2019
While numerous studies have demonstrated the efficacy of CW-TSCPC, comparisons between them are often difficult given the varying patient populations and laser settings. For example, in a study of refractory glaucoma cases, Murphy et al. found CW-TSCPC to be most effective in patients with chronic angle-closure glaucoma (CACG). CACG patients experienced the highest success rate (93%), and the lowest re-treatment (13%) and hypotony rates (0%) compared to those with other glaucoma diagnoses.Citation16
Moreover, there is evidence that preoperative IOP has an effect on the absolute reduction in IOP.Citation17,Citation18 Specifically, Vernon et al. observed that 94% of eyes with an initial IOP > 30 mmHg obtained at least a 30% reduction at the last follow-up, while only 75% of eyes with an initial IOP ≤ 30 mmHg achieved such a reduction. Additionally, Hauber et al. found a significant, direct linear correlation between the total amount of energy applied to the ciliary body and the percentage of patients who achieved successful outcomes. When using aggressive settings [2.0 W, 2 s, 25.6 burns] they observed an average 13 mmHg reduction in IOP.Citation19
CW-TSCPC has also been shown to reduce patients’ topical and systemic glaucoma medication use, particularly the use of oral acetazolamide for IOP control post-treatment. Vernon et al. demonstrated an 80% reduction in eyes requiring oral acetazolamide, while Rotchford et al. observed a 55% reduction.Citation18,Citation20 The average reduction in glaucoma medications following CW-TSCPC based on the studies featured in is approximately 1.2. However, it must be noted that with patients coming off of acetazolamide, there is an occasional increase in use of topical medications for IOP control that is not clearly reported across studies. Overall, CW-TSCPC has proven effective in absolute IOP control as well as in decreasing the medication burden in patients with refractory glaucoma.
Although effective, CW-TSCPC is not without complications. Specifically, postoperative pupillary abnormalities, hyphema, inflammation, hypotony, retreatments, drop in visual acuity, pain, lens subluxation, staphyloma formation, scleral perforation, and even sympathetic ophthalmia have been reported.Citation9,Citation18,Citation21-Citation24 The rates of hyphema were highest in patients with neovascular glaucoma (NVG), and occurred in between 0 and 5% of eyes portrayed in .Citation25 Hypotony and subsequent phthisis is another commonly feared complication of CW-TSCPC. Vernon et al. hypothesized that risks of hypotony and phthisis are directly proportional to the amount of laser energy delivered during CW-TSCPC, while other studies have shown that the underlying type of glaucoma seems to be more highly correlated with complication rates, particularly NVG.Citation20,Citation26-Citation28 Indeed, a staggering 76% of the patients that developed hypotony in a study by Iliev and Gerber had underlying NVG.Citation28
Retreatment is another important factor when discussing the efficacy and safety of CW-TSCPC. The need for retreatment has been most commonly described in younger patients, posttraumatic cases, and patients who have secondary glaucoma following vitreoretinal surgery.Citation13 Retreatment rates in the studies from range from 20 to 60%, with the highest rates seen in studies that used lower energy settings.Citation20,Citation29 However, Vernon and Pucci believe that using lower doses of laser energy is the optimal approach, given that this allows for titration of the dose of cycloablation to the individual eye in terms of the number of treatment episodes, thus minimizing risk of hypotony and phthisis.Citation18
Importantly, CW-TSCPC has also been associated with a decrease in final visual acuity (VA) and is therefore commonly reserved for patients with poor visual potential. However, recent studies have shed more light on this topic, suggesting CW-TSCPC treatment can be extended to patients with good vision. When used in patients with visual acuity >20/60, Ghosh et al. found that 39.1% of eyes retained their preoperative VA, 24% had a loss of two lines or more and 10.9% experienced some improvement in VA after 2 years of follow up.Citation30 Rotchford et al. also evaluated the effects of CW-TSCPC in patients with good visual acuity (≥20/60), but used a follow-up period of 5 years. At last follow up, 30.6% of patients had lost 2 or more lines of visual acuity, which is consistent with reported rates of vision loss following trabeculectomy or tube-shunt surgery.Citation20,Citation31
Taken together, these studies suggest that final visual acuity is minimally compromised due to CW-TSCPC alone, and that glaucoma progression, in and of itself, was typically the most frequent cause of decreased visual acuity in these patients. The overall trend of these studies, as outlined in , suggests that CW-TSCPC is an effective method of IOP control despite its associated risks and complications. It should be noted that while rates of VA decline were comparable between CW-TSCPC, trabeculectomy and tube-shunt surgery, MP-TLT appears to put patients at significantly less risk for postoperative VA decline ( and ). The more favorable progression in visual acuity following MP-TLT is among the reasons it has been preferred over CW-TSCPC in patients with good vision potential.
Table 2. Selection of MicroPulse Transscleral Cyclophotocoagulation (MP-TSCPC) Studies from 2010–2020
The use of CW-TSCPC in refractory cases of glaucoma due to NVG or secondary glaucoma in post-penetrating keratoplasty (PK) patients is of particular value, as the IOP in these patient populations can be significantly more challenging to control. Yildirim et al. prospectively compared the long-term IOP reduction in a cohort of NVG patients who received treatment with either CW-TSCPC or an Ahmed glaucoma valve (AGV). Surgical success was defined as a postoperative IOP between 5 and 21 mmHg without additional glaucoma surgery or loss of light perception vision.Citation32 Overall, no significant differences were found between the two groups: after 24 months, the probability of success was 61.18% in the CW-TSCPC group, and 59.26% in the AGV group. Additionally, visual acuity decreased in 24% of eyes in the CW-TSCPC group and 27% of eyes in the AGV group.Citation32 CW-TSCPC appears to be warranted for use in NVG patients and may be similar in efficacy to AGVs.
CW-TSCPC has also proven to be a useful tool in patients with secondary glaucoma post PK. A study by Shah et al. found a median 16 mmHg reduction in IOP from a baseline median of 33 mmHg, though most patients required 2 CW-TSCPC treatments to control IOP.Citation33 The mean number of glaucoma medications before and after CW-TSCPC in this study was 2.6 and 1.8, respectively (P < .001), at a median of 30.5 months of follow-up time. Visual acuity improved (> two Snellen lines of acuity) in three patients (11%) and remained the same (± one Snellen line) in 17 patients (61%). Of the 7 patients (26%) at final follow-up who lost vision (> two Snellen lines or decrease in one low-vision category), two (7%) of these lost vision due to the laser treatment (uveitis, cystoid macular edema (CME)) and the other five’s vision loss was associated with ongoing disease processes.Citation33 Additionally, of the 19 patients (68%) with originally clear grafts, three grafts (16%) developed opacification. Opacification was secondary to graft rejection and late endothelial failure. Six grafts (21%) had signs of rejection with two of these occurring more than 3 months after cyclodiode treatment; three of these rejection episodes (50%) were reversible with intensive corticosteroid treatment.Citation33
There is also a role for CW-TSCPC in the management of pediatric glaucoma, which remains notoriously difficult to manage. While angle-based and filtering procedures are usually the core of pediatric glaucoma management, surgery for childhood glaucoma has a substantial failure rate compared to that of adults.Citation34 Studies of CW-TSCPC in children have demonstrated clinically useful reductions in IOP in cases of refractory pediatric glaucoma with low complication rates, however retreatment is often needed to maintain control of IOP.Citation35,Citation36 Specifically, Kirwan et al. reported that after one treatment only 37% of pediatric patients had an IOP <22 mmHg or a 30% reduction from baseline at 12 months. However, with repeat treatment 72% of patients experienced such a reduction at 12 months post diode laser.Citation36 As such, CW-TSCPC may have a more significant role as an adjunct to surgery or in managing patients for whom surgery is undesirable due to increased risk of complications.
In summary, CW-TSCPC can be an excellent tool for IOP control in patients with difficult-to-manage glaucoma, but it is again not without its complications due to less-selective tissue ablation and collateral damage to other structures. Future direction for CW-TSCPC has been focused on more selective tissue ablation to allow for use in mild and moderate stages of glaucoma.
MicroPulse Transscleral Laser Treatment (MP-TLT)
Introduced to the U.S. market in 2015, MP-TLT (IRIDEX Corp., Mountainview, CA) is the newest form of diode-laser technology (). It has quickly emerged as a promising alternative to CW-TSCPC due to its comparable efficacy and potentially more favorable safety profile. In contrast to CW-TSCPC, MP-TLT utilizes repetitive pulses of energy separated by periods of rest, which allows for more targeted treatment of the pigmented tissue in the ciliary processes.
Figure 2. (a) The Generation 1 MicroPulse Probe. (b) The Generation 2 MicroPulse Probe. (c) The Generation 1 MicroPulse Probe in use
While no prior histopathological studies have described the actual mechanism of action for MP-TLT, it appears that IOP reduction is achieved through a few distinct pathways. One proposed mechanism is that MP-TLT primarily targets the pigmented tissue in the ciliary body epithelium. The adjacent non-pigmented structures are given time to recover during the “off cycle”, which prevents them from reaching the coagulative threshold and subsequently minimizes collateral tissue damage.Citation37,Citation38 Another mechanism proposed by Johnstone et al. postulates that that the pigmented epithelium is not necessarily involved in the IOP-reducing effect of MP-TLT. Rather, the authors suggest that the mechanism of the laser is similar to that of pilocarpine, and it induces contraction of the ciliary muscles, leading to posterior and inward movement of the scleral spur and trabecular meshwork. This histological study showed that the changes caused by the laser were confined to the longitudinal bundles of ciliary muscle, with no visible changes to the ciliary epithelium.Citation39
In addition to increasing the amount of outflow through the trabecular meshwork, there is evidence that MP-TLT also increases aqueous humor outflow via the uveoscleral pathway. In a prospective study of 22 patients who underwent the procedure, Barac et al. demonstrated that an increase in foveolar choroidal thickness following MP-TLT was associated with a positive response to the treatment.Citation40 As the variations in choroidal thickness observed here were likely due to a rise in uveoscleral outflow, it appears that some of the IOP-reducing ability of MP-TLT can be attributed to the increase in aqueous humor drainage through the uveoscleral pathway.Citation40 The authors reported no changes to the visual acuity of the patients.
Studies of MP-TLT have consistently demonstrated its ability to effectively reduce IOP in a wide array of glaucoma types (). Similar to studies of CW-TSCPC, direct comparison between studies of MP-TLT can be difficult given the differences between patient populations and the variability in laser settings used. Prior studies have indicated that the IOP-lowering effect at 1 year can vary widely, ranging from 6.9 to 12.6 mmHg.Citation37,Citation41-Citation47 One plausible explanation for the wide range in reported IOP reduction is the difference in mean baseline IOP, as the magnitude of IOP reduction in MP-TLT also appears to be strongly correlated with baseline IOP. For example, Garcia et al. reported a mean IOP reduction of 6.9 mm Hg from a baseline of 22.2 mm Hg, while Emanuel et al. reported a mean reduction of 16.6 mm Hg from a baseline of 27.7 mm Hg.Citation41 The majority of MP-TLT studies evaluated have used an IOP reduction ≥20% from baseline as the criteria for success. Once again, the reported success rates (at 3–12 months postoperatively) vary widely, ranging from 67.1% by Zaroour et al. to 93.1% by Garcia et al.Citation42,Citation46
In addition to lowering IOP, MP-TLT has proven to be effective in reducing the medication burden of glaucoma patients. It has consistently been able to reduce the amount of glaucoma medications, both oral and topical, needed by patients across all studies examined, with a mean reduction at 1 year ranging from 0.5 to 1.6 medications.Citation37,Citation42,Citation45,Citation47-Citation49 In a study by Tan et al., all 6 patients on oral acetazolamide were able to stop taking it following the procedure, while 17/65 (26%) patients in a study by Zaarour et al. were able to discontinue oral medication use.Citation37,Citation48
MP-TLT is a potentially valuable treatment tool for specific types of glaucoma patients, especially post-keratoplasty eyes and pediatric patients. In a retrospective study of 61 such eyes, Subramaniam et al. reported that the graft survival rate was 94% at 1 year, and 81% at 2 years following the initial laser treatment.Citation50 Many patients included in this study had previously undergone glaucoma filtration surgery. The authors noted that the graft failure rate observed here was unremarkable, given that prior filtration surgery is a major risk factor for graft failure. They reported a mean IOP reduction of 13.0 mm Hg at 12 months postoperatively from a baseline of 28.0 mmHg.Citation50 A handful of recent studies have explored a role for MP-TLT in the management of pediatric glaucoma. In a study of 36 pediatric glaucoma eyes, Elhefney et al. reported that IOP was reduced from 37.5 mmHg to 20.0 mmHg, and medications reduced from 2.6 to 1.7, 15 months after MP-TLT.Citation51 Sixty-six percent of eyes required a second treatment session to maintain control of IOP, and no major complications were observed in any patients.Citation51
Notably, Abdelrahman et al. compared CW-TSCPC and MP-TLT in a cohort of pediatric refractory glaucoma patients. With success defined as an IOP between 5 and 21 mmHg in the absence no vision-threatening complications at 6 months, the authors reported a greater success rate in the MP-TLT (71%) group compared to the CW-TSCPC (46%) group, however, this difference was not statistically significant. Moreover, they did not observe any significant complications in the MP-TLT group, while there were cases of phthisis bulbi (2) and severe pain and uveitis in the CW-TSCPC group.Citation52 While prospective randomized trials are needed to truly compare these procedures in pediatric glaucoma management, MP-TLT may be equally effective with a lower risk of complications.
There is currently a lack of consensus on the influence of a history of prior glaucoma surgery on the success rates of MP-TLT. For example, Garcia et al. found that prior incisional glaucoma surgery was positively correlated with the success rates of MP-TLT and patients who had previously undergone trabeculectomy, tube shunt surgery, or a combination thereof, had a success rate of 67.6% versus a success rate of 41.4% for patients had not had these procedures.Citation42 Interestingly, a history of Minimally-Invasive Glaucoma surgery (MIGS) did not seem to have this same effect, as no differences in IOP reduction were found between a subgroup of patients that underwent previous MIGS and a subgroup that had not.Citation42
The notion that prior traditional glaucoma surgery has a favorable effect on the success of MP-TLT is disputed by results reported by Zaarour et al. who reported no difference in the success rates of patients who had previous incisional glaucoma surgery and those who had not.Citation46 Differences between the studies in the laser settings and population of glaucoma patients studied can potentially explain this discrepancy. For example, the laser settings used by Garcia et al., which demonstrated a positive correlation between prior incisional surgery and success rates, were in some cases tailored based on the severity of glaucoma.Citation42 In contrast, Zaarour et al. used standardized settings throughout.Citation46 Garcia et al also included a substantially greater proportion of primary open-angle glaucoma (POAG) patients, 56.9% versus 34.7%.Citation42,Citation46 These differences could potentially account for the differences reported in the analyses of patients who underwent prior incisional surgery.
As MP-TLT is still a relatively new technique, the optimal laser settings have yet to be defined. There is considerable variation between studies in the laser settings used with regard to both power and duration of treatment. The power settings in the studies examined in ranged from 1.6–2.4 W, while the total treatment duration ranged from 100 to 360 s. There is also variability in the probe motion used by providers when performing MP-TLT. The sweeping or painting technique is currently recommended by the manufacturer, in which the probe is moved in a slow, continuous back-and-forth sliding motion along the arcs around the limbus. However, some studies have demonstrated promising results using the discrete spot or stop-and-continue techniques.Citation45,Citation53 In a population of refractory glaucoma patients who had previously undergone MP-TLT under more standard settings, Ting et al. reported favorable outcomes using both the sweeping and discrete spot techniques.Citation53
The effectiveness of higher-than-usual MP-TLT settings has recently been demonstrated by Anand et al.Citation45 The higher-than-usual settings in this study were defined as treating at least 180 seconds per hemisphere with a power between 2 and 2.4 W, along with a mix of the sweeping and stop-and-continue techniques. The authors observed a mean IOP reduction of 12.3 mmHg 6 months postoperatively, and an 8.9 mmHg reduction after 12 months. This was slightly better than the IOP reductions described by Yelenskiy et al. and Nguyen et al. who reported mean 12-month reductions of 6 mmHg (27%) and 7.6 mmHg (30%), respectively.Citation44,Citation54 Given the variability in laser power, duration, probe motion and patient populations used among studies of MP-TLT, the optimum laser settings of MP-TLT remains elusive.
While MP-TLT has proven to be effective in controlling IOP, its IOP-lowering effect appears to wane over time. For example, Zaroour et al. reported that while 86.7% of patients achieved surgical success (defined as an IOP reduction ≥20% from baseline) at 1-month post-MP-TLT treatment, the success rate dropped to 61.7% at the 6-month visit and 56.7% at the 12-month visit.Citation46 Additionally, Garcia et al. demonstrated a success rate of 93.1% at 3-months, which dropped to 67.4% at 6 months and 59.6% at 12 months.Citation42 A similar decline in the success rate was observed by Anand et al. using the higher-than-usual settings, dropping from 80% at 6-weeks postoperatively to 69% at 1-year.Citation45 Interestingly, it appears that these patients treated with the higher-than-usual settings did not experience as drastic a decline in success rate compared to those in other studies, which may indicate a potential influence of the laser settings used on the longevity of MP-TLT treatment.Citation45
The recent shift in favor of MP-TLT over CW-TSCPC is largely due to the sentiment that it results in fewer postoperative complications, while being equally as effective. As expected, the rates of postoperative complications seem to be comparatively lower in MP-TLT than in CW-TSCPC. While the highest reported rate of prolonged hypotony was just 6% in the reviewed studies of MP-TLT, it has been reported in up to 18% of patients who received CW-TSCPC.Citation28 MP-TLT also appears to put patients at significantly less risk of losing visual acuity following the procedure. Decline in visual acuity has been reported in up to 64% of patients following CW-TSCPC, while the highest reported rate of visual acuity loss following MP-TLT was 41%.Citation37,Citation42,Citation44-Citation48 Incidences of prolonged anterior chamber (AC) inflammation (defined as 1+ cell or flare for >3 months) have been reported in as few as 0.8%, but up to 10% of patients.Citation37,Citation41,Citation42,Citation45-Citation49,Citation55 Rates of CME have consistently been minimal across studies of MP-TLT with the majority of studies reporting no cases of CME post operatively, and even when reported the incidence rate has varied from 1–6%. Persistent mydriasis was not a commonly reported complication in studies of MP-TLT. When persistent mydriasis was reported, the incidence rate ranged from 0 to 3.2%, and was significantly more common following MP-TLT in Asian patients.Citation56 While MP-TLT does appear to have a more favorable safety profile than CW-TSCPC, rates of subconjunctival hemorrhage are of potential concern.Citation57 In a prospective case series of 36 eyes, subconjunctival hemorrhage was reported in 32.3% of patients postoperatively, which appears to be higher than the rate seen in other cyclodestructive techniques. Additionally, the incidence of neurotrophic keratitis (NK) should be monitored. In a case study by Perez et al, the authors reported that two patients still developed NK postoperatively even when the 3 and 9 o’clock positions were avoided during treatment.Citation58 The occurrence of NK was also noted as a concern in an animal study evaluating the safety of MP-TLT in dogs.Citation59 Notably, the higher-than-usual settings used by Anand et al. did not appear to put patients at an increased risk of postoperative complications, as the rates of hypotony (6%), prolonged inflammation (4%), and visual decline (2%) were comparable to those reported in other studies.Citation37,Citation42,Citation44,Citation45,Citation47,Citation49,Citation54
All of the studies included in this review used the Generation 1 MP3 probe (IRIDEX Corp., Mountainview, CA) (). IRIDEX is currently in the process of phasing out the Generation 1 probe and shifting to the Generation 2 MP3 probe. According to the manufacturers, the Generation 2 MP3 probe has a new foot plate design but all other components of the laser are the same (). However, there are currently no studies that have studied the new probe’s efficacy or optimal laser settings in a clinical setting.
Endoscopic Cyclophotocoagulation (ECP)
ECP was originally developed by Martin Uram in 1992.Citation60–Citation62 The ECP probe (Endo Optiks, Little Silver, NJ) combines a diode endolaser, aiming beam, light source, and endoscope into a single intraocular probe (). This allows for targeted, controlled ablation of the ciliary processes with direct visualization and titration of power. Histologically, ECP-related tissue injury has been demonstrated to be effectively limited to the ciliary processes and associated capillary bed, sparing the ciliary muscle and stromal tissue.Citation55,Citation63 This is in contrast to histological changes observed following treatment with Nd:YAG and diode CW-TSCPC, in which significant disruption of the cells through the ciliary processes and a loss of blood vessels within the stroma has been observed.Citation64
Figure 3. (a) The endoscopic cyclophotocoagulation probe (ECP) (Endo Optiks, Little Silver, NJ) entering an eye. (b) Two intraoperative views of ciliary process during ECP treatment. Photographs from the archives of Dr. David Solá-Del Valle
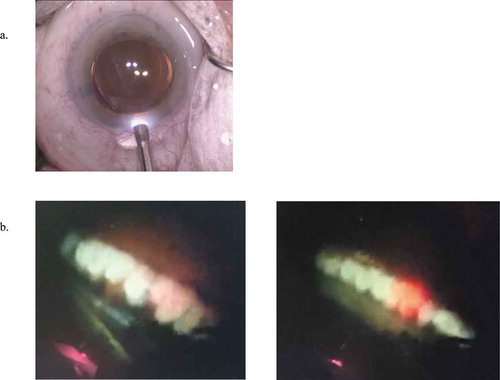
Numerous studies have demonstrated the effective IOP-lowering ability of ECP in a variety of glaucoma patients (). Most commonly, ECP is performed in conjunction with phacoemulsification cataract surgery (phaco-ECP). Specifically, reports of IOP reductions range from 2.7–11.5 mmHg, or 14.9%-46.9%, in cohorts of POAG patients depending on whether ECP is done alone or in combination with cataract surgery.Citation63,Citation65-Citation68
Table 3. Selection of Endoscopic Cyclophotocoagulation (ECP) studies from 2007–2018
Studies comparing phacoemulsification -ECP (phaco- ECP) to phacoemulsification alone have illuminated the IOP-lowering effect of ECP independent of the known effect of phacoemulsification. For example, Francis et al. observed a 2.7 mmHg reduction (17%) in IOP in open-angle glaucoma (OAG) patients 36 months after phaco-ECP, and only a 0.9 mmHg reduction (6%) in patients that underwent phacoemulsification alone. Similarly, Bartolome et al. observed a 23% IOP reduction 12 months after the combined procedure, and an 11% reduction after cataract surgery alone.Citation67
ECP treatment can be extended beyond its role in conjunction with cataract surgery, as it has also proven effective in treating cases of refractory glaucoma and NVG as a stand-alone procedure. Specifically, Francis et al. observed a 36% (8.7 mmHg) IOP reduction 18 months following ECP treatment in a group of patients with uncontrolled IOP and a prior tube shunt surgery.Citation64 In a similar manner, Murakami et al. compared pseudophakic patients with refractory glaucoma and a prior tube shunt who underwent ECP or a second tube shunt surgery to control IOP.Citation69 Throughout the 12-month follow-up period, no significant differences in postoperative IOP or glaucoma medication use were observed between those that underwent ECP and those that underwent an additional tube shunt surgery.Citation69
Similar efficacy was observed in a cohort of NVG patients. Specifically, NVG patients underwent a combination pars plana vitrectomy/panretinal photocoagulation/ECP or the current standard of care for patients with NVG, which included panretinal photocoagulation, filtration surgery, pars plana vitrectomy, or AGV placement. After 12 months, IOP was reduced by 28.4 mmHg in the combined ECP group, and 11.7 mmHg in the Ahmed valve group.Citation70
The effectiveness of ECP in cases of pediatric glaucoma has also been briefly studied. In a study of 35 eyes of patients under 16 years of age with glaucoma following cataract surgery, the success rate of ECP was 54% after a mean follow-up period of 7.2 years (treatment failure defined as consecutive IOP >24 mmHg, an additional glaucoma procedure, or visually significant complications). The average final IOP was 18.9 mmHg, compared to 33.9 mmHg preoperatively. The authors also reported that visual acuity was preserved in these patients from baseline to last follow-up.Citation71 Additionally, Glaser et al. reported success rates of 64%, 36%, and 16% at 1, 3, and 5 years, respectfully, following a single ECP treatment in 80 eyes with pediatric glaucoma. Similarly, treatment failure was defined as IOP >24 mmHg at 2 consecutive visits, any additional glaucoma surgery, sight-threatening complications, or progression to no light-perception vision.Citation72
In addition to effectively lowering IOP, ECP can provide significant reductions in patients’ topical and systemic glaucoma medication burden. In POAG patients, reductions in glaucoma medication use range from 0.4 to 1.4.Citation63,Citation65-Citation68,Citation73 Moreover, in cases of refractory glaucoma, ECP has been demonstrated to reduce glaucoma medication use by 1.5–1.7.Citation64,Citation69 Additionally, NVG patients that underwent treatment that included ECP experienced a 2.6 reduction in medications 12 months postoperatively, while the Ahmed glaucoma valve group experienced an increase in medications.Citation70
A number of studies have looked at the long-term effectiveness of ECP. Francis et al. demonstrated that phaco-ECP resulted in significant reductions in IOP up to 36 months postoperatively.Citation65 However, the authors reported that the initial downward trend in IOP seemed to level off at 12 months postoperatively in both phaco-ECP and phacoemulsification alone groups. Throughout the next 2 to 3 years, the eyes that underwent phaco-ECP remained stable at this level, while those that had cataract extraction alone showed regression towards the baseline IOP.
Furthermore, Bartolomé et al. noted that IOP initially decreased after phaco-ECP, then began to gradually rise 1 week following surgery. However, at 12 months postoperatively, IOP was still significantly reduced at 16.8 mmHg compared to 21.45 mmHg at baseline.Citation67 Additionally, Kaplan-Meier survival data revealed that although the success rate (defined as IOP <21 mmHg and at least a 20% reduction in IOP from baseline) declined over time, it remained relatively high at 69.6% 12 months postoperatively. This was significantly greater than the success rate of the phacoemulsification alone group, which was 40% at 12 months postoperatively.Citation67 Similarly, in a group of patients who had ECP with a failed prior tube shunt, Francis et al. found that IOP was reduced by 43% at month 1.Citation64 This gradually decreased over the 24-month follow-up period to 25%, however, mean IOP remained statistically significantly reduced from baseline throughout the 24-month follow-up period.
As previously mentioned, direct visualization of the ciliary processes provides a more targeted approach, limiting the tissue injury following ECP to the aqueous humor-producing cells and associated capillary bed. It is postulated that minimal collateral damage to the surrounding tissues during ECP contributes to its favorable safety profile. Specifically, complications associated with ECP in over 5000 eyes reported by the ECP Collaborative Study Group included: IOP spike (14.5%), hyphema (3.8%), choroidal detachment (0.36%), visual acuity decline (1.03%), CME (0.7%), and hypotony and phthisis (0.12%).Citation74
Similarly, low rates of hypotony, phthisis, and CME were observed in the studies reviewed in this article. For example, rates of CME ranged from 0 to 5.8%. Only one study reported incidences of prolonged hypotony that progressed to phthisis bulbi, occurring in 2 (7.4%) cases of both ECP-treated and control group NVG patients.Citation70 In this study, patients were diagnosed with phthisis if their IOP remained under 5 mmHg for 3 consecutive visits any time after 6 months after their initial treatment.Citation57 Additionally, no differences in postoperative complication rates were observed in studies that compared phaco-ECP to phacoemulsification alone.Citation64,Citation67
Compared to TS-CPC, which is associated with less-selective tissue ablation, ECP confers lower rates of hypotony or phthisis.Citation55,Citation75 When comparing the complication rates of the studies outlined in –, there are substantially fewer reports of hypotony or phthisis following ECP compared to both variants of TS-CPC. Excluding studies that primarily focused on patients with underlying NVG, as this subset of glaucoma tends to experience greater rates of postoperative complications following CPC, reported rates of hypotony are up to 9% with CW TS-CPC, 6% with MP-TLT, and 0% with ECP. Moreover, a decline in visual acuity is often observed following TS-CPC.Citation15,Citation30,Citation76 This effect is rarely seen with ECP, as it is most often performed in conjunction with cataract surgery, thereby maintaining or improving patients’ visual acuity.Citation23,Citation73
There is some evidence that the efficacy of ECP, with regard to IOP and medication reduction, may be dependent on the degrees of ciliary processes treated. For example, of the studies reviewed, anywhere from 200 to 360 degrees of ciliary processes were treated, signifying a lack of clinical consensus on the optimal treatment area.Citation66,Citation73 However, in a study investigating the efficacy of 240° versus 360° of treatment, Kahook et al. observed significantly greater reductions in IOP and glaucoma medication use in the 360° group.Citation63 Specifically, 360° of treatment conferred an additional reduction of 3.9 mmHg and 1.5 medications compared to 240° of treatment. Notably, 360° treatment did not add to the postoperative complication rate. Treating 360° of ciliary processes requires a second corneal incision and may add surgical time to the case, but the positive IOP lowering effect and few complications reported with 360° treatment warrant further investigation.
In addition to phacoemulsification, ECP can be combined with other MIGS that increase aqueous humor outflow. Simultaneously increasing aqueous outflow and decreasing aqueous production may potentially confer additional reductions in IOP and glaucoma medication usage. For example, the ICE procedure—combined iStent, phacoemulsification, and ECP— has been compared to phacoemulsification and iStent alone.Citation61 Specifically, Ferguson et al. reported a statistically significant greater reduction (7.49 mmHg) in the ICE group compared to the iStent and phacoemulsification group (4.66 mmHg) after 12 months. Both groups achieved statistically significant reductions in glaucoma medications after 12 months. However, despite similar numbers of medications at baseline in both groups, the reduction in medications at 12 months was significantly greater in the iStent and phacoemulsification group compared to the ICE group: 1.78 to 1.10 and 1.68 to 0.63, respectively.Citation77
ECP and phacoemulsification can also be combined with Kahook dual blade (KDB) goniotomy in what has been named the PEcK procedure. In a similar manner to ICE, this dual-mechanism procedure simultaneously reduces aqueous production and increases aqueous outflow. Preliminary, unpublished data by Klug et al. suggests that this combination procedure reduced IOP by 5.6 mmHg, and glaucoma medication use by 1.9, 6 months postoperatively. Average treatment parameters included 195º (120–250º) of ciliary process treated with 0.36 (0.2–0.55) W of power, and 4–5 clock hours of goniotomy. Mild hyphema, corneal edema, and anterior chamber inflammation were present, but all resolved spontaneously within 3 months postoperatively. No serious complications were observed.
High-intensity Focused Ultrasound Cyclodestruction (HIFU)
Transscleral ultrasound cyclodestruction utilizes focused ultrasound beams rather than laser energy to induce cyclodestruction of the ciliary body epithelium. HIFU primarily reduces IOP by decreasing aqueous humor production following thermic necrosis of the ciliary body epithelium. Unlike its laser counterparts, ultrasound absorption does not depend on the pigmentation of the ciliary body epithelium. The deposition of energy is therefore better controlled, avoiding much of the concern about collateral damage to surrounding tissues.Citation78
In addition to its effects on the ciliary body, HIFU has been demonstrated to induce significant modifications to the scleral and conjunctival anatomy, thereby increasing uveoscleral outflow through the supraciliary and suprachoroidal spaces.Citation79,Citation80 Specifically, Rouland and Aptel observed an increase in aqueous humor outflow due to a wider uveoscleral pathway, visible in ultrasound biomicroscopy pictures.Citation79 Moreover, using anterior segment optical coherence tomography and in vivo confocal microscopy, Mastropasqua et al. observed increases in intrascleral hyporeflective spaces and conjunctival microcysts at the site of treatment, suggesting aqueous humor passage through the sclera and the conjunctiva.Citation80
In the 1980s, HIFU technology was proposed as a potentially safer alternative to ciliary body destruction with an early commercially available device (Therapeutic Ultrasound System; Sonocare, Inc., Ridgewood, NJ). However, due to its large apparatus, operating times up to 2 hours, and poor safety profile, the device was not widely adopted and interest in the technique progressively waned.Citation81,Citation82 More recently, interest in ultrasound technology for glaucoma treatment has been revived with the development of a miniaturized HIFU device, the EyeOP1 (EyeTechCare, Rillieux-la-Pape, France).
The EyeOP1 device allows for a safer, faster, and more precise procedure. It is a ring comprised of six active piezoelectric transducers operating at a frequency of 21 MHz to achieve a rapid, selective coagulation of the ciliary body.Citation79 It has a particularly favorable safety profile, as ultrasound induces injury with much more controlled energy absorption than the laser modalities.Citation3,Citation83,Citation84 HIFU also involves a much slower temperature rise compared to TS-CPC, thus eliminating the risks involved with tissue explosion.Citation3
The initial pilot study using HIFU and the EyeOP1 device examined patients with refractory glaucoma and limited visual potential.Citation84 In this study, mean IOP was reduced by 13 mmHg 6 months postoperatively, and surgical success (IOP reduction ≥ 20% and IOP > 5 mmHg) was obtained in 83% of patients. A follow-up study demonstrated that HIFU was also an effective treatment option for POAG patients with much less advanced disease, displaying an average 7 mmHg (24%) reduction in IOP 12 months postoperatively.Citation79 More recently, these same authors have reported reductions in IOP ranging from 7.5 to 8.6 mmHg (32–33%) in similar cohorts of patients with mild-to-moderate disease.Citation79,Citation83 Average reductions in glaucoma medication burden across these studies range from 0 to 0.7.Citation84
Similar to its laser counterparts, HIFU may be more successful in particular subsets of glaucoma patients and with optimal treatment settings. Specifically, Giannaccare et al. observed an average 9.9 mmHg reduction after 6 months in patients with various types of refractory glaucoma.Citation85 However, the authors reported that the average percent reduction in IOP was highest in eyes with angle-closure glaucoma (38%), followed by those with NVG (26.2%), and lastly open-angle glaucoma (20%).Citation85
Additionally, Giannaccare et al. performed a sub-analysis regarding the ultrasound exposure time. Patients were treated with either 4, 6, or 8 seconds of ultrasound.Citation85 Those that underwent 8 seconds of treatment showed a significantly greater reduction in IOP than the other two groups. A similar dose-escalation study was performed examining 4 versus 6 seconds of exposure time. After 12 months, Denis et al. did not find a significant difference in the success rates (greater than 20% IOP decrease and IOP > 5 mmHg) of patients that underwent 4 versus 6 seconds of treatment. Perhaps a more substantial increase in exposure time, such as 8 seconds, is needed to reveal dose effects.
All studies outlined in report similar rates of postoperative complications following HIFU. In a discussion of the pilot, first and second multi-center, and current studies, Aptel et al. described the most common postoperative complications.Citation85–Citation88 No major intra or postoperative complications such as prolonged hypotony or phthisis occurred in any study. Superficial punctate keratitis was the most common complication, observed up to 46.4% of the time. Transitory cases of CME and anterior inflammation were also observed. Moreover, visual acuity remained stable across all studies.Citation86 In sum, while HIFU is yet to be available in the United States, its success abroad highlights the promise of this technology in safely and effectively treating a variety of glaucoma patients.
Table 4. Selection of High-Intensity Focused Ultrasound Cyclodestruction (HIFU) Studies from 2011–2017
CONCLUSION
With advances in technology, cyclodestruction has evolved from a procedure that used to be reserved for refractory glaucoma, to one that can be offered to almost any glaucoma patient under the right circumstances. The exciting advances in the realm of cyclodestruction have allowed for favorable IOP reduction with minimal postoperative complications, owing to more targeted destruction of the ciliary body.
It is our belief that the role of cyclodestruction in glaucoma treatment will only continue to expand, as it can be used in conjunction with other known IOP-lowering procedures, from phacoemulsification and MIGS, to incisional glaucoma surgery. Currently, there are no large randomized clinical trials published to help guide clinicians’ use of these cyclodestructive modalities. As the use of cyclodestructive procedures continues to expand, it will become increasingly imperative to set forth guidelines outlining optimal patient selection and cyclodestructive device settings through such trials.
DISCLOSURE STATEMENT
The authors of this paper have no relevant conflicts to disclose.
REFERENCES
- Quigley H, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi:10.1136/bjo.2005.081224.
- Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: A review. J Am Med Assoc. 2014;311(18):1901–1911. doi:10.1001/jama.2014.3192.
- Dastiridou AI, Katsanos A, Denis P, et al. Cyclodestructive procedures in glaucoma: a review of current and emerging options. Adv Ther. 2018;35(12):2103–2127. doi:10.1007/s12325-018-0837-3.
- Histological QH. Physiological studies of cyclocryotherapy in primate and human eyes. Am J Ophthalmol. 1976;85:722–732.
- Walton DS, Grant WM. Penetrating cyclodiathermy for filtration. Arch Ophthalmol. 1970;83(1):47–48. doi:10.1001/archopht.1970.00990030049008.
- Weekers R, Lavergne G, Watillon M, Gilson M, Legros AM. Effects of photocoagulation of ciliary body upon ocular tension. Am J Ophthalmol. 1961;52(2):156–163. doi:10.1016/0002-9394(61)91110-2.
- Beckman H, Kinoshita A, Rota A, Sugar H. Transscleral Ruby laser irradiation of the ciliary body in the treatment of intractable glaucoma. Am Acad Ophthalmol Otolaryngol. 1972;76(2):423–436.
- Beckman H, Sugar H. Neodymium laser cyclocoagulation. Arch Ophthalmol. 1973;90:27–28. doi:10.1001/archopht.1973.01000050029006.
- Oguri A, Sogano S, Yamamoto T, Kitazawa Y. Incidence of elevation of intraocular pressure over time and associated factors in normal-tension glaucoma. J Glaucoma. 1998;7(2):117–120. doi:10.1097/00061198-199804000-00009.
- Youn J, Cox TA, Herndon LW, Allingham RR, Shields MB. A clinical comparison of transscleral cyclophotocoagulation with neodymium: YAG and semiconductor diode lasers. Am J Ophthalmol. 1998;126(5):640–647. doi:10.1016/S0002-9394(98)00228-1.
- Lin P, Wollstein G, Glavas I, Schuman J. Contact transscleral neodymium: yttrium-aluminum-garnetlaser cyclophotocoagulation: long-term outcomes. Ophthalmology. 2004;111(22):2137–2143. doi:10.1016/j.ophtha.2004.05.027.
- Peyman GA, Naguib KS, Gaasterland D. Trans-scleral application of a semiconductor laser. Lasers Surg Med. 1990;10(6):569–575. doi:10.1002/lsm.1900100609.
- Ndulue J, Rahmatnejad K, Sanvicente C, Wizov S, Moster M. Evolution of Cyclophotocoagulation. J Ophthalmic Vis Res. 2018;13(1):55–61. doi:10.4103/jovr.jovr.
- Schubert HD. The influence of exposure duration in transscleral Nd: yAGLaser cyclophotocoagulation. Am J Ophthalmol. 1993;115(5):684–685. doi:10.1016/S0002-9394(14)71483-7.
- Frezzotti P, Mittica V, Martone G, et al. Longterm follow-up of diode laser transscleral cyclophotocoagulation in the treatment of refractory glaucoma. Acta Ophthalmol. 2010;88(1):150–155. doi:10.1111/j.1755-3768.2008.01354.x.
- Murphy C, Burnett C, Spry P, Broadway D, Diamond J. A two centre study of the dose-response relation for transscleral diode laser cyclophotocoagulation in refractory glaucoma. Br J Ophthalmol. 2003;87(10):1252–1257. doi:10.1136/bjo.87.10.1252.
- Egbert P, Fiadoyor S, Budenz D, Dadzie P, Byrd S. Diode laser transscleral cyclophotocoagulation as a primary surgical treatment for primary open-angle glaucoma. Arch Ophthalmol. 2001;119:658–662. doi:10.3760/cma.j..2095-0160.2016.07.018.
- Vernon S, Koppens J, Menon J, Negi A. Diode laser cycloablation in adult glaucoma: long-term results of a standard protocol and review of current literature. Clin Exp Ophthalmol. 2006;34(5):411–420. doi:10.1111/j.1442-9071.2006.01241.x.
- Hauber FA, Scherer WJ. Influence of total energy delivery on success rate after contact diode laser transscleral cyclophotocoagulation: a retrospective case review and meta-analysis. J Glaucoma. 2002;11:329–333. doi:10.1097/01.IJG.0000021460.34376.B0.
- Rotchford AP, Jayasawal R, Madhusudhan S, Ho S, King AJ, Vernon SA. Transscleral diode laser cycloablation in patients with good vision. Br J Ophthalmol. 2010;94(9):1180–1183. doi:10.1136/bjo.2008.145565.
- Williams AL, Moster MR, Rahmatnejad K, et al. Clinical efficacy and safety profile of micropulse transscleral cyclophotocoagulation in refractory glaucoma. J Glaucoma. 2018;27(5):445–449. doi:10.1097/IJG.0000000000000934.
- Albahlal A, Al Dhibi H, Al Shahwan S, Khandekar R, Edward DP. Sympathetic ophthalmia following diode laser cyclophotocoagulation. Br J Ophthalmol. 2014;98(8):1101–1106. doi:10.1136/bjophthalmol-2013-304257.
- Bloom PA, Tsai J, Sharma K, et al. “Cyclodiode”: trans-scleral diode laser cyclophotocoagulation in the treatment of advanced refractory glaucoma. Ophthalmology. 1997;104(9):1508–1520. doi:10.1016/S0161-6420(97)30109-2.
- Kosoko O, Gaasterland DE, Pollack IP, Enger CL. Long-term outcome of initial ciliary ablation with contact diode laser transscleral cyclophotocoagulation for severe glaucoma. The diode laser ciliary ablation study group. Ophthalmology. 1996;103(8):1294–1302. doi:10.1016/S0161-6420(96)30508-3.
- Noureddin BN, Zein W, Haddad C. Diode laser transcleral cyclophotocoagulation for refractory glaucoma: a 1 year follow-up of patients treated using an aggressive protocol. Eye. 2006;20(20):329–335. doi:10.1038/sj.eye.6701875.
- Schlote T, Derse M, Rassmann K, Nicaeus T, Dietz K, Thiel HJ. Efficacy and safety of contact transscleral diode laser cyclophotocoagulation for advanced glaucoma. J Glaucoma. 2001;10(4):294–301. doi:10.1097/00061198-200108000-00009.
- Ramli N, Htoon HM, Ho CL, Aung T, Perera S. Risk factors for hypotony after transscleral diode cyclophotocoagulation. J Glaucoma. 2012;21(3):169–173. doi:10.1097/IJG.0b013e318207091a.
- Iliev ME, Gerber S. Long-term outcome of trans-scleral diode laser cyclophotocoagulation in refractory glaucoma. Br J Ophthalmol. 2007;91(12):1631–1635. doi:10.1136/bjo.2007.116533.
- Pucci V, Tappainer F, Borin S, Bellucci R. Long-term follow-up after transscleral diode laser photocoagulation in refractory glaucoma. Ophthalmologica. 2003;217:279–283. doi:10.1159/000070635.
- Ghosh S, Manvikar S, Ray-Chaudhuri N, Birch M. Efficacy of transscleral diode laser cyclophotocoagulation in patients with good visual acuity. Eur J Ophthalmol. 2014;24(3):375–381. doi:10.5301/ejo.5000389.
- Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL. Treatment outcomes in the tube versus trabeculectomy study after one year of follow-up. Am J Ophthalmol. 2007;143(1). doi:10.1016/j.ajo.2006.07.020.
- Yildrim N, Yalva I, Sahin A, Ozer A, Bozca T. A comparative study between diode laser cyclophotocoagulation and the ahmed glaucoma implant in neovascular glaucoma. J Glaucoma. 2009;18(3):192–196. doi:10.1097/IJG.0b013e31817d235c.
- Shah P, Lee G, Kirwan J, et al. Cyclodiode photocoagulation for refractory glaucoma after penetrating keratoplasty. Ophthalmology. 2001;108(11):1986–1991. doi:10.1016/S0161-6420(01)00767-9.
- Papadopoulos M, Khaw PT. Advances in the management of paediatric glaucoma. Eye. 2007;21(10):1319–1325. doi:10.1038/sj.eye.6702850.
- Autrata R, Rehurek J. Laser-assisted subepithelial keratectomy for myopia: two-year follow-up. J Cart Refract Surg. 2003;29(4):661–668. doi:10.1016/S0886-3350(02)01897-7.
- Kirwan JF, Shah P, Khaw PT. Diode laser cyclophotocoagulation: role in the management of refractory pediatric glaucomas. Ophthalmology. 2002;109(2):316-323. doi:10.1016/s0161-6420(01)00898-3
- Tan AM, Chockalingam M, Aquino MC, Lim ZIL, See JLS, Chew PT. Micropulse transscleral diode laser cyclophotocoagulation in the treatment of refractory glaucoma. Clin Exp Ophthalmol. 2010;38(3):266–272. doi:10.1111/j.1442-9071.2010.02238.x.
- Desmettre TJ, Mordon SR, Buzawa DM, Mainster MA. Micropulse and continuous wave diode retinal photocoagulation: visible and subvisible lesion parameters. Br J Ophthalmol. 2006;90(6):709–712. doi:10.1136/bjo.2005.086942.
- Johnstone M, Wang R, Padilla S, Wen K. Transcleral laser induces aqueous outflow pathway motion and reorganization. Poster presented at: The 27th Annual AGS Meeting; 2017; Coronado, CA, USA.
- Barac R, Vuzitas M, Balta F. Choroidal thickness increase after micropulse transscleral cyclophotocoagulation. Rom J Ophthalmol. 2018;61(2):144–148. doi:10.22336/rjo.2018.21.
- Emanuel ME, Grover DS, Fellman RL, et al. Micropulse cyclophotocoagulation: initial results in refractory glaucoma. J Glaucoma. 2017;26(8):726–729. doi:10.1097/IJG.0000000000000715.
- Garcia GA, Nguyen CV, Yelenskiy A, et al. Micropulse transscleral diode laser cyclophotocoagulation in refractory glaucoma. Ophthalmol Glaucoma. 2019;2(6):402–412. doi:10.1016/j.ogla.2019.08.009.
- Kuchar S, Moster MR, Reamer CB, Waisbourd M. Treatment outcomes of micropulse transscleral cyclophotocoagulation in advanced glaucoma. Lasers Med Sci. 2016;31(2):393–396. doi:10.1007/s10103-015-1856-9.
- Anand N, Nirappel A, Klug E, Chachanidze M, Solá-Del Valle D Outcomes of Iridex MicroPulse P3 (MP3) with higher-than-usual settings for the management of elevated eye pressure. Poster presented at: The 30th Annual AGS Meeting; 2020; Washington D.C., USA.
- Zaarour K, Abdelmassih Y, Arej N, Cherfan G, Tomey KF, Khoueir Z. Outcomes of micropulse transscleral cyclophotocoagulation in uncontrolled glaucoma patients. J Glaucoma. 2019;28(3):270–275. doi:10.1097/IJG.0000000000001174.
- Varikuti VNV, Shah P, Rai O, et al. Outcomes of micropulse transscleral cyclophotocoagulation in eyes with good central vision. J Glaucoma. 2019;28(10):901–905. doi:10.1097/IJG.0000000000001339.
- Aquino MCD, Barton K, Tan AMWT, et al. Micropulse versus continuous wave transscleral diode cyclophotocoagulation in refractory glaucoma: A randomized exploratory study. Clin Exp Ophthalmol. 2015;43(1):40–46. doi:10.1111/ceo.12360.
- Pastor S, Singh K, Lee D, et al. Cyclophotocoagulation: A report by the american academy of ophthalmology. Ophthalmoogy. 2001;108(11):2130–2138. doi:10.1016/S0161-6420(01)00889-2.
- Subramaniam K, Price M, Feng M, Price F Jr. Micropulse transscleral cyclophotocoagulation in keratoplasty eyes. Cornea. 2019;38(5):542–545. doi:10.1097/ICO.0000000000001977.
- Elhefney EM, Mokbel TH, Hagras SM, et al. Micropulsed diode laser cyclophotocoagulation in recurrent pediatric glaucoma. Eur J Ophthalmol. 2019;1–7. doi:10.1177/1120672119858226.
- Abdelrahman AM, El Sayed YM. Micropulse versus continuous wave transscleral cyclophotocoagulation in refractory pediatric glaucoma. J Glaucoma. 2018;27(10):900–905. doi:10.1097/IJG.0000000000001053.
- Ting KWY, Koh Teck Chang V, Aquino CM, Macasaet AM, Suwandono ME, Chew Tec Kuan P. MP3 plus: a modified micropulse trans-scleral cyclophototherapy technique for the treatment of refractory glaucoma. J Glaucoma. 2020;0000000000001443. doi:10.1097/IJG.0000000000001443.
- Yelenskiy A, Gillette TB, Arosemena A, et al. Patient outcomes following micropulse transscleral cyclophotocoagulation: intermediate-term results. J Glaucoma. 2018;27(10):920–925. doi:10.1097/IJG.0000000000001023.
- Francis B, Flowers B, Dastiridou AI, Yelenskiy A, Chopra V, Alvarado JA. Endoscopic cyclphotocoagulation and other cyclodestructive methods. Ophthalmol Glaucoma. 2019;2(6):413–421. doi:10.1016/j.ogla.2019.08.008.
- Radhakrishnan. Outcomes of micropulse cyclophotocoagulation - A multicenter review. Poster presented at: The 26th Annual AGS Meeting; 2016; Fort Lauderdale, FL.
- Hierro C, Ascencio D, Larrea C, Roman J. Micropulse transscleral cyclophotocoagulation in refractory glaucoma. 6 month follow-up. Poster presented at: ARVO 2019; 2019; Vancouver, BC..
- Perez CI, Han Y, Rose-Nussbaumer J, Ou Y, Hsia YC. Neurotrophic keratitis after micropulse transscleral diode laser cyclophotocoagulation. Am J Ophthalmol Case Rep. 2019;15(May2018):100469. doi:10.1016/j.ajoc.2019.100469.
- Sebbag L, Allbaugh RA, Strauss RA, et al. MicroPulseTM transscleral cyclophotocoagulation in the treatment of canine glaucoma: preliminary results (12 dogs). Vet Ophthalmol. 2019;22(4):407–414. doi:10.1111/vop.12603.
- Tan JCH, Francis BA, Noecker R, Uram M, Dustin L, Chopra V. Endoscopic cyclophotocoagulation and pars plana ablation (ecp-plus) to treat refractory glaucoma. J Glaucoma. 2016;25(3):e117–e122. doi:10.1097/IJG.0000000000000278.
- Uram M. Combined phacoemulsification, endoscopic ciliary process photocoagulation, and intraocular lens implantation in glaucoma management. Ophthalmic Surg. 1995;26:346–352.
- Uram M. Ophthalmic laser microendoscope ciliary process ablation in the management of neovascular glaucoma. Ophthalmology. 1992;99(12):1823–1828. doi:10.1016/S0161-6420(92)31718-X.
- Pantcheva MB, Kahook MY, Schuman JS, Noecker RJ. Comparison of acute structural and histopathological changes in human autopsy eyes after endoscopic cyclophotocoagulation and trans-scleral cyclophotocoagulation. Br J Ophthalmol. 2007;91(2):248–252. doi:10.1136/bjo.2006.103580.
- Francis BA, Kawji AS, Vo NT, Dustin L, Chopra V. Endoscopic cyclophotocoagulation (ECP) in the management of uncontrolled glaucoma with prior aqueous tube shunt. J Glaucoma. 2011;20(8):523–527. doi:10.1097/IJG.0b013e3181f46337.
- Francis BA, Berke SJ, Dustin L, Noecker R. Endoscopic cyclophotocoagulation combined with phacoemulsification versus phacoemulsification alone in medically controlled glaucoma. J Cataract Refract Surg. 2014;40(8):1313–1321. doi:10.1016/j.jcrs.2014.06.021.
- Roberts SJ, Mulvahill M, SooHoo JR, Pantcheva MB, Kahook MY, Seibold LK. Efficacy of combined cataract extraction and endoscopic cyclophotocoagulation for the reduction of intraocular pressure and medication burden. Int J Ophthalmol. 2016;9(5):693–698. doi:10.18240/ijo.2016.05.09.
- Pérez Bartolomé F, Rodrigues IA, Goyal S, et al. Phacoemulsification plus endoscopic cyclophotocoagulation versus phacoemulsification alone in primary open-angle glaucoma. Eur J Ophthalmol. 2018;28(2):168–174. doi:10.5301/ejo.5001034.
- Bloom PA, Clement CI, King A, et al. A comparison between tube surgery, ND:YAG laser and diode laser cyclophotocoagulation in the management of refractory glaucoma. Biomed Res Int. 2013;2013(1):1–11. doi:10.1155/2013/371951.
- Murakami Y, Akil H, Chahal J, et al. Endoscopic cyclophotocoagulation versus second glaucoma drainage device after prior aqueous tube shunt surgery. Clin Exp Ophthalmol. 2017;45(3):241–246. doi:10.1111/ceo.12828.
- Marra KV, Wagley S, Omar A, et al. Case-matched comparison of vitrectomy, peripheral retinal endolaser, and endocyclophotocoagulation versus standard care in neovascular glaucoma. Retina. 2015;35(6):1072–1083. doi:10.1097/IAE.0000000000000449.
- Cantor AJ, Wang J, Li S, Neely DE, Plager DA. Long-term efficacy of endoscopic cyclophotocoagulation in the management of glaucoma following cataract surgery in children. J Aapos. 2018;22(3):188–191. doi:10.1016/j.jaapos.2018.01.014.
- Glaser TS, Mulvihill MS, Freedman SF. Endoscopic cyclophotocoagulation (ECP) for childhood glaucoma: a large single-center cohort experience. J Aapos. 2019;23(2):84.e1-84.e7. doi:10.1016/j.jaapos.2018.10.014.
- Izquierdo Villavicencio JC, Gonzalez Mendez AL, Ramirez Jimenez I, et al. Clinical results of endocyclophotocoagulation in patients with cataract and open-angle glaucoma at Oftalmosalud Eye Institute, Lima-Peru. J Clin Exp Ophthalmol. 2018;9:6. doi:10.4172/2155-9570.1000762.
- The ECP Collaborative Study Group. Complications of ECP: a large, long term, multicenter study. Ocul Surg News. 2006.
- Ishida K. Update on results and complications of cyclophotocoagulation. Curr Opin Ophthalmol. 2013;24(2):102–110. doi:10.1097/ICU.0b013e32835d9335.
- Tabibian D, Wride N, Birch M, Figueiredo FC. Contact transscleral cyclodiode laser treatment for refractory glaucoma after penetrating keratoplasty: retrospective long-term outcomes. J Glaucoma. 2019;28(5):440–446. doi:10.1097/IJG.0000000000001205.
- Ferguson TJ, Swan R, Sudhagoni R, Berdahl JP. Microbypass stent implantation with cataract extraction and endocyclophotocoagulation versus microbypass stent with cataract extraction for glaucoma. J Cataract Refract Surg. 2017;43(3):377–382. doi:10.1016/j.jcrs.2016.12.020.
- Lim KS. Ultrasound cycloplasty in glaucoma - mechanisms of action and their possible impact on intraocular pressure. Eur Ophthalmic Rev. 2017;11(1):35–39. doi:10.17925/EOR.2017.11.01.35.
- Aptel F, Rouland J. Ultrasound ciliary plasty to treat glaucoma: efficacy and safety results on 152 patients. Poster presented at: 2017 European Association for Vision and Eye Research Conference; 2017; Nice, Province Alpes Cote.
- Mastropasqua R, Agnifili L, Fasanella V, et al. Uveo-scleral outflow pathways after ultrasonic cyclocoagulation in refractory glaucoma: an anterior segment optical coherence tomography and in vivo confocal study. Br J Ophthalmol. 2016;100(12):1668–1675. doi:10.1136/bjophthalmol-2015-308069.
- Burgess SE, Silverman RH, Coleman DJ, et al. Treatment of glaucoma with high-intensity focused ultrasound. Ophthalmology. 1986;93(6):831–838. doi:10.1016/S0161-6420(86)33672-8.
- Coleman DJ, Lizzi FL, Driller J, et al. Therapeutic ultrasound in the treatment of glaucoma. Ophthalmology. 1985;92(3):347–353. doi:10.1016/S0161-6420(85)34028-9.
- Denis P, Aptel F, Rouland JF, et al. Cyclocoagulation of the ciliary bodies by high-intensity focused ultrasound: A 12-month multicenter study. Investig Ophthalmol Vis Sci. 2015;56(2):1089–1096. doi:10.1167/iovs.14-14973.
- Charrel T, Aptel F, Birer A, et al. Development of a miniaturized HIFU device for glaucoma treatment with conformal coagulation of the ciliary bodies. Ultrasound Med Biol. 2011;37(5):742–754. doi:10.1016/j.ultrasmedbio.2011.01.017.
- Giannaccare G, Vagge A, Gizzi C, et al. High-intensity focused ultrasound treatment in patients with refractory glaucoma. Graefe’s Arch Clin Exp Ophthalmol. 2017;255(3):599–605. doi:10.1007/s00417-016-3563-z.
- Aptel F, Denis P, Rouland JF, Renard JP, Bron A. Multicenter clinical trial of high-intensity focused ultrasound treatment in glaucoma patients without previous filtering surgery. Acta Ophthalmol. 2016;94(5):e268–e277. doi:10.1111/aos.12913.
- Aptel F, Dupuy C, Rouland JF. Treatment of refractory open-angle glaucoma using ultrasonic circular cyclocoagulation: A prospective case series. Curr Med Res Opin. 2014;30(8):1599–1605. doi:10.1185/03007995.2014.910509.
- Berke SJ. Endolaser cyclophotocoagulation in glaucoma management. Tech Ophthalmol. 2006;4(2):74–81. doi:10.1097/00145756-200606000-00008.

