ABSTRACT
Background: Topical prostaglandin analogs (PGAs) are widely approved and preferred first-line options for glaucoma and elevated intraocular pressure (IOP). However, prostaglandin-associated periorbitopathy syndrome (PAPS) is now a well-recognized clinical and cosmetic concern for patients receiving PGAs, especially during long-term and unilateral therapy. PGA-associated periocular changes occur in a substantial proportion of patients, with older patients (>60 years) at greater risk of clinical presentation. PAPS may hinder long-term management of glaucoma, including treatment adherence, ophthalmic surgery outcomes, and reliable IOP measurements. Recommendation: New therapeutic approaches may address this unmet clinical need. Omidenepag isopropyl (OMDI) is a novel, non-prostaglandin, selective EP2 receptor agonist in ongoing development, which provides a unique pharmacological mechanism of action. OMDI appears to provide IOP reductions comparable to PGAs, but without PAPS-related undesirable effects. OMDI may offer a suitable long-term option for patients who demonstrate decreased efficacy, or failure, of PGAs, plus patients with significant PAPS, while fulfilling international guidelines.
INTRODUCTION
Defining Prostaglandin-associated Periorbitopathy Syndrome (PAPS)
The progressive optic neuropathy associated with glaucoma results in loss of visual acuity and constriction of visual fields. Topical prostaglandin analogs (PGAs) are widely approved and preferred as a first-line treatment option for the management of glaucoma and ocular hypertension (OHT). These agents include latanoprost (0.005%), travoprost (0.004%), bimatoprost (0.01% and 0.03%), and tafluprost (0.0015%), and are characterized by a potent intraocular pressure (IOP)-lowering efficacy, few systemic side effects, and good adherence associated with once-daily regimens.1,Citation2 However, PGAs have been reported to induce periocular changes and development of local periorbitopathic side effects early, in >40% of patients treated for ≥3 months, and increases over time, in >60% of patients after just 6 months of topical treatment.Citation2,Citation3 In fact, the development of early-onset periorbitopathy has even been identified as early as within a month of PGA initiation.Citation4
The term prostaglandin-associated periorbitopathy (PAP) was first coined by Dr Stanley Berke in 2012,Citation5 and is now well recognized as comprising a significant constellation of clinical and cosmetic changes in any one of up to ten factors (see , ).6,Citation7 Notable signs of PAP include flattening of the lower eyelid bags (FLEB), deepening of the upper eyelid sulcus (DUES), upper eyelid ptosis, orbital fat atrophy, and ciliary hypertrichosis.Citation1,Citation8,Citation9 Upper eyelid retraction or eyelid redness have also been reported as less recognized aspects of the PAP spectrum.Citation10
Table 1. Clinical and cosmetic signs of prostaglandin-associated periorbitopathy syndrome (PAPS)
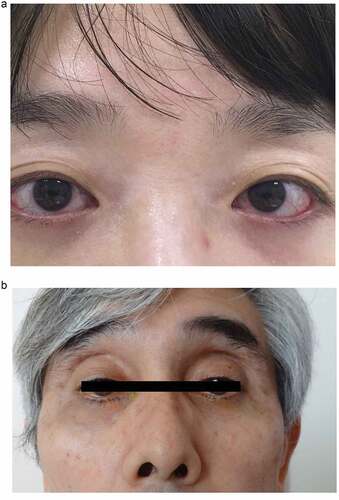
Figure 1. A case of DUES caused by treatment with benzalkonium chloride (BAK)-preserved tafluprost ophthalmic solution.
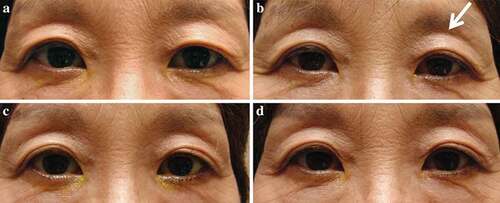
Figure 2. Notable signs of PAPS.
In 2015, Sarnoff and Gotkin referred to the constellation of PAP symptoms as prostaglandin-associated periorbitopathy syndrome (PAPS).Citation11 In one study, the prevalence of PAPS (defined as the presence of DUES and at least three additional clinical manifestations) was reported as >40% in patients treated with a PGA for ≥3 months, with older patients (>60 years) demonstrating up to three times the risk of clinical presentation (OR 3.0; 95% CI: 1.2–7.8).Citation3
The most prominent feature of PAPS is the appearance of, or worsening of, DUES,Citation6,Citation10,Citation12 which is one of the most significant adverse cosmetic outcomes associated typically with application of bimatoprost and travoprost,Citation1,Citation12 although it has also been observed with induction of topical treatment with latanoprost and tafluprost (see ).Citation6,Citation13 Retrospective studies and case series suggest that development of DUES is associated with the use of all available PGAs, with incidence varying depending on the agent.Citation4,Citation14 In a small series of Japanese patients (n = 25) newly diagnosed with open-angle glaucoma (OAG), 60% were reported to have developed DUES as early as 3 months after switching from latanoprost to bimatoprost. The incidence of DUES was significantly higher in older patients and in non-myopic eyes (p < .01), but not related to sex or IOP reduction. In patients assessed by a clinician as having DUES, over half also self-reported their cosmetic changes at 6 months.Citation1,Citation12
In addition, the choice of pre-operative PGA and cosmetic changes associated with the presence of DUES can have a significant impact on ophthalmic clinical measures (such as IOP measurement) and surgical outcomes (for example, with trabeculectomy).Citation15,Citation16 In a small, retrospective study, following trabeculectomy for OAG, patients with PGA-associated DUES before surgery were observed to be at a significantly higher risk of recurrent IOP elevation up to 24 months post-surgery than patients without DUES (p < .0001). Bimatoprost use was found to be the highest risk (and only significant independent factor) for post-trabeculectomy recurrence of IOP elevation.Citation15
Long-term use of PGAs (with preservatives) may also have some biomechanical and pathological impact on the ocular surface, including changes in corneal hysteresis and corneal resistance factor, decreases in central corneal thickness, delays in corneal wound healing, and occurrences of conjunctival hyperemia and dry eye disease.Citation17 Clinical data have previously demonstrated numerous intraoperative situations in which eyelid tissues appear abnormal in patients on long-term treatment with a PGA, often adding complexity to ptosis repair surgery.Citation18
In patients treated with PGA for unilateral glaucoma, PAPS may cause visible facial asymmetry,Citation2 potentially reducing adherence to treatment as well as patients’ confidence. In addition, PAPS may also hinder successful and long-term clinical management of glaucoma, including filtration surgery, and even reliable IOP measurements. Thus, PAPS may be of significant clinical concern for patients receiving treatment with PGAs, especially for those receiving long-term treatment and the elderly.
With bilateral PGA treatment, significant reductions in patient interpupillary distance (IPD) may provide an indicator of developing PAP.Citation19 Following PGA instillation, IPDs have demonstrated significant shortening (–0.80 ± 2.1 mm) compared with control patients (p < .001); change in IPD after bimatoprost instillation (–2.20 ± 0.97 mm) was significantly (p < .001) greater than with other PGAs, including travoprost, latanoprost, and tafluprost (–0.65 ± 2.09 mm). Over 85% of patients receiving bimatoprost demonstrated IPD decreases of ≥2 mm.19
It is proposed that management of PAPS and associated symptoms can usually be achieved by simple discontinuation of the causative therapy, which often results in partial-to-complete reversal of PAP characteristics (as early as 4–6 weeks).Citation20 Much like the onset of PAPS, observation of PAP reversal is more noticeable in those treated unilaterally, so thorough documentation and measurements can be helpful in assessing the extent of atrophy reversal.
This review aims to highlight an unmet clinical need in patients with PAPS requiring lowering of IOP, and evaluate new clinical solutions – including a novel, non-prostaglandin, selective EP2 (a prostaglandin E receptor) agonist.
PAPS AND NEW CLINICAL SOLUTIONS
Non-prostaglandin Selective EP2 Agonists
For more than two decades, EP2 agonists have been the subject of anti-glaucoma research and development, leading to the commercial approval of omidenepag isopropyl (OMDI) in Japan (known as EYBELIS™).Citation21
OMDI is a novel, non-PGA derivative currently in Phase III global clinical development for lowering IOP, which has demonstrated improvements in signs and symptoms of PAPS in patients switched from PGA therapy.Citation7,Citation8,Citation22 OMDI provides an alternative pharmacological mechanism of action to PGAs, lacking measurable binding to the prostaglandin F (FP) receptor, and instead selectively binding to EP2: a G-protein-coupled receptor expressed widely in ocular tissues, including the trabecular meshwork and ciliary body involved in conventional and uveoscleral outflow.Citation7 OMDI demonstrates a similar pharmacokinetic profile across Japanese and Caucasian subjects.Citation23
PGAs Mechanism of Action
PGAs have high specificity for the FP receptor, which has a crucial role in reducing IOP by both increasing the outflow and decreasing resistance to outflow of aqueous fluid from the eyes.Citation11,Citation24 Long-term, repeated treatment with prostaglandin F2α (PGF2α) in preclinical models provides a long-lasting reduction in IOP, which persists following treatment cessation. This long-term effect is likely due to expansion of the intermuscular spaces in the ciliary body through extracellular matrix remodelling.Citation25
In addition, PGA exposure is hypothesized to influence expression of matrix metalloproteinase (MMP) and tissue inhibitors of metalloproteinases (TIMP) in a tissue-specific manner, with decreased expression of certain MMPs in collagen correlating to increased periorbitopathy.Citation26 PGA-mediated activation of MMP promoters leads to alterations in MMP and TIMP levels, which are responsible for remodeling the extracellular matrix (ECM) by increasing uveoscleral and trabecular outflow, and ultimately resulting in reduction of IOP. However, remodeling of the ECM scaffold has been postulated to play a role in PAPS by increasing fibrous tissue in pterygium epithelial cells, and in conjunctival scarring, indicating a time-dependent effect of PGAs on the tissue ultrastructure that maintains ocular and periorbital physiology. Such dysregulation in the ECM scaffold has a role in eyelid malpositions and may have implications for ptosis and PAP through alterations of MMP/TIMP dynamics.Citation26
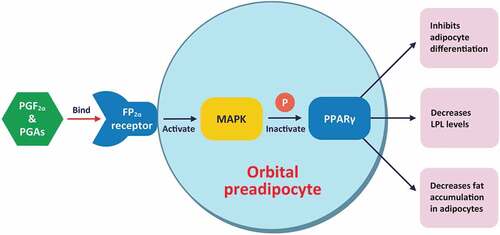
In preclinical models, PGAs inhibit adipogenesis through FP receptor stimulation, which may lead directly to reduced orbital fat.Citation27 PGF2α and PGAs bind to the prostaglandin F2α receptor (FP2α) on orbital preadipocytes and activate mitogen-activated protein kinase (MAPK), resulting in phosphorylation and inactivation of peroxisome proliferator activated receptor gamma (PPARγ) – leading to inhibition of adipocyte differentiation, decreased lipoprotein lipase (LPL) levels (a marker for adipocyte differentiation), and decreased fat accumulation within adipocytes (see ).Citation11
Figure 3. Mechanism of action and impact of PGAs on adipose changes in PAP.
PGAs are also associated with eyelash growth and increased pigmentation of the periorbital skin. Exposure promotes cell growth, inducing a growth phase in resting follicles and hypertrophic follicle changes. These changes result in increased length and thickness of lashes, additional lash rows, conversion of vellus to terminal hairs in canthal areas, as well as increased growth and pigmentation of ancillary hairs around the eyelids.Citation28
OMDI Mechanism of Action
Prostaglandin E2 (PGE2) is an endogenous lipid with potent IOP-lowering effects; it has four distinct receptors: EP1, EP2, EP3, and EP4.Citation21,Citation25 OMDI is an isopropyl ester derivative that hydrolyzes to an active EP2 agonist metabolite during corneal penetration, which targets the intracellular signaling cascade and elevates cyclic adenosine monophosphate levels (see ).Citation29,Citation32,Citation33
Figure 4. Receptor-based mechanism of action.
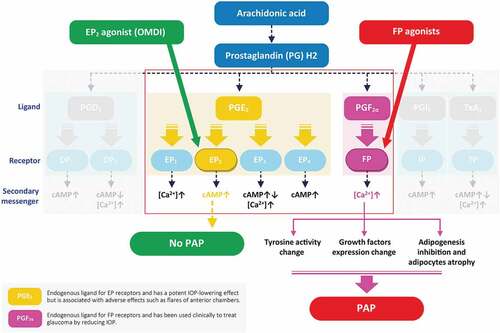
Available PGAs for the reduction of IOP have a strong binding affinity for FP, but very weak or no binding affinity to the EP2 receptor.Citation22 Elevations in Ca2+ detected with the PGF2α pathway following PGA application are associated with elevated tyrosinase activity and associated iris pigmentationCitation30; by comparison, OMDI is not associated with elevated tyrosinase activity, as it has no effect on any other prostaglandin receptors.
The IOP-lowering effect of OMDI is induced by enhancement of both conventional and uveoscleral outflow routes, potentially through relaxation of the ciliary muscle, which constitutes part of the uveoscleral outflow apparatus. Thus, the impact of an EP2 agonist would include the enhancement of drainage due to an increase in the ciliary intermuscular space via relaxation.Citation22 Notably, EP2 agonists promote similar effects (to those described earlier with PGF2α analogs) by upregulating MMPs and remodeling the ECM to produce intermuscular spaces in the ciliary body for aqueous egress.Citation34 In addition to the enhancement of outflow of aqueous humor via uveoscleral outflow,Citation31 EP2 receptor agonists may facilitate conventional trabecular outflow pathways by decreasing the quantity of collagenous materials, resulting in stable IOP reductions.Citation22,Citation35,Citation36 Preclinical models of OHT demonstrate that OMDI significantly increased the rates of both aqueous facility and uveoscleral outflow by 71% and 176%, compared with vehicle (p < .05 for both).Citation36 In dose-finding studies, OMDI significantly decreased mean diurnal IOP from baseline in patients with primary OAG or OHT in both eyes, resulting in clinically relevant reductions that were superior to placebo and non-inferior to latanoprost (p < .005).Citation37
In contrast to FP agonists, EP2 agonists do not promote eyelash growth and do not inhibit adipogenesis. OMDI studies report no significant effects on eyelash growth or the eyelash growth cycle over 52 weeks of treatment.Citation7,Citation38 A key difference is that OMDI affects adipogenesis of 3T3-L1 cells in a different manner to PGAs, suggesting it would not be associated with the characteristic orbital fat atrophy observed in PGA-mediated DUES (as shown in ).Citation32,Citation39
Thus, a selective EP2 agonist has the potential to lower IOP similar to current PGF2α analogs, but with PAPS being unlikely with the former.Citation34
OMDI PHASE III STUDIES IN LOWERING IOP
Multiple Phase II studies of OMDI have been conducted (in the United States and Japan). These dose-finding studies have demonstrated OMDI to be generally well tolerated with clinically relevant IOP-lowering effects in patients with primary OAG and OHT. In these studies, maximum IOP reductions were achieved within 1 week of treatment initiation, demonstrating an early onset of action that was maintained for up to 3 months.Citation37
OMDI 0.002% has been approved by authorities in Japan (2018) for the treatment of glaucoma and OHT to lower IOP; a global clinical development program is ongoing and includes four Phase III studies. The randomized, investigator-masked, active-controlled, parallel-group AYAME study demonstrated that once-daily OMDI was non-inferior to once-daily latanoprost (0.005%) in reducing IOP in Japanese patients with primary OAG or OHT (n = 190) for up to 4 weeks.Citation40 The ongoing, non-inferiority PEONY study is designed to examine mean diurnal IOP reduction at 3 months versus latanoprost (n = 370).Citation41
Of the two open-label studies, the 52-week, single-arm RENGE study primarily assessed change in mean diurnal IOP in Japanese patients with primary OAG (including normal tension glaucoma and exfoliation glaucoma), or OHT in both eyes; OMDI treatment resulted in sustained IOP reductions over 12 months across patients with either normal (≥16 to <22 mmHg) or hypertensive IOP (≥22 to ≤34 mmHg) at baseline.Citation38 The multicenter FUJI study reported clinically significant reductions in IOP from baseline at Week 4 (−2.99 mmHg, p < .0001) in patients switched to OMDI following low or no response to latanoprost 0.005% during an 8-week run-in period (≤15% IOP reduction over 8 weeks).Citation42
Important Safety Considerations
Phase III clinical studies highlight that OMDI 0.002% is generally well tolerated in the IOP-managed patient population with no serious safety concerns presenting in those that are complete. In the Phase II and III studies, OMDI was associated with conjunctival hyperemia, a common side effect of FP receptor agonists. In the 4-week AYAME study, the incidence of conjunctival hyperemia was 24.5% (compared with 10.4% in the latanoprost group) but was reported as mild, transient, and not associated with treatment discontinuation.Citation40 Conjunctival hyperemia is a well-recognized side effect of ocular hypotensive treatment, with varying incidences in FP agonists (between 4% and 50% of patients), as well as being observed in up to 20% of patients receiving brimonidine and up to 53% of patients receiving netarsudil.Citation40 The incidence observed in the AYAME study, as well as in both the FUJI and RENGE studies (7.7% and 14.6–18.9%, respectively),Citation38,Citation42 falls within this typical range.Citation40 The exact cause of OMDI-induced hyperemia is currently unknown, yet short-term assessment (up to 6 hours after administration of one drop of OMDI) of conjunctival hyperemia patterns suggests that OMDI predominantly affects small blood vessels only.Citation43 A longer-term study would be required to determine the exact time course of hyperemia during OMDI treatment.Citation40
Increased central corneal thickness (CCT) was reported more frequently following OMDI than latanoprost in the AYAME study.Citation40 CCT change from baseline ranged from 13.0–18.7 µm (approximately 3%; mean 15 µm) and remained constant throughout the study period. Investigators considered these increases in CCT not to be clinically significant, being within the range of physiological change that occurs after one night of sleep (3–8% overnight corneal swelling). There was no reported corneal edema associated with CCT changes, as evaluated by slit-lamp microscopy, and no impact on visual acuity was observed.Citation40
IMPROVEMENTS IN PAPS SIGNS IN PATIENTS SWITCHING FROM PGA THERAPY TO OMDI
Notably, within the clinical trials previously mentioned, none of the eyes treated with OMDI experienced abnormal eyelash growth or PAPS.Citation34 Patients switched from a long-term, conventional PGA to OMDI have reported significant improvements in the signs of PAPS.Citation7,Citation8 A recent Japanese case series described patients (n = 11 eyes) with PAP that were prospectively switched from conventional PGAs (mean duration of 65 months [range, 24–112 months]) to OMDI and were assessed 3 months and 6 months later. At 6 months, three patients show improvements in DUES, two in FLEB, and two in ciliary hypertrichosis.Citation8 Recovery of periorbital skin hyperpigmentation was the most dramatic change reported, with eight patients showing resolution at 6 months. There was no improvement in upper eyelid ptosis in any patient across the 6-month study duration.Citation8
In a longer-term follow-up study, lessening of DUES was the most observed improvement following a 12-month switch to OMDI; patients (n = 12) had previously received PGAs for a mean duration of 61 months (range, 24–115 months). Reduction in DUES was observed in ≤50% of patients and was most frequent in prior users of bimatoprost and travoprost, with the majority of responders showing a reduction in DUES by Month 3 of treatment.Citation7 However, it is important to consider that the lessening of DUES may be due to cessation of conventional PGAs alone, and not OMDI directly.
Improvements in FLEB and periorbital skin hyperpigmentation were also demonstrated over the 12-month study, whereas recovery of ciliary hypertrichosis was the least observed sign with ≤8% of patients improved by study completion. It was considered that the impact of PGA on the eyelash growth cycle may be long-lasting.Citation7 Patients included in the 12-month follow-up study were also monitored for IOP, which was maintained within expected levels. No significant changes in IOP were observed during the follow-up period, with mean IOP measurements of 16.5 ± 1.5 mmHg, 15.4 ± 1.9 mmHg, 15.5 ± 1.7 mmHg, and 15.9 ± 1.8 mmHg at baseline, and 3, 6 and 12 months, respectively (p = .394 by one-way ANOVA).Citation7
DISCUSSION
IOP-lowering treatment with PGAs is a standard of care for primary OAG and OHT. Now, after over two decades of first-line use, the consequences of long-term exposure have become more familiar.Citation34 PAPS is an often-overlooked consequence that appears following long-term PGA use, with most side effects being cosmetic in nature – including eyelash growth, periorbital skin pigmentation, DUES, and FLEB. While not typically a reason to avoid or discontinue therapy in many patients, the occurrence of these manifestations can limit the use of PGAs, especially in unilateral therapy and younger patients who are more concerned about cosmetic appearances.Citation34 Within glaucoma, patient-delivered regimens rely heavily on treatment adherence, and therefore the reversal or avoidance of PAPS remains a clinical unmet need.
Alongside the visually disconcerting manifestations of PAPS, which is often more noticeable in unilateral treatment, PAPS may also be asymptomatic and be associated with a variety of functional limitations, pertaining to clinical practice and surgery – such as overestimation of IOP measurement due to tight orbit syndrome. Severe cases of PAPS, although relatively uncommon, have the potential to hinder eyedrop retention and lead to complexity in ophthalmic surgery.Citation34
One option typically considered to manage PAPS is the discontinuation of the causative therapy. However, cessation of treatment is associated with a range of inherent issues, such as increasing IOP and worsening of glaucoma itself, as well as a need to resort to other topical or systemic medications, which may be less efficacious and be associated with other side effects linked to long-term management. In addition, PGA-therapy switching itself is unlikely to be a long-term solution for PAPS. Outcomes from a prospective, open-label study in Japanese patients with primary OAG suggest that switching from bimatoprost to latanoprost offers a potential avenue for reversing DUES, although the study included a limited self-reporting population (n = 13) over a 6-month follow-up, and not all patients experienced improvements.Citation12
Following failure or resistance to first-line treatments for IOP reduction, both the European Glaucoma Society and Asia-Pacific Glaucoma Society recommend that patients are switched to an alternative drug class rather than considering same-class combination therapy.Citation44,Citation45 Clinical studies indicate that OMDI 0.002% may provide an effective solution for switching patients with glaucoma from PGA monotherapy.Citation40,Citation42 It is suggested that OMDI may not cause PAPS, likely due to its unique mechanism of action, which does not inhibit adipogenesis, and therefore offers benefits to many patients.Citation34 Outcomes from the Phase III AYAME study suggest that OMDI may provide IOP-lowering benefits comparable with those of latanoprost.Citation40 However, no data exist directly comparing the IOP-lowering effects of other PGAs and OMDI. Further research may be necessary to characterize the IOP-lowering benefits of OMDI in comparison with other first-line treatments in glaucoma and the long-term efficacy and safety profile of this drug.
In children and young adults with a history of childhood glaucoma, mild-to-moderate changes in the ocular adnexa have been reported following long-term PGA exposure.Citation46 Incidence of eyelash trichomegaly and hypertrichosis (76%), high upper eyelid crease (69%), upper eyelid ptosis (52%), and superior sulcus hollowing (52%) were the most frequently observed features of PAPS associated with PGA treatment in this young patient population.Citation46 Moreover, both patients and their caregivers are often sensitive to cosmetic orbital changes. The impact of potential future decades of cumulative PGA exposure on both physical appearance and normal development should also be considered, especially when initiating monocular therapy.Citation46 Thus, management of unilateral glaucoma may be a key clinical consideration in preventing asymmetrical DUES, with OMDI providing an important treatment option for relatively young patients requiring long-term IOP management.
Initial opportunities for OMDI treatment could include management of OHT, and patients with OAG receiving monotherapy who do not require significant IOP lowering. For example, good candidates, for a different class of eye drops other than PGAs, could include patients who already have deep-set eyes at baseline or those who need only unilateral treatment. In addition, the reductions in uveoscleral flow rate associated with OMDI administration may provide benefits for patients with angle-closure glaucoma; current clinical evaluations have predominantly included patients with primary OAG and OHT. Yet, research has also demonstrated significant IOP-lowering efficacy in treatment-naïve patients with normal tension glaucoma.Citation47
Further long-term clinical data are required to fully elucidate the value and position of OMDI in the treatment paradigm of IOP-lowering (including to confirm any clinically meaningful differences to PGAs) and PAPS resolution, as well as to evaluate its additivity to other classes of IOP-lowering therapy.
CONCLUSIONS
The evolution and subsequent impact of PAPS are significant clinical concerns among patients receiving long-term treatment with PGAs, especially those with unilateral therapy. There is an unmet need for medical therapy that is more effective and/or well tolerated, and which could be considered a practical long-term option. OMDI appears to provide IOP reductions comparable with those of PGAs with a markedly different safety profile and to lower IOP in non- or low responders to latanoprost. Although the modest IOP reductions reported in comparative studies may limit the appeal of OMDI for first-line treatment versus PGAs, the avoidance of PAPS and associated long-term cosmetic and clinical consequences should be key clinical factors to consider a switch in glaucoma treatment. The addition of OMDI to the IOP-lowering armamentarium provides an alternative for lessening PAPS typically associated with PGAs while also fulfilling international guideline criteria following PGA recalcitrancy or failure. Further research and large head-to-head comparisons of OMDI are required, with particular emphasis on extended time frames, to identify any significant clinical benefit OMDI may offer over standard-of-care treatments.
LITERATURE SEARCH
Electronic search of the PubMed (MEDLINE) database was performed for the years 2010–2021 with the keywords ‘prostaglandin’, ‘PGA’, ‘periorbitopathy’, ‘PAP’, ‘PAPS’, ‘omidenepag isopropyl’, ‘OMDI’, ‘glaucoma’, ‘intraocular pressure’, and ‘IOP’. Some articles that were not found by the PubMed search were taken from the citations of other articles and books. Case reports were only included if they contributed new information about characteristics, diagnosis or treatment of the disease. We included reports of orbital Only articles with English translation were included.
Acknowledgments
Medical writing support was provided by Synergy Vision.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aihara M, Shirato S, Sakata R. Incidence of deepening of the upper eyelid sulcus after switching from latanoprost to bimatoprost. Jpn J Ophthalmol. 2011;55(6):600–604. doi:10.1007/s10384-011-0075-6.
- Manju M, Pauly M. Prostaglandin-associated periorbitopathy: a prospective study in Indian eyes. Kerala J Ophthalmol. 2020;32(1):36–40. doi:10.4103/kjo.kjo_90_19.
- Patradul C, Tantisevi V, Manassakorn A. Factors related to prostaglandin-associated periorbitopathy in glaucoma patients. Asia Pac J Ophthalmol. 2017;6(3):238–242. doi:10.22608/APO.2016108.
- Kucukevcilioglu M, Bayer A, Uysal Y, et al. Prostaglandin associated periorbitopathy in patients using bimatoprost, latanoprost and travoprost. Clin Exp Ophthalmol. 2014;42(2):126–131.doi:10.1111/ceo.12163.
- Berke S. PAP: new concerns for prostaglandin use. Review of Ophthalmology 2012. Available at: reviewofophthalmology.com/article/pap-new-concerns-for-prostaglandin-use [Last accessed 28 January 2021].
- Sakata R, Shirato S, Miyata K, et al. Incidence of deepening of the upper eyelid sulcus on treatment with a tafluprost ophthalmic solution. Jpn J Ophthalmol. 2014;58(2):212–217.doi:10.1007/s10384-013-0299-8.
- Oogi S, Nakakura S, Terao E, et al. One-year follow-up study of changes in prostaglandin-associated periorbital syndrome after switch from conventional prostaglandin F2alfa to omidenepag isopropyl. Cureus. 2020;12(8):e10064.doi:10.7759/cureus.10064.
- Nakakura S, Terao E, Fujisawa Y, et al. Changes in prostaglandin-associated periorbital syndrome after switch from conventional prostaglandin F2α treatment to omidenepag isopropyl in 11 consecutive patients. J Glaucoma. 2020;29(4):326–328.doi:10.1097/IJG.0000000000001442.
- Shah M, Lee G, Lefebvre DR, et al. A cross-sectional survey of the association between bilateral topical prostaglandin analogue use and ocular adnexal features. PLoS ONE. 2013;8(5):e61638.doi:10.1371/journal.pone.0061638.
- Rabinowitz MP, Katz LJ, Moster MR, et al. Unilateral prostaglandin-associated periorbitopathy: a syndrome involving upper eyelid retraction distinguishable from the aging sunken eyelid. Ophthalmic Plast Reconstr Surg. 2015;31(5):373–378.doi:10.1097/IOP.0000000000000351.
- Sarnoff DS, Gotkin RH. Bimatoprost-induced chemical blepharoplasty. J Drugs Dermatol. 2015;14:472–477.
- Sakata R, Shirato S, Miyata K, et al. Recovery from deepening of the upper eyelid sulcus after switching from bimatoprost to latanoprost. Jpn J Ophthalmol. 2013;57(2):179–184.doi:10.1007/s10384-012-0219-3.
- Sakata R, Shirato S, Miyata K, et al. Incidence of deepening of the upper eyelid sulcus in prostaglandin associated periorbitopathy with a latanoprost ophthalmic solution. Eye. 2014;28(12):1446–1451.doi:10.1038/eye.2014.224.
- Inoue K, Shiokawa M, Wakakura M, et al. Deepening of the upper eyelid sulcus caused by 5 types of prostaglandin analogs. J Glaucoma. 2013;22(8):626–631.doi:10.1097/IJG.0b013e31824d8d7c.
- Miki T, Naito T, Fujiwara M, et al. Effects of pre-surgical administration of prostaglandin analogs on the outcome of trabeculectomy. PLoS ONE. 2017;12(7):e0181550.doi:10.1371/journal.pone.0181550.
- Yaoeda K, Fukushima A, Shirakashi M, et al. Factors associated with fluctuations in repeated measurements of intraocular pressure using the Goldmann applanation tonometer in Japanese patients with primary open-angle glaucoma. Clin Ophthalmol. 2018;12:1473–1478. doi:10.2147/OPTH.S174277.
- Shen W, Huang B, Yang J. Ocular surface changes in prostaglandin analogue-treated patients. J Ophthalmol. 2019;9798272. doi:10.1155/2019/9798272.
- Sweeney AR, Williams KJ, Dermarkarian CR, et al. Topical prostaglandin analog use is associated with increased failure rate of ptosis repair. Ophthalmology. 2020;27(2):276–278.doi:10.1016/j.ophtha.2019.09.007.
- Sano I, Takahashi H, Inoda S, et al. Shortening of interpupillary distance after instillation of topical prostaglandin analog eye drops. Am J Ophthalmol. 2019;206:11–16. doi:10.1016/j.ajo.2019.03.013.
- Peplinski LS, Smith KA. Deepening of lid sulcus from topical bimatoprost therapy. Optom Vis Sci. 2004;81(8):574–577. doi:10.1097/01.opx.0000141791.16683.4a.
- Woodward DF, Wang JW, Stamer WD, et al. Antiglaucoma EP2 agonists: a long road that led somewhere. J Ocul Pharmacol Ther. 2019;35(9):469–474.doi:10.1089/jop.2019.0041.
- Kirihara T, Taniguchi T, Yamamura K, et al. Pharmacologic characterization of omidenepag isopropyl, a novel selective EP2 receptor agonist, as an ocular hypotensive agent. Invest Ophthalmol Vis Sci. 2018;59(1):145–153.doi:10.1167/iovs.17-22745.
- Aihara M, Lu F, Kawata H, et al. Pharmacokinetics, safety, and intraocular pressure-lowering profile of omidenepag isopropyl, a selective, nonprostaglandin, prostanoid EP2 receptor agonist, in healthy Japanese and Caucasian volunteers (Phase I study). J Ocul Pharmacol Ther. 2019;35(10):542–550.doi:10.1089/jop.2019.0044.
- Ota T, Aihara A, Saeki T, et al. The IOP-lowering effects and mechanism of action of tafluprost in prostanoid receptor-deficient mice. Br J Ophthalmol. 2007;91(5):673–676.doi:10.1136/bjo.2006.105585.
- Doucette LP, Walter MA. Prostaglandins in the eye: function, expression, and roles in glaucoma. Ophthalmic Genet. 2017;38(2):108–116. doi:10.3109/13816810.2016.1164193.
- Karli S, Ayala-Haedo JA, Feuer WJ, et al. Effect of prostaglandin analogs on matrix metalloproteinases and tissue inhibitor of metalloproteinases in eyelid muscle specimens. Clin Ophthalmol. 2018;12:2039–2046. doi:10.2147/OPTH.S178106.
- Taketani Y, Yamagishi R, Fujishiro T, et al. Activation of the prostanoid FP receptor inhibits adipogenesis leading to deepening of the upper eyelid sulcus in prostaglandin-associated periorbitopathy. Invest Ophthalmol Vis Sci. 2014;55(3):1269–1276.doi:10.11667/iovs.13-13589.
- Johnstone MA, Albert DM. Prostaglandin-induced hair growth. Surv Ophthalmol. 2002;47(Suppl 1):S185–202. doi:10.1016/s0039-6257(02)00307-7.
- Mohan S, Ahmad AS, Glushakov AV, et al. Putative role of prostaglandin receptor in intracerebral hemorrhage. Front Neurol. 2012;3:145. doi:10.3389/fneur.2012.00145.
- Dutkiewicz R, Albert DM, Levin LA. Effects of latanoprost on tyrosinase activity and mitotic index of cultured melanoma lines. Exp Eye Res. 2000;70(5):563–569. doi:10.1006/exer.1999.0819.
- Fuwa M, Toris CB, Fan S, et al. Effects of a novel selective EP2 receptor agonist, omidenepag isopropyl, on aqueous humor dynamics in laser-induced ocular hypertensive monkeys. J Ocular Pharmacol Ther. 2018;34(7):531–537.doi:10.1089/jop.2017.0146.
- Ida Y, Hikage F, Umetsu A, et al. Omidenepag, a non‑prostanoid EP2 receptor agonist, induces enlargement of the 3D organoid of 3T3‑L1 cells. Sci Rep. 2020;10:16018. doi:10.1038/s41598-020-72538-x.
- Yan K, Gao LN, Cui YL, et al. The cyclic AMP signaling pathway: exploring targets for successful drug discovery (Review). Mol Med Rep. 2016;13(5):3715–3723.doi:10.3892/mmr.2016.5005.
- Aihara M, Aung T, Bacharach J, et al. Omidenepag isopropyl ophthalmic solution for open-angle glaucoma and ocular hypertension: an update. Exp Rev Ophthalmol. 2021;16:243–250. doi:10.1080/17469899.2021.1935241.
- Duggan S. Omidenepag isopropyl ophthalmic solution 0.002%: first global approval. Drugs. 2018;78(18):1925–1929. doi:10.1007/s40265-018-1016-1.
- Ferro Desideri L, Cutolo CA, Barra F, et al. Omidenepag isopropyl for the treatment of glaucoma and ocular hypertension. Drugs Today (Barc). 2019;55(6):377–384.doi:10.1358/dot.2019.55.6.2984806.
- Aihara M, Lu F, Kawata H, et al. Phase 2, randomized, dose-finding studies of omidenepag isopropyl, a selective EP2 agonist, in patients with primary open-angle glaucoma or ocular hypertension. J Glaucoma. 2019;28(5):375–385.doi:10.1097/IJG.0000000000001221.
- Aihara M, Lu F, Kawata H, et al. IOP-lowering efficacy and long-term safety of omidenepag isopropyl 0.002%, a selective EP2 agonist, in OAG and OHT. Poster PT-WT-127. Presented at World Glaucoma Congress (WGC) 2019, March 27-30, Melbourne, Australia. Available at: worldglaucomacongress.org/wp-content/uploads/ninja-forms/8/WGC-AYAME-RENGE-poster_Draft-2_12Mar2019.pdf [Last Accessed 18 September 2020].
- Yamamoto Y, Taniguchi T, Inazumi T, et al. Effects of the selective EP2 receptor agonist omidenepag on adipocyte differentiation in 3T3-L1 Cells. J Ocul Pharmacol Ther. 2020;36(3):162–169.doi:10.1089/jop.2019.0079.
- Aihara M, Lu F, Kawata H, et al. Omidenepag isopropyl versus latanoprost in primary open-angle glaucoma and ocular hypertension: the Phase 3 AYAME study. Am J Ophthalmol. 2020;220:53–63. doi:10.1016/j.ajo.2020.06.003.
- Clinical trials.gov. A Phase III study assessing the safety and efficacy of DE-117 ophthalmic solution compared with latanoprost ophthalmic solution in subjects with OAG or OHT (PEONY study). Available at: https://clinicaltrials.gov/ct2/show/NCT02981446 [Last Accessed 28 January 2021].
- Aihara M, Ropo A, Lu F, et al. Intraocular pressure‑lowering effect of omidenepag isopropyl in latanoprost non‑/low‑responder patients with primary open‑angle glaucoma or ocular hypertension: the FUJI study. Jpn J Ophthalmol. 2020;64(4):398–406.doi:10.1007/s10384-020-00748-x.
- Terao E, Nakakura S, Nagata Y, et al. Evaluation of patterns and correlations of the degree of conjunctival hyperemia induced by omidenepag isopropyl 0.002% and ripasudil 0.4%. Cureus. 2020;12(9):e10368.doi:10.7759/cureus.10368.
- Asia-Pacific Glaucoma Society (APGS). Asia-Pacific Glaucoma Guidelines [Internet]. Third ed. Amsterdam, The Netherlands: Kugler Publications. Available at: https://apglaucomasociety.org/Public/Public/Default.aspx [Last Accessed. 2016.
- European Glaucoma Society (EGS). Terminology and guidelines for glaucoma, 4th Edition - Chapter 3: treatment principles and options. Supported by the EGS Foundation. Br J Ophthalmol. 2017;101:130–195. doi:10.1136/bjophthalmol-2016-EGSguideline.003.
- Kim JS, Blizzard S, Woodward JA, et al. Prostaglandin-associated periorbitopathy in children and young adults with glaucoma. Ophthalmol Glaucoma. 2020;3(4):288–294.doi:10.1016/j.ogla.2020.03.009.
- Inoue K, Inoue J, Kunimatsu-Sanuki S, et al. Short-term efficacy and safety on omidenepag isopropyl in patients with normal-tension glaucoma. Clin Ophthalmol. 2020;14:2943–2949. doi:10.2147/OPTH.S271789.
