ABSTRACT
Purpose
Certain peripheral retinal degenerations pose a significant risk to vision and require prompt detection and management. Other historically “benign” peripheral lesions are being recognised as clinically significant due to their associations with ocular and systemic disorders. Assessment and documentation of these entities however can be difficult due to challenges in visualisation of the peripheral retina. This review addresses this by providing a series of clinical examples of these entities visualised with a variety of ocular imaging technologies.
Methods
A literature search was performed in Embase, Medline, and Google Scholar. We identified and analysed all papers referring to peripheral retinal degenerations and the peripheral retina, as well as reference lists of retrieved articles until August 2019.
Results
Using ocular imaging technologies including ultra-widefield imaging and peripheral optical coherence tomography, we comprehensively describe current evidence and knowledge of a number of peripheral retinal degenerations and anomalies including microcystoid, pavingstone, lattice, snail track, snowflake and reticular pigmentary degenerations, peripheral drusen, white without pressure, retinal holes and vitreoretinal tufts. A summary of these entities is also provided as a short and easily interpretable chairside guide to facilitate the translation of this evidence base into clinical practice.
Conclusion
While ocular technologies are useful in visualising peripheral retinal degenerations, the current evidence is fragmented throughout the literature and there is a paucity of information on imaging of “benign” peripheral lesions. This review facilitates a multimodal imaging approach to evaluating peripheral lesions.
Introduction
Peripheral retinal degenerations associated with retinal breaks, tears, and detachments can pose a significant risk to ocular health and functional vision and require prompt detection and clinical management.Citation1 Other peripheral lesions such as microcystoid, pavingstone, and reticular degeneration are typically considered benign but may become clinically significant in terms of their associations with ocular and systemic disorders.Citation2–8 Similarly, there are a number of normal peripheral retinal features which are not associated with disease but it can be misdiagnosed as more sinister lesions. With up to 31% of individuals having a peripheral retinal degeneration,Citation5 a thorough understanding of these ‘benign’ peripheral retina entities is essential for eye-care practitioners to ensure appropriate diagnosis and management.
Advances in ocular imaging have allowed better visualisation and documentation of the peripheral retina and its interface with the vitreous and choroid. Techniques including ultra-wide field (UWF) imaging, fundus autofluorescence (FAF), infrared imaging, and optical coherence tomography (OCT) have all contributed to both improved detection and diagnosis of these changes as well as our understanding of their aetiology and prognosis. Specific descriptions of peripheral lesions using ocular imaging modalities are however scattered throughout the literature with many descriptions found as case series, limiting the effective translation of this knowledge into clinical practice. This is particularly problematic considering the growing emphasis for digital modes of practice, which involve remote assessment of patients through ocular imaging or integration of artificial intelligence assistance systems based on imaging results. Indeed in other ocular diseases, such as macular disease, knowledge of multimodal imaging insights is necessary for appropriate management of all stages of diseaseCitation9 and settings with clinicians highly trained in ocular imaging interpretation can lead to better patient outcomes, particularly for low risk retinal lesions.Citation10,Citation11
The purpose of this review is to comprehensively describe current evidence and knowledge of normal peripheral retinal anomalies and benign peripheral retinal degenerations using clinical ocular imaging. We provide representative case images so presentation of these entities using different imaging modalities are clear and within a single reference. Findings are then summarised into a chairside guide with visual examples to aid the translation of this comprehensive review into clinical practice.
Visualisation of the peripheral retina
Definition of the Retinal Periphery
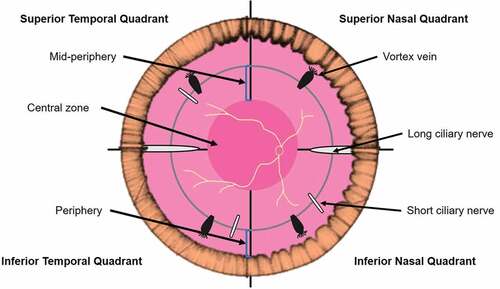
The retinal periphery has been defined as the area from the ora serrata to the equator. It has also been defined geometrically or using retinal landmarks such as vortex veins (). The geometric equator of the globe has been described as a ‘vertically orientated circle around the eye, perpendicular to the geometric axis.’Citation12 The equatorial zone is frequently defined as the circumferential area extending 2.5 mm posterior and anterior to the geometric equator.Citation13,Citation14 Clinically, artificial boundaries delimited by the retinal vascular arcades and vortex veins have been used to define the central retina, mid-periphery and far peripheral retina, respectively.Citation15 Self-devisedCitation16 and adapted grading gridsCitation17,Citation18 have also been utilised to define peripheral retinal zones.
Imaging Techniques for the Retinal Periphery
The long-standing gold standard for examination of the peripheral retina is a dilated indirect ophthalmoscopic or contact lens assisted fundus examination, supplemented by scleral indentation.Citation19 However, techniques and devices to capture an image of the peripheral retina have significantly progressed over time; from retinal drawings to montaging of multiple fields using fundus photography to ultrawide field (UWF) imaging (a history of these developments were recently summarised by Quinn et al).Citation20
Currently, UWF imaging dominates peripheral retinal imaging mostly for its wide field of view: 200 degree or 82.5% of the total retinal area and varying excitation lasers that allows the capture of this view as a colour image (using red (633 nm) and green (523 nm) lasers) or as an autofluorescent or fluorescein angiogram image.Citation21 These varying modes are useful for determining the relative involvement of the retinal pigment epithelium (RPE), choroid and other retinal layers. Other advantages of UWF imaging include being a non-contact, non-mydriatic methodCitation21 whilst still demonstrating equivalent outcomes compared to dilated fundus examination.Citation22 A number of studies also suggest UWF imaging detects significant peripheral retinal findings, which have consequences on disease grading and patient management.Citation5
UWF imaging, however, does have some limitations including eyelashes and eyelid artefacts, which can particularly affect the view of the inferior retina, camera reflectance artefacts and distortion and reduced resolution which are most significant in the far temporal and nasal periphery.Citation20 Development of the projection-based view, however, should account for the latter and studies of Croft et al.Citation23 and Sagong et al.Citation24 suggest after accounting for distortion and axial length, UWFI images can provide accurate measurements of retinal features.
Aside from en face views of the peripheral retina, cross-sectional views of the peripheral retina are also achievable using OCT – either steered via eccentric fixationCitation25 or through specifically designed wide-field OCTs.Citation25–27 The former has been reported to allow visualisation across the entire horizontal retinaCitation28–30 but is limited by dilation, patient compliance, OCT devices steering capabilities and resolution issues from off-axis imaging. The latter allows for up to a 55 degree field of view using commercial devices and 80 degree field of view using a research device based on ‘in-house’ modifications in a single image.Citation25
Visualisation of Normal Features of the Retinal Periphery
Despite the widespread acceptability of UWF imaging and OCT for imaging the peripheral retina, there are limited descriptions in the literature of the visualisation of normal peripheral retinal features using these techniques. The sections below describe the appearance of dentate processes, oral bays, oral pearls, pars plana cysts and meridional folds using these techniques.
Dentate Processes (Or Ora Tooth)
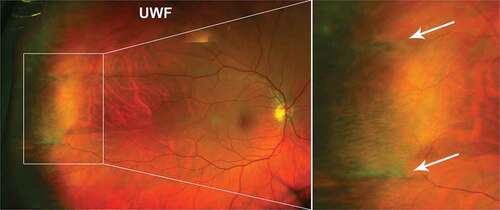
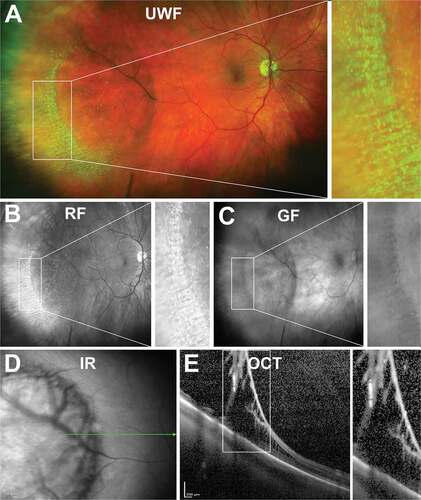
Dentate processes are normal anterior extensions of retina that project 0.5–2.5 mm from the adjacent retina circumferentially. Large dentate processes project further onto the pars plana of the ciliary body, and giant processes extend to the pars plicata. On UWF pseudocolour imaging, dentate processes appear as thin projections in the peripheral retina, also visible in red-free but less so in green-free UWF images (). Histologically, dentate processes may have retinal thinning (particularly towards anterior edge), broad areas of attachment between RPE and retina and cystoid degeneration.Citation31 A small study (48 eyes without visual complaints related to retinal disease) reported large dentate processes in 91% of asymptomatic eyes, and giant processes in 19% of eyes mostly in the supero-nasal quadrant.Citation32
Figure 2. Dentate processes (arrow) visible in the temporal peripheral retina of a 31 year old Caucasian female using (A) UWF imaging, and separated as the (B) red-free image and (C) green-free image. A meridional fold is also present (asterisk). Abbreviations: UWF, ultra-widefield; RF, red-free; GF, green-free.
Oral Bays
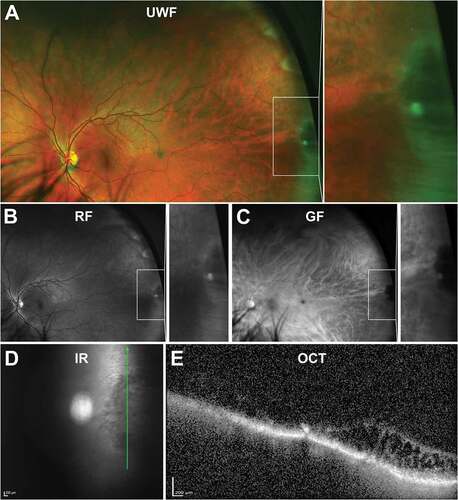
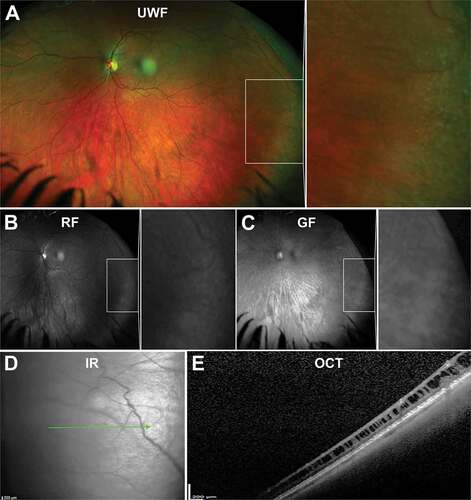
Oral bays are small Islands of pars plana surrounded by retina due to meeting of two adjacent dentate processes. They are visible on UWF imaging with red and green separated images sometimes helpful in distinguishing the lesion border (–c)). They are a normal variant of the ora serrata and extend posteriorly, covered by ciliary epithelium from the pars plana.Citation33 They may extend 0.5–2.5 mm into the retina and tend to occur along the horizontal meridian, occasionally inferior but never in the superior clock hours. Enclosed or partially enclosed bays are anatomical variations where there is full or partial separation of the oral bay from the retina, respectively,Citation31 and peripheral OCT can be useful for detecting this ()). Visualisation of these entities may also be important prognostically as retinal tears are associated with oral bays in 0.5% of eyes based on post-mortem analysis of 1000 eyes (50% of eyes with tears had partially enclosed oral bays, 8% had enclosed oral bays).Citation34 In addition, this study found all eyes with oral bays and a posterior vitreous detachment contained at least one retinal tear associated with a bay. Enclosed and partially enclosed oral bays occur in 4% and 0.6% of eyes, respectively.Citation34
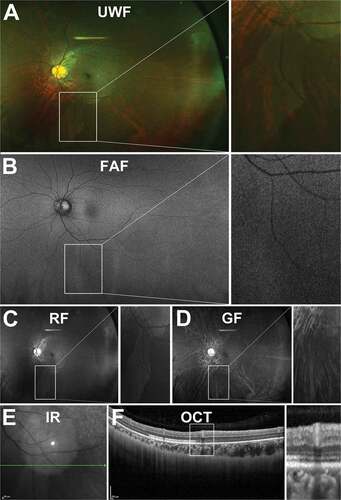
Figure 3. 31 year old Caucasian male with an oral bay and oral pearl in the far temporal peripheral retina visible on (A) UWF imaging and associated (B) red-free and (C) green-free images. (D) En face, infra-red imaging and (E) peripheral OCT through the structure demonstrates separation of the retinal layers at the bay. Abbreviations: IF, infra-red; OCT, optical coherence tomography. All other abbreviations as in .
Oral Pearls
Oral pearls are drusen-like structures that appear at the junction of retina and ciliary epithelium or along dentate processes.Citation35 Histologically, they initially appear brown or black and form under RPE cells, firmly adhering to the inner surface of Bruch’s membrane.Citation35 In UWF imaging, however, they may appear as bright lesions along dentate processes (). Epidemiologically, oral pearls appear in approximately 20% of eyes with no sex or age predilection (based on post-mortem analysis of 700 consecutive eyes).
Pars Plana Cysts
Pars plana cysts are smooth, bullous elevations occurring between the non-pigmented and pigmented ciliary epithelium.Citation33,Citation36 Smaller cysts are typically round, whereas large cysts are oval or oblong in shape.Citation37 Most cysts are situated at the posterior pars plana, though large ones may extend anteriorly and occupy an area greater than half an oral bay. They may present as isolated entities, separated by areas of intact ciliary epithelium or be confluent in shape with an irregular internal wall profile. They may also present as a clustered lesion where the continuous internal lamina exhibits multiple convexities towards the vitreous in eyes with retinoschisis. Cysts contain hyaluronic acid (similar to cystoid spaces and retinoschisis),Citation38 and are semi-transparent although pigment granules are also often present within cells of the inner cyst wall.Citation39 Visualisation is important as associations of pars plana cysts with microcystoid degeneration,Citation36,Citation39 chorioretinal scars,Citation39 retinal detachmentCitation40 and retinoschisisCitation38 have been reported in the literature. A post-mortem study found pars plana cysts were present in 18% (28/154) of subjects with increased prevalence with age.Citation39
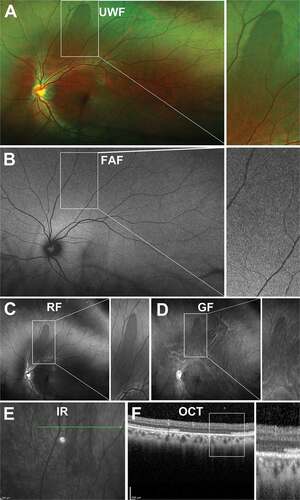
Meridional Folds
Meridional folds appear as a radial ridge or linear roll of redundant, elevated retinal tissue ranging from 0.5 to 1.5 disc diameters in length and found perpendicular to and crossing the ora serrata ().Citation31,Citation33 They may involve all retinal layers as visualised on OCT.Citation41 Meridional folds are relatively common, found in 16%–28% of eyes, commonly bilaterally, with multiple folds possible per eye.Citation32,Citation41 Meridional folds have not been significantly associated with any peripheral retinal pathology although it has been suggested that small retinal holes can develop at the posterior termination due to vitreoretinal traction. It is of interest that in mathematical origami, small folds (“crumpling”) are required in order to transform a flat sheet into a curved surface,Citation42 so it is perhaps to be expected that retinal folds might exist as part of the normal retinal architecture. An analysis of how this might be achieved during retinal embryogenesis and development has yet to be carried out.
Visualisation of peripheral retinal degenerations without vitreous involvement
Beyond normal peripheral retinal features, a number of peripheral retinal degenerations and anomalies exist which are considered benign and have no vitreous involvement or association with sight-threatening disease. These include microcystoid (or peripheral cystoid) degeneration, pavingstone (or cobblestone) degeneration, reticular pigmentary (or honeycomb) degeneration, peripheral drusen, white without pressure and dark without pressure lesions. The prevalence of these entities varies, with many associated with age and some associated with other ocular diseases, such as myopia and AMD (Supplementary Table S1). The sections below describe the appearance of these entities using peripheral imaging and their relative aetiology and prognosis.
Microcystoid Degeneration
Presentation on Ocular Imaging
Early microcystoid degeneration presents as red dots with indistinct boundaries at the ora serrata, particularly at the dentate processes ()).Citation43 Individual cystoid units are generally 0.15 mm or less in size, with the largest lesions observed near the ora serrata.Citation44 A greater visible red choroidal reflex through the central portion of cysts can often be observed, particularly using red-free UWF imaging due to thin inner walls separating the fluid-filled cavities within cystoid units ()). UWF imaging is also useful to capture the formation of retinal holes, which can occur in late degeneration due to enlargement and coalescence of cysts.Citation43
Figure 5. 32 year old Caucasian female with microcystoid degeneration. (A) UWF imaging demonstrates microcystoid degeneration in the infero - temporal peripheral retina and (B) red –free and (C) green-free images highlight the increased choroidal reflex due to the cysts. (D) On infra-red imaging, microcystoid degeneration can appear as a hazy area. (E) Peripheral OCT through the structure highlights separation of the inner retinal layers typical in reticular microcystoid degeneration. Abbreviations as in .
A variant known as reticular microcystoid degeneration appears as patterns of arborizing, reticular-like lines on the surface of cystoid degeneration.Citation45 Foos et al.Citation46 also reported that reticular microcystoid degeneration was often observed posterior to typical microcystoid degeneration behind the ora serrata. Fine arborizing blood vessels originating from subsurface retinal vasculature have been observed macroscopically throughout reticular lesions,Citation45 as opposed to typical microcystoid degeneration that only presents with large blood vessels.Citation46 Red and green separated UWF imaging as well as angiography, however, have found no obvious abnormalities in overall retinal or choroidal circulation in eyes with microcystoid degeneration.Citation45
Further differentiation between typical and reticular variants can be achieved with peripheral OCT where cystoid spaces of typical microcystoid degeneration form in the outer plexiform layer and the underlying RPE layer appearing more granularCitation41,Citation43,Citation45 whilst reticular microcystoid degeneration results in loss of retinal nerve fibre layer in the early stages, replaced by intercommunicating lacunae ()).
Location and Distribution
Microcystoid degeneration is typically bilateral and symmetrical with a predilection for the superior and inferior quadrants and sparing of the temporal and nasal quadrants (although Rutnin et al. reported greater severity of microcystoid degeneration temporally vs nasally in 90 subjects).Citation41,Citation44 Reticular microcystoid degeneration has been reported commonly anterior to the ora serrata although 25% have been described posterior to the equator.Citation45
Aetiology
Several theories have been proposed to explain microcystoid degeneration pathogenesis. Foos et al.Citation46 postulated the disproportionate growth of peripheral fundus and pars plana postnatally may be responsible for the relatively increased severity of typical microcystoid degeneration in the temporal quadrant, compared to the nasal. These findings were based on measurement of RPE cell densities across the fundus in neonates and adults from 2 months to 74 years of age.Citation47 However, the RPE growth rates in the superior and inferior quadrants, which are more commonly affected by microcystoid degeneration, were not reported. O’Malley et al.Citation44 reported that the youngest case observed was in a 6-day-old child, which suggests there may be congenital factors involved, particularly as microcystoid degeneration tends to be symmetrical between the eyes.
Alternatively, microcystoid degeneration could result from the pulling of the ciliary muscles and simultaneous reaction of zonules creating an opposing, tractional effect on the retina.Citation48 However, this mechanism would operate near the ora serrata and does not explain the equatorial extension of microcystoid degeneration in some cases.Citation46 Similarly, a vascular aetiology has been considered as there are senescent changes in blood vessels with age. However, histological and angiography studies have found no significant changes in blood vessels in eyes with these lesions.Citation45,Citation46
Prognosis
Microcystoid degeneration is assumed to be benign with no association with refractive error,Citation44,Citation46 vitreous detachment,Citation48 systemic conditions or increased risk of retinal breaks or holes.Citation44 However, accurate visualisation may be important as postmortem analysis found eyes with retinoschisis were always surrounded by microcystoid degeneration,Citation49 suggesting extensive microcystoid degeneration may lead to retinoschisis through coalescence of vacuoles, which eventually splits the retina.Citation43 However, the prevalence of retinoschisis among patients with microcystoid degeneration is very low, with O’Malley et al. (1967) identifying just 12 cases in 1000 autopsy eyes. Late microcystoid degeneration is also associated with the possible formation of retinal holes.Citation43
Pavingstone Degeneration
Presentation on Ocular Imaging
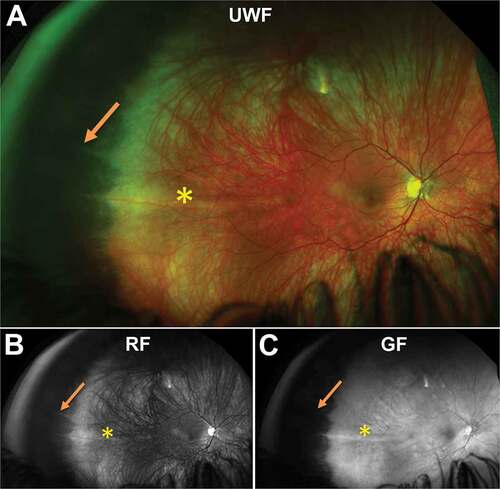
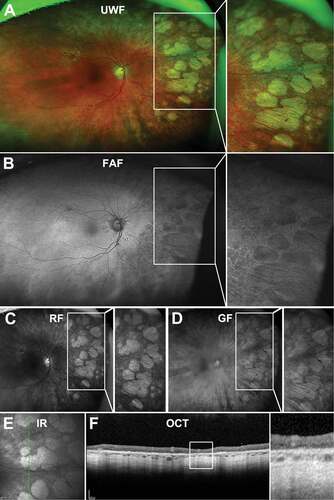
On UWF imaging, pavingstone degeneration appears as multiple distinct or confluent, round areas of depigmentation in the peripheral retina varying from 0.1 to 1.5 mm in size which may join to form larger areas of atrophy ()). Some lesions have been described to present with hyperpigmented cuffs with histology indicating this may be a result of RPE proliferation with RPE cells increased in number, height, and layering at lesion borders.
Figure 6. 87 year old Caucasian female with pavingstone degeneration. (A) UWF imaging demonstrates extensive lesions in the nasal retina which appear as (B) hypofluorescent lesions on FAF. (C) Red and (D) green-free images highlight the increased visibility of the choroid underneath lesions. (E) On infra-red imaging, pavingstone degeneration appears as hyporeflective lesions. (F) Peripheral OCT highlights thinning of the retina at lesions and subsequent increased choroidal visibility. Abbreviations as in .
On FAF, pavingstone degeneration lesions have been associated with nummular FAF patterns: small-to-medium areas of discrete, uniformly decreased autofluorescence ()).Citation16,Citation18 Nummular FAF patterns were also significantly associated with areas of RPE atrophy resembling a cobblestone pattern in 69.8% of 110 AMD and control subjects.Citation16
Within lesions, outer retinal thinning due to RPE atrophy occurs resulting in increased choroidal visibility, which can be imaged using UWF colour separated images or peripheral OCT (). Distinct outer retinal thinning can also be well visualised using OCT ()). No correlation, however, appears to exist between retinal thinning and size of the lesions and the choroid is also variably affected, from mild capillary wall thickening to total loss of blood vessels below large lesions.Citation50
Location and Distribution
Pavingstone degeneration lesions are typically located anterior to the equator and tend to increase in number from the equator to ora serrata.Citation18,Citation44 Lesions are concentrated between 5 and 7 o’clock in the infero-temporal quadrant with nasal horizontal meridians spared.Citation43,Citation44
Aetiology
The aetiology of pavingstone degeneration is unknown, although O’Malley et al.Citation50 suggested that the lesions may develop from decreased vascular flow in the choriocapillaris leading to areas of relative round areas of ischaemia corresponding to the structural organisation of anastamosed choroidal arteries. This hypothesis is derived from knowledge of the organisational structure of choroidal arteries, where the terminal branches supply an area approximately 1.5–2 mm in diameter, similar to the size of the largest pavingstone lesions found peripherally.Citation50
Prognosis
Pavingstone degeneration appears to be benign, not associated with retinal breaks or schisis or loss in visual function.Citation50 Despite a higher prevalence in individuals with AMD or homozygotes of the AMD-related ARMS2 SNP (Supplementary table S1), there is still insufficient evidence to link pavingstone degeneration to AMD.
Peripheral Drusen
Presentation on Ocular Imaging
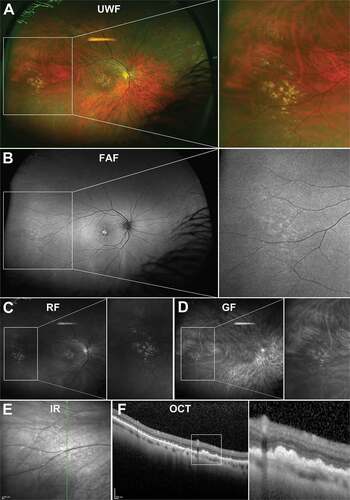
Peripheral drusen are small in diameter and display a similar appearance to macular drusen on UWF colour imaging () and (c–d)) and on OCT () although peripheral drusen have been noted to be more reflective than macular drusen.Citation51 On UWF FAF imaging however, peripheral drusen has been associated with some specific patterns.Citation16,Citation52 Tan et al.Citation16 described small discrete areas of increased hyperfluorescence known as granular hyperfluorescent FAF patterns to be significantly associated with peripheral drusen in intermediate to advanced AMD eyes. This finding, however, should be interpreted with caution as this is the most commonly observed FAF pattern in AMDCitation16 and FAF abnormalities are common with increasing age. Focal, pinpoint, hyperfluorescent FAF patterns have also been correlated with peripheral retinal drusen ()) and may be related to increased lipofuscin content in drusen.Citation53 Corbelli et al.Citation51 described both hypo- and hyper-autofluorescence for peripheral drusen and indicated the modality was associated with low intergrader agreement.
Figure 7. (A) UWF imaging of peripheral drusen in the nasal and temporal retina in a 66 year old Caucasian female which appear as (B) hyperfluorescent spots on FAF and (C) hyperreflective lesions on red-free and (D) green free imaging. (E) Infra-red imaging of peripheral drusen in a 56 year old Caucasian male which appear as reflective lesions. (F) OCT through peripheral drusen indicate similar morphology to macular drusen and location in the sub-RPE space. Abbreviations as in .
Location and Distribution
Some reports observe peripheral drusen predominantly in the superior quadrant,Citation8,Citation54 while others suggest lower prevalence in the temporal quadrant.Citation17,Citation51,Citation55 A recent study of healthy eyes (n = 98) suggests variations in prevalence with eccentricity with 48% of eyes having mid-peripheral drusen while only 21% and 9% of eyes had drusen in the far superior and inferior periphery, respectively.Citation18
Aetiology
Peripheral drusen are thought to have a distinct aetiology to macular drusen based on differing reflective properties and histological composition.Citation51,Citation56 Peripheral drusen may have an association with complement dysfunction as their prevalence is increased in AMD,Citation4,Citation5,Citation7,Citation8 Alzheimer’s disease,Citation3,Citation6 and chronic kidney disease.Citation2 This is also supported by significant correlations between peripheral drusen and homozygosity for the AMD risk allele CFH Y402H.Citation4 A genetic origin is also supported by Postel et al. (2005) who found peripheral drusen was correlated with multiplex probands in multiplex families.Citation57
Prognosis
The presence of peripheral drusen has not been associated with other peripheral retina degenerations. However, in AMD, peripheral drusen presence has been associated with disease severity with a retrospective analysis of 2103 subjects including 840 monozygotic and dizygotic male twin pairs reporting a significant association between peripheral drusen and AMD grade.Citation8 Peripheral drusen presence has also been associated with AMD polymorphisms, suggesting it could have future implications in the management of this disease.
Reticular Pigmentary Degeneration
Presentation on Ocular Imaging
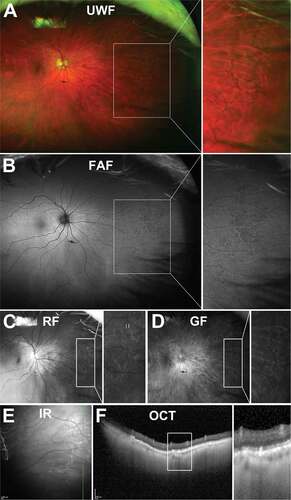
Reticular pigmentary degeneration appears as a net-like arrangement of hyperpigmented lines that may form complete or incomplete, irregular polygons on en face imaging ()). The polygons are typically 5 or 6-sided and measure about 0.75 mm in size.Citation58,Citation59 Hyperpigmented lines are frequently associated with homogenous drusen, which have hyperpigmented borders.Citation59,Citation60
Figure 8. 73 year old Caucasian female with reticular pigmentary degeneration. (A) UWF imaging demonstrates reticular pigmentary degeneration in the nasal far periphery and magnification of this area demonstrates the typical “honeycomb” appearance. (B) On FAF, these structures can appear predominantly hypofluorescent. (C) Red-free and (D) green-free images highlight reticular pigmentary degeneration as light and dark areas respectively pointing to RPE involvement. (E) Infra-red imaging shows hyperreflective lesions corresponding to reticular pigmentary degeneration (F) OCT imaging highlights RPE involvement and outer retinal disruption of reticular pigmentary degeneration. Abbreviations as in .
Fluorescein angiography demonstrates early hyperfluorescence of the sharp borders in reticular pigmentary degeneration and no dye leakage in into the late phase. This window defect hyperfluorescence corelates with attenuation of pigment within the RPE cells or RPE atrophy.Citation14 Friberg et al.Citation61 observed that peripheral reticular pigmentary findings on fluorescein angiography were only present in the same location on UWF photographs in 46% of patients suggesting UWF imaging may underestimate the extent of reticular pigmentary changes. Bae et al.Citation58 however, found a similar frequency of reticular degeneration on UWF imaging and fluorescein angiography. Interestingly, fluorescein angiography also revealed variations with age with linear, geometric patterns observed in the nasal quadrants individuals aged 50–70 years and complete polygon patterns in both nasal and temporal quadrants in individuals aged over 70 years.Citation62
FAF imaging supports the hypothesis of pigment attenuation of the RPE with areas of reticular pigmentary degeneration exhibiting hypofluorescence ()). A high correlation between mottled FAF patterns and reticular pigmentary degeneration has also been identified in AMD cohorts, though the significance of this finding is unclear.Citation16 On OCT imaging, disruptions to the outer retinal layers in reticular pigmentary degeneration are evident at the intersections of hyperpigmented lines ()).
Location and Distribution
Reticular pigmentary degeneration is typically found anterior to the equator, although it can occasionally appear posteriorly or extend anteriorly into the ora zone.Citation13,Citation14 Reticular pigmentary degeneration tends to involve more than one quadrant, and not infrequently, the circumference of the equatorial region.Citation14 Humphrey et al.Citation60 identified 360 degree involvement in 43% of cases. The superior and nasal quadrants are affected more frequently than the temporal quadrant, and both eyes tend to be symmetrically involved.Citation14,Citation58,Citation60
Aetiology
Bae et al.Citation58 suggested a hypothesis of choroidal vascular insufficiency being involved as choroidal filling times are significantly delayed in reticular pigmentary degeneration. A vascular aetiology could also explain the prevalence of reticular pigmentary degeneration in the nasal retina as blood flow to this quadrant is 3 times less than the temporal retina potentially making it less resistant to vascular-related aging changes.Citation63 Similarly, the equatorial predilection of reticular pigmentary degeneration lesions may relate to the ‘watershed’ zone between anterior and posterior choroidal circulation that may leave the equator susceptible to impaired perfusion.Citation58,Citation64 Several histological and fluorescein angiography imaging studies, however, have found no evidence of abnormal choroidal or retinal vasculature in areas of reticular pigmentary degeneration.Citation14,Citation59
Alternatively, reticular pigmentary degeneration may originate from RPE dysfunction as histologically, reticular pigment lines can be correlated to RPE cells that are taller, thicker, multi-layered and exhibit increased pigment content compared to normal RPE.Citation14 Similarly, hyperpigmented borders of drusen correspond to aggregation of RPE.Citation13 This may explain the links of reticular pigmentary degeneration with AMD grade,Citation8 severity,Citation14 and delayed dark adaptation in AMD patients.Citation65
Prognosis
Reticular pigmentary degeneration is considered benign and has not been associated with retinal breaks or detachment. Associations with increased grade, severity, and scotopic visual function deficits in AMD suggest reticular pigmentary degeneration may play a role in this disease that requires monitoring. Care should also be taken as the similar presentation and location of these lesions can resemble the bone spicule pigmentation associated with Retinitis Pigmentosa.
White Without Pressure (WWOP)
Presentation on Ocular Imaging
White without pressure (WWOP) presents as irregular, translucent areas in the retinal periphery sometimes with a red-brown border ())Citation66,Citation67 Areas of tissue with a normal appearance may be present within WWOP lesions and lesions can appear to move over time with scalloped edges indicating a more migratory nature of lesionsCitation68 although there are no reported cases of WWOP developing into distinct fundus lesions.
Figure 9. 22 year old Asian female with WWOP. (A) UWF imaging demonstrates irregular patches in the retinal periphery which are (B) hypofluorescent on FAF. Patches are visible on (C) red-free but less so on (D) green-free imaging. (E) Infrared imaging and (F) accompanying peripheral OCT at the margin of the lesion highlight the alterations to the ellipsoid zone in the outer retina. Abbreviations as in .
WWOP is associated with some specific fluorescein angiography patterns including multiple pinpoint areas of hyperfluorescence and increased peripheral fluorescence (50% of eyes with WWOP compared to 6% of non-WWOP eyes).Citation69 These patterns may result from greater tension from the vitreous on peripheral blood vessels, which are less supported by the thinner overlying nerve fibre layer, resulting in diminished blood flow and consequent blanching of the underlying retinal area.Citation69 Pinpoint hyperfluorescence could also represent peripheral leakage from overlying traction.
FAF findings demonstrate relative hypofluorescence within WWOP lesions ()) suggesting potentially reduced lipofuscin density in RPE cells.Citation70 Altered infrared reflectance, specifically outer retinal hyper-reflectance has also been described at the border of WWOP lesions and may represent the ellipsoid portion of photoreceptor inner segments ()).Citation70,Citation71 Near infra-red imaging also showed hyper-reflectance of WWOP lesions.Citation71 Changes in the ellipsoid zone may also be visualised using OCT ()).Citation70
Location and Distribution
Lesions are usually located beyond the equator, although changes may occur near the major retinal vascular arcades.Citation67 A small case series observed that WWOP presented at or posterior to the equator, separated from the ora by white with pressure (WWP).Citation72 Smaller, focal areas may also be visualised, particularly in regions of vitreous base attachment and ora serrata. WWOP most frequently involves the infero-temporal quadrant and supero-temporal quadrants.Citation66,Citation67,Citation72,Citation73
Aetiology
Initial theories suggested WWOP may be an optical illusion caused by variations in the angle of the light beam during examinationCitation74 or the result of a light reflex from dense vitreous collagen, prominent inner limiting membrane or vitreous base.Citation68,Citation75 More recently, however, WWOP has been hypothesised to result from altered interactions between the vitreous base and retina due to age, myopia, or other ocular conditions affecting the vitreoretinal interface. This is supported by associations between WWOP and retinopathy of prematurityCitation76 and familial exudative vitreoretinopathy,Citation77,Citation78 conditions, which both exhibit vitreoretinal signs and traction that can lead to retinal detachment or retinal folds.Citation79 Higher frequency of WWOP in younger myopes and moderate myopes casts doubt on the above theory as one would expect a higher frequency in older myopes if WWOP was caused by inward tractional force of the expanding globe on the peripheral retina in myopia.Citation71,Citation80 Other associations with WWOP are also difficult to explain with this theory including increased incidence of ‘retinal patches’ correlating with WWOP and dark without pressure (DWOP) in a cross-sectional observational study of 389 Jamaican children with sickle cell disease.Citation81
Prognosis
WWOP has not been significantly associated with vitreoretinal interactions and retinal breaks.Citation66 Migration or alteration in shape of WWOP lesions over time has been observed,Citation68 but no permanent retinal changes have been observed in areas previously affected by WWOP,Citation70 and no visual acuity loss has been reported in association with WWOP.
Dark without Pressure (DWOP)
Presentation on Ocular Imaging
There is limited description of DWOP lesions in the literature, but in general DWOP pressure lesions present as the converse of WWOP lesions, typically appearing as dark, flat, pigmented lesions with well-delineated, though not always regular, borders on UWF imaging ()).Citation82,Citation83 Lesions are known to be mobile, transient and can vary from one to several disc diameters in size.
Figure 10. 29 year old Asian male with DWOP. (A) UWF imaging demonstrates irregular patches in the retinal periphery which show no alteration on (B) FAF but are visible on (C) red-free, less so on (D) green-free imaging. (E) Infrared imaging demonstrates DWOP as hyporeflective region and (F) peripheral OCT at the margin of the lesion highlights the alterations in reflectivity of the ellipsoid zone in the outer retina in DWOP. Abbreviations as in .
Converse to WWOP, DWOP lesions are hypofluorescent on FAF ()) and hyporeflective on infrared imaging, though the cause of this is unknown ()).Citation70 On OCT imaging, lesions correspond to hyporeflective ellipsoid zone ()).Citation70,Citation82,Citation84,Citation85 Imaging with fluorescein angiography and OCTA indicate normal retinal and choroidal vasculature and circulation in areas of DWOP.Citation82–84
Location and Distribution
DWOP lesions typically present near the posterior pole, mid-periphery or in a parapapillary location, orientated circumferentially or radially.Citation70,Citation83,Citation86
Aetiology
As with WWOP, DWOP has been suggested to result from abnormal vitreo-retinal interactions with Chang et al. suggesting a resolution of DWOP lesion over 3 years in one case was attributed to the breakdown of vitreous with age.Citation82 However, DWOP has not been associated with vitreo-retinal alterations on OCT imagingCitation82 and reported in subjects aged 12 or younger without sign of vitreous collapse.Citation83 Growth and shrinkage of DWOP has also been shown to occur without evidence of vitreous detachment or vitreoretinal adhesions.Citation83,Citation87
More likely, retinal changes may be a result of intrinsic alterations in photoreceptors based on changes to the ellipsoid zone over DWOP lesions.Citation84 Fawzi et al.Citation70 proposed that WWOP and DWOP may arise from the varying photopigment densities within the lesions. However, this is inconsistent with changes being observed in the ellipsoid zone which mostly contains organelles of the inner segment of photoreceptors. Nevertheless, photoreceptor associations could explain the association with lesions, such as CHRPE which can disrupt photoreceptor organisation. The appearance of DWOP in younger including paediatric populations also suggests development potentially during ocular development.Citation86
Prognosis
As with WWOP, DWOP lesions appear to be benign without no associations with visual function changes in two separate cases studies.Citation70,Citation83
Visualisation of peripheral lesions predisposing to retinal detachment
There also exist several peripheral retinal degenerations that predispose to retinal detachment: lattice degeneration, snail track degeneration, acquired retinoschisis, retinal holes, vitreoretinal tufts, and snowflake degeneration. As with benign peripheral retinal degenerations, the prevalence of some abovementioned degenerations varies, often with association with age and refractive error (Supplementary Table 2). These entities, which can play an important role in appropriate patient management, are described and illustrated here.
Lattice Degeneration
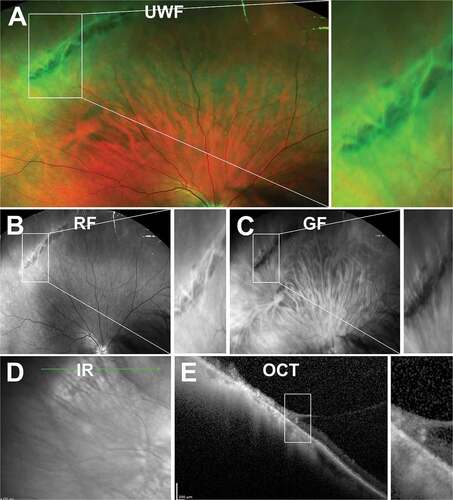
Presentation on Ocular Imaging
Lattice degeneration appears as elongated, round, or oval whitish peripheral lesions, 0.25–0.5 disc diameters in widthCitation88 and usually parallel to the ora serrata and between the equator and vitreous base on UWF imaging ()). Citation88,Citation89 Lesions can have a variable appearance; from a light shimmering image to a dark appearance and are not usually continuous with multiple rows present. Lesions may also vary in pigmentation, with the pigmented lesions displaying patchy retinal thinning and hyper-reflectivity as a result of increased pigment, whilst non-pigmented lesions demonstrate no retinal thinning.Citation90 As the lesions grow, underlying blood vessels become sheathed or attenuate as they pass through lattice lesion – easily visualised using red or green-free UWF imaging )). Areas with significant thinning can mimic or develop into retinal holes.Citation91 Other features include white lines (7–14%), atrophic holes (18–45%), RPE hyperplasia (82–92%), and yellow spots (80%).
Figure 11. A 60-year-old East Asian male with lattice degeneration in the superior-temporal peripheral retina of the right eye. (A) UWF imaging highlights an oval-shaped area of whitened retina with irregular borders and internal RPE hyperplasia and (B) red-free and (C) green-free imaging suggests that the internal hyperpigmentation is located in the RPE layer with no choroidal involvement. (D) Infrared imaging demonstrates irregular reflectivity in the region of degeneration and shows the orientation of the lesion to be largely parallel to the ora serrata and (E) peripheral OCT, with the line scan orientated radially through the lesion, highlights the overlying vitreoretinal attachment at the anterior and posterior aspects and shows a retinal break at the anterior edge of the lesion, internal disorganisation of the retinal layers, and irregular hypo-reflectivity in the outer retinal layers likely related to the overlying vitreoretinal traction. Abbreviations as in .
At the margins of lesions, exaggerated vitreoretinal attachments, U-shaped vitreous traction, and underlying focal detachments have been reported which can be well visualised using peripheral OCT ()). Citation89 Thinning of the neurosensory retina and liquefaction of the overlying vitreous is also clearly visualised by OCT Surface reflectivity and hyper-reflective dots on OCT imaging can also represent changes in surface cell morphology and adherent gel vitreous, respectively ()). Citation90,Citation91 Histology studies indicate lesions are associated with thinning of the inner retina although this can extend to affect the full thickness of the retina and overlying lacunae of liquefied vitreous.Citation89
Location and Distribution
Lattice degeneration lesions is more commonly located anterior to the equator with 78.7% of lesions situated 1–3 disc diameters from the ora serrata. Lattice degeneration is bilateral in 33.7–61.3% of cases.Citation1,Citation88,Citation89,Citation92–98
Aetiology
A small number of studies have found altered retinal circulation near or within lattice lesions. Tolentino et al.Citation45 found arterioles were occluded at the posterior edge of severe lattice lesions in 10 subjects and Sato et al.Citation99 reported avascularity around and within lattice degeneration lesions and leakage from choroidal blood vessels. It is not known if circulation disturbances precede or develop due to lattice degeneration.
A genetic origin has also been proposed with a controlled, prospective comparative study examining 634 Japanese patients with lattice degeneration to 734 healthy controls finding SNP rs4760608 and rs1793953 for the collagen type II alpha 1 gene were significantly associated with lattice degeneration, while a retrospective study comparing genotypes of 574 Japanese subjects to 608 controls reported a strong association between SNP rs7558081 and lattice degeneration.Citation100,Citation101 A further 11 SNPs showed significant evidence of association with lattice degeneration.Citation102
Prognosis
It has been postulated that lattice degeneration may arise from degenerative processes, which could lead to atrophic holes and vitreoretinal adhesions, subsequently increasing the risk of retinal breaks and detachment.Citation1,Citation89,Citation103 Prophylactic treatment, however, is only suggested when the detachments are progressive or symptomatic,Citation1 and locally liquefied vitreous lacunae may be a source of subretinal fluid flow through atrophic holes within lattice degeneration lesions leading to significant degeneration. Indeed, whilst retinal holes are frequently present in eyes with lattice degeneration lesions (18–26%)Citation88,Citation89 and increase in incidence with age,Citation88 Tillery et al.Citation104 observed that only 2.8% of retinal detachments were due to retinal holes in lattice degeneration lesions. When detachments did occur, they tended to be slowly progressive as evidenced by pigment demarcation lines, and occurred most frequently in the inferior quadrant.Citation104 Similarly, in 276 consecutive patients, ByerCitation105 found clinical retinal detachment in 1.08% of patients over a follow-up period of 1–25 years and clinical or progressive subclinical retinal detachment in 2% of eyes with atrophic holes. ByerCitation106 also observed that only 2 of 137 atrophic holes enlarged over time, which also suggests that atrophic holes associated with lattice degeneration are a low-risk lesion.Citation1 Furthermore, Foos et al.Citation107 reported no association between lattice degeneration and central vitreous lesions in autopsy eyes suggesting no subretinal fluid flow. Interestingly, whilst ByerCitation105 reported a < 1% chance of detachment in eyes with lattice degeneration with a history of retinal detachment, Folk et al.Citation108 reported a higher incidence of retinal detachment (5.1%) in the fellow eye of patients with positive history of phakic lattice-related retinal detachment with bilateral lattice lesions. These findings suggest that a history of retinal detachment, which is lattice-related could increase the risk of detachment in lattice degeneration.
A greater level of retinal detachment has been noted for lattice degeneration in high myopes.Citation109 Prophylactic treatment, however, does not reduce the risk of new retinal detachment in the fellow eye of high myopes compared to low and non-myopes.Citation108
Snail Track Degeneration
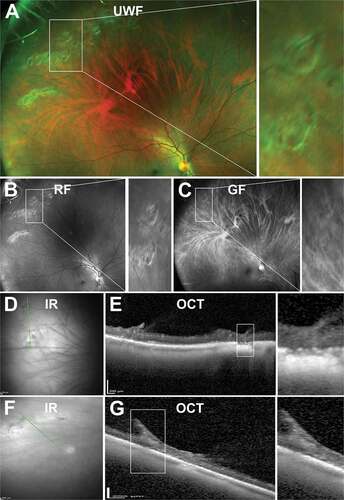
Presentation on Ocular Imaging
There are limited descriptions of snail track degeneration in the literature. Using UWF imaging, snail track degeneration appears as closely-spaced shiny, crinkled, or frosted bands of retina composed of pin-point glistening white dots resembling the track left by a snail ()).Citation110 These lesions can be also well visualised on red free UWF imaging ()). On OCT, lesions appear as irregular thinning and a wrinkled curvilinear inner retinal surface potentially without traction or condensation of overlying vitreous ()).Citation90
Figure 12. 29 year old Caucasian male with snail track degeneration in the superotemperal peripheral retina. (A) UWF imaging (B) red-free and (C) green-free imaging highlights closely spaced circular lesions forming a ‘track’. (D) Infra red imaging and associated (E) peripheral OCT highlights thinning and a wrinkled inner retina. In some cases, (F - G) traction can be observed. Abbreviations as in .
Location and Distribution
Snail track degeneration tends to distribute evenly between the four quadrants,Citation111,Citation112 with reasonable uniformity and symmetry.Citation113 In a familial case study of 11 members however, lesions always presented in the supero-temporal and infero-temporal quadrants, with infero-nasal quadrants being least involved. 80% of lesions were anterior to the mid-equator.Citation114 Shukla et al.Citation115 reported an 58.8% bilaterality in a cohort of 500 individuals.
Aetiology
There is suggestion that snail track degeneration represents an early or mild form of lattice degenerationCitation106,Citation115 however histological study suggests lattice is predominantly associated with lipid inclusions and intraretinal fibrils in glial cells, whilst snail track degeneration is associated with organelle degeneration within retinal neurons.Citation116 Histology also suggests no involvement of the vitreoretinal interface in snail-track degenerationCitation116 and there is no clinical evidence of snail-track degeneration progressing to lattice degeneration in the literature. A familial tendency was observed in 2 of the 4 families studied in a small case series,Citation114 although there is no other evidence of genetic aetiology in the literature.
Prognosis
Snail track lesions have not been associated with an increased risk of retinal breaks or retinal detachment although small sample size is a major limiting factor in a number of these studies.Citation110,Citation113,Citation117. A small case series noted snail track lesions had a similar appearance to snowflake degeneration and therefore care is needed to distinguish between the two, as snowflake degeneration can become clinically significant when presenting with other lesions, such as lattice.Citation118
Acquired Retinoschisis
Presentation on Ocular Imaging
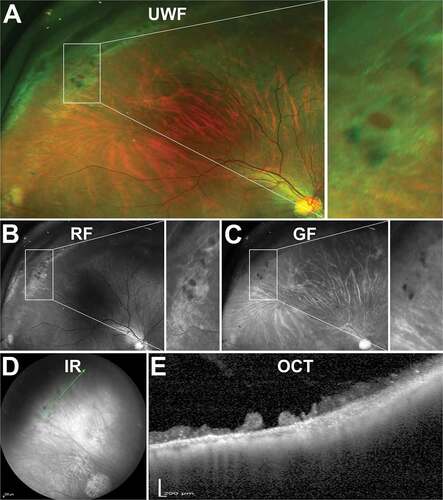
With en face imaging, retinoschisis appears as an elevated area of retina that may be shallow or bullous in shape representing typical and reticular types respectively ()).Citation119 Reticular retinoschisis may also exhibit a thin arborising reticular pattern on its surface from fine retinal blood vessels.Citation119,Citation120 Other features of retinoschisis include glistening white dots on the inner retinal surface, microaneurysmal, telangiectatic changes, vessel occlusion and white lines along schisis areas representing thickened blood vessel walls that are usually patented with a visible blood column.Citation119,Citation121,Citation122
Figure 13. 86 year old Caucasian male with degenerative retinoschisis. (A) UWF, (B) red-free, (C) green-free and (D) infra-red imaging highlight the transparent dome-shaped elevation of the retina associated with retinoschisis. (E) Peripheral OCT highlights the splitting of the inner retinal layers and remnants of intraretinal pillars. Abbreviations as in .
Retinoschisis represents a splitting of the neurosensory retina which is well visualised using OCT (). Loss of tissue from the inner plexiform to outer nuclear layer should be evident with the presence of intraretinal pillars of varying size, transversing the inner and outer leaf also common.Citation119,Citation123,Citation124 The remaining outer layer may be variable in thickness with an irregular surfaceCitation124 or exhibit a break with a rolled edge thought to result from contraction of glial fibrous layer on the inner surface of the outer layer.Citation119,Citation125 In some instances, a double schisis cavity where two cavities are present within the affected area separated by the outer plexiform layer can occur.
Location and Distribution
Typical retinoschisis is usually found anterior to the equator, whereas reticular retinoschisis often extends posteriorly and frequently has outer retinal layer holes. Both types of retinoschisis are usually found in the temporal fundus quadrants,Citation38,Citation126 with equal involvement of upper and lower temporal quadrants,Citation38 though infero-temporal preponderance has been reported.Citation120,Citation121 Lesions are bilateral in 62.5% – 82.1% of reported cases in the literature.Citation119,Citation121
Aetiology
Histochemical studies show that schisis cavities are typically filled with hyaluronic acid produced by Müller cells.Citation127 Fluid appears to be secreted at the pars plana level and passes into the schisis cavity based on pars plana cysts being more numerous and close to areas corresponding to retinoschisis.Citation38 Straatsma et al.Citation119 observed that a narrow band of typical microcystoid degeneration was present between the ora serrata and anterior border of the retinoschisis in all retinoschisis cases suggesting these two peripheral degenerations may be associated. This is supported by both entities usually occurring in the inferior temporal quadrant.
Posterior vitreous traction and/or detachment has also been suggested to play a role but evidence for this is limited to a single study, which observed 70% of schisis detachments were associated with a posterior vitreous detachment on presentation.Citation123 It should be noted that a retrospective OCT study of 500 cases reported 3 cases of schisis detachments were associated with a posterior vitreous detachment and 2 were not.Citation128
Prognosis
There appears to be a low risk of retinal breaks and detachment following retinoschisis with Byer et al.Citation120 observing only 11.4% of 218 eyes with retinoschisis to develop a new break and 0.05% of eyes progressing to symptomatic retinal detachment over an average follow-up period of 9.1 years.Citation120 Most schisis cavities remained stable with only 3.2% of cases showing posterior progression. Visual acuity was unaffected in the 6.4% of cases where schisis-detachments occurred.Citation120 Similarly, the Scottish Retinal Detachment Study estimated the incidence of progressive schisis retinal detachment to be 0.85/million,Citation123 with 1.6% of rhegmatogenous retinal detachment associated with retinoschisis.Citation129 Interestingly, Byer also observed that retinoschisis lesions spontaneously disappeared in 2.3% of 218 cases and schisis cavity height decreased in 4.1% of cases. Other case reports have shown similar findings with flattening of schisis areas associated with the appearance of large outer leaf breaks.Citation130,Citation131
Due to its relatively non-progressive nature, treatment is usually reserved for schisis detachments with posterior extensions of subretinal fluid nearing the macula as a result of holes in the outer layer.Citation122 A small case series identified that vision loss occurred in all subjects with outer leaf breaks where the schisis extended to within 10 degrees of the fovea, whereas central vision was preserved in those without breaks.Citation132 Other indications for surgery proposed include four disc diameters or more extension from the ora serrata on diagnosis, more than 4 disc diameters posterior progression on follow-up, 10% or more change in visual field defect,Citation126 or retinal breaks in both inner and outer layers.Citation119
Retinal Holes
Presentation on Ocular Imaging
Retinal holes are full thickness retinal breaks, which appear as round or horseshoe shaped, red lesions commonly associated with a surrounding white cuff, which may result from intraretinal oedema or surrounding subretinal fluid. They are generally divided into two types – operculated holes, which form when focal vitreoretinal traction completely avulses a circular or oval piece of retinal tissue, known as the operculum that floats above the retinal surface () and atrophic holes, which form due to progressive localised retinal thinning, including within areas of lattice degeneration or, less commonly, in an area of otherwise normal retina without vitreoretinal traction ().Citation1,Citation133,Citation134 OCT imaging can be particularly useful for the differentiation of retinal holes, with peripheral atrophic holes taking on a similar appearance to idiopathic macular holes with flat or everted edges and in some cases with cystoid changes in the surrounding retina (). This similarity possibly explains their similarly innocuous nature.Citation90 For operculated holes, ChoudhryCitation29 described vitreous adhesion at the inner retinal surface in cases where the operculum was partially attached or in retinal holes has an inverted V-shaped profile. Hole with a flat-shaped profile did not show any vitreous adhesion or subretinal fluid. ChoudhryCitation29 also noted that varying degrees of cystoid degeneration can be seen in the surrounding retina and hyper-reflective material in the subretinal space using OCT.
Figure 14. 84-year-old Caucasian male with operculated retinal hole. (A) UWF imaging, (B) red and (C) green-free and (D) infra-red imaging show a round lesion with a surrounding cuff and an adjacent operculum. (E) Peripheral OCT highlights the flat profile of the full-thickness hole with no associated subretinal fluid. Abbreviations as in .
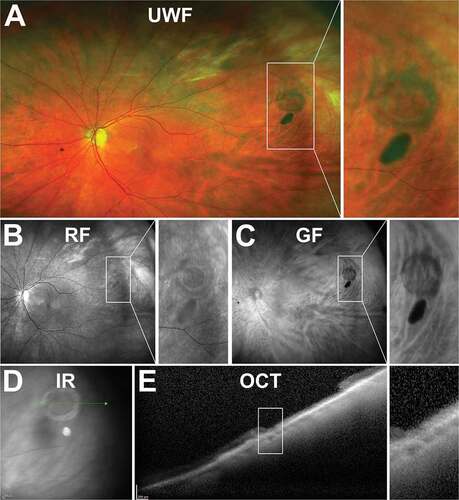
Figure 15. 23 year old Caucasian female with atrophic retinal hole within an area of lattice degeneration. (A) UWF imaging, (B) red and (C) green-free and (D) infra-red imaging show similar characteristics to operculated holes of a round lesion with a surrounding cuff. (E) Peripheral OCT highlights the full-thickness loss of retinal tissue at the site of the hole but no sign of overlying or displaced tissue. Abbreviations as in .
Location and Distribution
Neumann et al.Citation135 found that round retinal breaks were most prevalent in the superotemporal periphery, followed by the inferotemporal then superonasal periphery and, least commonly, inferonasally.
Aetiology
The majority of retinal holes occur as a secondary complication of other retinal lesions, such as retinal thinning within areas of lattice degeneration, retinoschisis, meridional fold, zonular traction tuft or avulsion of a vitreoretinal tuft.Citation133 Atrophic holes are generally thought to be unrelated to vitretinal traction, however recent OCT studies have found that some retinal holes, particularly operculated or partly operculated holes associated with subretinal fluid, have an overlying vitreous attachment at the site of the retinal hole.Citation29,Citation136
Prognosis
The risk of progression of a retinal hole in an asymptomatic patient is considered low.Citation1,Citation137 Byer reported on a series of consecutive phakic patients over 1–33 years whom had asymptomatic retinal breaks. Of 162 patients with round holes without operculum, 19 patients had a subclinical retinal detachment at baseline or follow-up and none developed a clinical retinal detachment over the follow-up period. Of 32 patients with operculated retinal holes, no detachments were presented.Citation138
Neumann et al.Citation135 followed 153 untreated retinal breaks in 102 patients, and 3 patients developed a detachment, all within 6 weeks of the first visit. In symptomatic patients, DavisCitation139 also found that retinal detachment usually occurred within 6 weeks of symptom onset in patients with a newly operculated hole or tear. The presence of symptoms was the single most important factor in determining progression risk.
Atrophic holes within lattice degeneration are associated with up to 60% of retinal detachments compared to isolated retinal holesCitation94. These detachments may progress slowly and insidiously without symptoms until the macula is involved.Citation140 ByerCitation105 found subclinical retinal detachments in 6.7% of eyes with atrophic holes within lattice degeneration but clinical detachment or progressive subclinical detachment in only 2% of eyes during the follow-up period. The American Academy of Ophthalmology preferred practice patterns also suggests “an eye that has atrophic round holes within lattice lesions, has minimal subretinal fluid without progression, or lacks evidence of posterior vitreous detachment (PVD) does not require treatment.”Citation1 On the other hand, in a Taiwanese study, 21.1% of 1032 cases of rhegmatogenous retinal detachment were attributed to atrophic holes within lattice degeneration and 20.2% was due to atrophic holes only.Citation141 In a Finnish study of 342 consecutive patients for retinal detachment surgery, retinal holes were found in 17.3% of retinal detachment eyes.Citation142 Thus, when round holes are associated with lattice degeneration, they should be monitored for risk of progression to retinal detachment where treatment may be indicated.Citation1,Citation140
Vitreoretinal Tufts
Presentation on Ocular Imaging
Non-cystic vitreoretinal tufts generally appear as short (<0.1 mm) and thin projections and can be difficult to visualise in the peripheral retina (). Cystic vitreoretinal tufts, however, are generally larger than their non-cystic counterparts (0.1–1.0 mm diameter),Citation143,Citation144 appearing as round or oval shaped discrete, lesions with grey/white nodules of varying size and often with a pigmented base ().
Figure 16. 47 year old Caucasian male with non-cystic vitreoretinal tuft. (A) UWF imaging indicates the well demarcated lesion in the superonasal peripheral retina, visible on (B) red-free and (C) green-free images. (D) On infrared imaging, the lesion appears dark and corresponding (E) peripheral OCT highlights the hyperreflective apical tip of the tuft extending into the vitreous with disturbance to the underlying photoreceptor and RPE layers. Abbreviations as in .
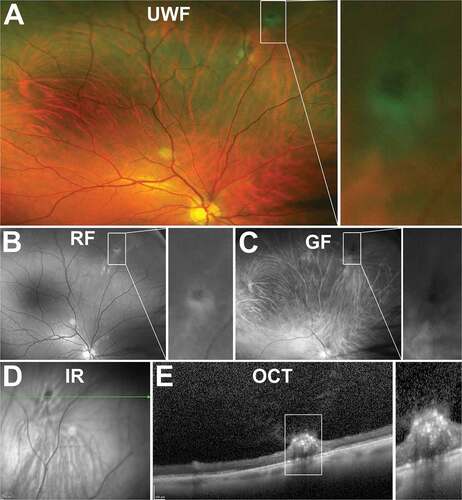
Figure 17. 43 year old Asian female with multiple cystic vitreoretinal tufts in the superior peripheral retina. (A) On UWF imaging, tufts appeared as bright, sharp lesions which remained visible on (B) red-free and (C) green-free images. (D) Infrared imaging shows lesions are hyporeflective and (E) peripheral OCT demonstrates tufts have a large, hyper-reflective apical tip extending into the vitreous with internal cystic spaces and loss of reflectivity of the underlying inner and outer retinal layers. Abbreviations as in .
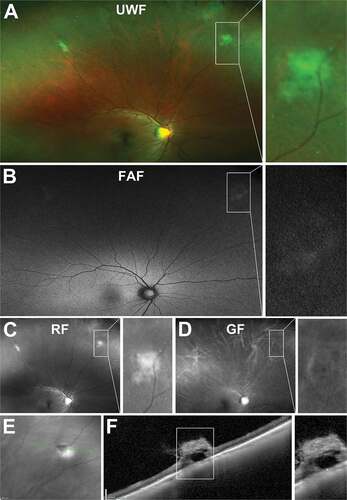
On UWF imaging, vitreoretinal tufts can appear as a round, well demarcated, cotton ball-like structures that may be moderately elevated ()).Citation143,Citation144 Alterations in the RPE can also aid visualisation using red and green separated images and FAF ( (b–d)). On IR imaging, lesions may appear hyporeflective (.Citation29 On peripheral OCT, a hyperreflective apical region, which likely reflects reactive gliosis can generally be observed as well as vitreous attachment at the apex and traction ()).Citation29,Citation145 Hyporeflective, microcystic spaces within the inner retina may also be seen allowing easy differentiation of non-cystic and cystic variants.Citation29 Photoreceptor and RPE reflectivity are usually absent. These findings are consistent with histology, which showed internal microcysts, glial cell proliferation, and photoreceptor loss in cystic vitreoretinal tufts.Citation143,Citation144,Citation146
Location and Distribution
Vitreoretinal tufts appear most commonly in the inferior and temporal peripheral retina and the equator.Citation147 Non-cystic vitreoretinal tufts have been reported with bilaterality in 50% of cases and multiple lesions in 64% of cases.Citation148 Cystic vitreoretinal tufts have been reported as unilateral in 80% of individuals.
Aetiology
Cystic vitreoretinal tufts are considered congenital although no hereditary factor has been confirmed. Cahill et al.Citation149 performed a case study of a single Irish family and found non-cystic retinal tufts in 11 family members inherited in an autosomal dominant pattern yet could not identify a causative gene. Nevertheless, any genetic associations with the disease may relate to Müller cell function as vitreoretinal tufts appear to mostly contain glial tissue. This is supported by the presence of vitreoretinal tufts in several cases of Neurofibromatosis Type 2 with tissue culture studies suggesting the causative mutation in the NF2 gene results in dysplastic Müller cells that lose polarity and accumulate in the epiretinal space, resulting in tuft formation.Citation150,Citation151 Chan et al. also confirmed high levels of the Müller cell stress protein and glial fibrillary acidic protein in histopathological samples of retinal tufts from individuals with neurofibromatosis 2.Citation150
Prognosis
Although non-cystic vitreoretinal tufts are not associated with retinal breaks, the tips of these lesions may break off and be observed as small floaters overlying the vitreous base. Cystic vitreoretinal tufts on the other hand may predispose to a retinal tear/operculated hole or detachment with PVD and it is estimated that 10% of rhegmatogenous retinal detachments are caused by retinal tears in areas of cystic retinal tufts.Citation152 In contrast, the risk of developing rhegmatogenous retinal detachment from a cystic retinal tuft is only around 0.28%.Citation143
Snowflake Degeneration
Presentation on Ocular Imaging
Snowflake degeneration is characterised clinically by the appearance of small crystalline deposits in the retina from the ora serrata to equator. HiroseCitation153 proposed four stages of the disease with distinct clinical appearances including (1) extensive white without pressure in the periphery (under 15 years old), (2) snowflake like, yellow-white spots in areas of white without pressure (15–25 years), (3) sheathing of retinal vessels and pigmentation due to RPE clumping posterior to the area of snowflake degeneration (25–50 years), and (4) increased pigmentation and disappearance of retinal vessels and less prominent snowflakes (over 50 years). Other ocular changes include optic nerve changes, waxy pallor, vitreous liquefaction, vitreous traction, early onset cataract, and corneal guttae.Citation153,Citation154
Aetiology
Although initially considered to be a variant of retinitis pigmentosa,Citation155 clinical and genetic studies confirm snowflake degeneration as a separate disease entityCitation156,Citation157 with the causative mutation localised to the KCNJ13 gene, which encodes the potassium channel Kir1.7, found in the RPE.Citation158,Citation159 Although the exact mechanism of disease is unknown, most models propose a loss of channel function leading to altered RPE polarisation and compromise of the blood-retinal barrier leading to crystalline deposits. Loss of Kir1.7 channel function may also explain the association of myopiaCitation157 as the channel is implicated in regulating refractive error.Citation159
Prognosis
Functional assessment of family case studies of snowflake degeneration suggests the disease is associated with elevated dark adaptation times and a reduced scotopic b-wave but unaffected photopic vision.Citation160 Changes to the vitreoretinal interface are also associated with increased traction on the retina and risk of detachment. This is supported by Lee et al.Citation157 who reported 21% of family members with snowflake degeneration exhibited retinal detachment. Poor outcomes of retinal detachment surgery have also been reported for two separate families with snowflake degeneration.Citation153,Citation161
Discussion
This review provides a comprehensive summary of a range of peripheral retinal degenerations and anomalies and their visualisation using ocular imaging technologies including UWF imaging and peripheral OCT. While many of these peripheral degenerations and anomalies are considered benign with no evidence of visual function being affected, some, including lattice degeneration, snail track degeneration, retinoschisis, vitreoretinal tufts, retinal holes, and snowflake degeneration may predispose to the risk of retinal detachment.Citation1 Others, such as peripheral reticular degeneration, have similar presentations to sight-threatening pathologies such as retinitis pigmentosa, and therefore require investigation for accurate diagnosis. Finally, recent literature suggests certain peripheral changes such as pavingstone degeneration and peripheral drusen are associated with AMD,Citation22,Citation51,Citation130; chronic kidney disease,Citation2 and Alzheimer’s disease.Citation162,Citation163 Thus, the use of ocular imaging to assist with appropriate detection and management of these entities may be of significance in clinical practice.
Imaging of peripheral retinal degenerations and anomalies have been described in studies scattered throughout the literature. In less common entities, such as snail-track degeneration, DWOP, and snowflake degeneration, evidence is mostly derived from case reports, which is limited and difficult to interpret. Indeed, aside from a single OCT image in Kothari et al.,Citation90 this review is the first to report advanced imaging of snail-track degeneration. Translating this work into clinical practice, provides a summary of the peripheral degenerations described in this review by ocular imaging and can act as a convenient chairside guide for clinicians to access this information in busy practice. Chairside references or guides have been used considerably as evidence-based clinical decision support tools in dentistry,Citation164,Citation165 and have become prevalent in eye-care with the Royal College of Ophthalmologists in the UK providing illustrative examples within several of their clinical guidelines.Citation166 Other international professional eye-care organisations have also published guides for a number of ocular conditionsCitation166,Citation167 and validation of such chairside references is becoming increasingly common.Citation167–169 This guide aims to provide examples of peripheral retinal degenerations supported by a validated, evidence-based review article for cross-referencing when needed.
Figure 18. Chairside guide of peripheral retinal degenerations including summary of presentation, epidemiology (if known), prognosis and appearance with ocular imaging.
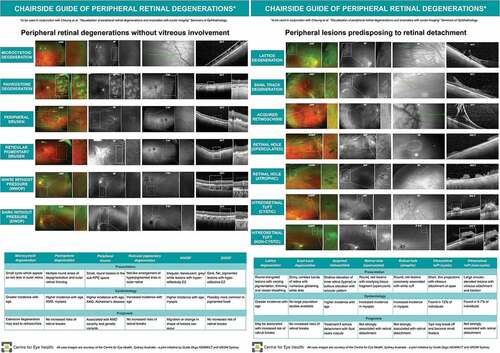
A difficulty associated with many peripheral retinal entities is that they are often uncommon incidental findingsCitation5 with unclear details for onset and natural progression. Consequently, the aetiology of many of these lesions remains unknown. Advances in ocular imaging are elucidating the aetiology of some of these entities. For example, WWOP and DWOP were originally suggested to be associated with abnormal vitreoretinal interactions, however OCT imaging showed evidence of involvement of the outer retina and photoreceptor layers.Citation70 OCT imaging has also delineated differences between pigmented and non-pigmented lattice degeneration, with the former potentially reflecting a more active form of the lesion, displaying retinal thinning and RPE hyperplasia.Citation90 For peripheral drusen, differences in reflectivity properties compared to macula drusen have been shown on OCT and support previous suggestions from histology that the aetiology of peripheral drusen differs from their macula counterparts.Citation51,Citation56
In terms of prognosis, ocular imaging may also be useful in identifying peripheral retinal lesions that require clinical intervention. Although most of the lesions discussed within this review are considered benign, some may predispose to the risk of retinal detachment. Chu et al.Citation170 demonstrated the power of ocular imaging, determining altered management for 5% of patients with peripheral retinal lesions following OCT imaging.
Finally, several peripheral retinal degenerations have been associated with other disease states. Aside from the well-known associations with myopia, genetic associations between peripheral drusen, reticular pigmentary degeneration and pavingstone degeneration with AMD suggest that some peripheral retinal degenerations may be a peripheral expression of AMD or share a similar aetiology. Indeed, we recently found that drusen and pigmentary changes typical in the macula of AMD patients, were also the most common peripheral findings for these patients.Citation5 Significant associations between peripheral drusenCitation22,Citation51,Citation130; chronic kidney disease,Citation2 and Alzheimer’s diseaseCitation162,Citation163 also suggest that complement pathway dysfunction may be a common factor in the aetiology of these conditions. Thus, convenient visualisation of peripheral retinal degenerations facilitated by ocular imaging may have growing future importance in the clinical assessment of these other ocular and systemic conditions.
Conclusion
This review provides an update on current knowledge of peripheral retinal degenerations, incorporating evidence from ocular imaging modalities. The review collates findings of some lesions from a number of clinical studies and case series, while rarer degenerations, such as snail track degeneration, dark without pressure and snowflake degeneration still lack high-quality studies and require further investigation to establish their epidemiology, aetiology, and prognosis. In addition, while the peripheral retinal lesions discussed here are typically considered benign, a review of current literature reveals that some have significant associations with ocular and systemic disorders. This association highlights the utility of their visualisation by ocular imaging as they may become a relevant part of the clinical picture for these conditions in the future. Finally, to facilitate the clinical translation of this work, a chairside guide is provided highlighting the main clinical findings of these lesions with illustrative examples.
Literature search
The initial literature search was conducted using EMBASE (Classic + Embase, 1947 to 2019), MEDLINE (1946 to 2019) databases and Google Scholar. The key terms “microcystoid AND degeneration”, “(pavingstone or cobblestone) AND degeneration”, “peripher* AND drusen”, “lattice AND degeneration AND retina”, “periph* AND reticular AND pigment*”, “snail track AND degeneration”, “white without AND pressure AND retin*”, “dark without AND pressure AND retin*”, “snowflake AND degeneration”, “periph* AND hole* AND retin*”, “(Degenerative OR Senile OR Acquired) AND retinoschisis”, “dentate AND process* AND retin*”, “oral AND bay* AND retin*”, “oral AND pearl AND retin*”, “pars plana AND cyst* AND retin* NOT vitrectomy”, and “meridional AND folds AND retin*” were searched. No date or language restrictions were applied in electronic searches, which were last searched on 25th August 2019. Reference lists were reviewed for additional relevant articles, which were then hand-searched individually. Non-English papers were reviewed as far as was translated and excluded if there was insufficient information regarding study design or results.
All case studies were of patients seen at the Centre for Eye Health, Sydney Australia who provided written, informed consent for the use of their anonymised images for research and education purposes.
Author contributions statement
RC performed the literature search and wrote a large proportion of the manuscript. LNS obtained case study examples and contributed significantly to manuscript writing and editing. PK wrote a portion of the manuscript. MC, AC, MK, MCM, and AL contributed to the literature review design and manuscript editing.
Supplemental Material
Download PDF (140.6 KB)Supplemental Material
Download PDF (147.7 KB)Acknowledgments
This work was supported, in part, by funding from the National Health and Medical Research Council (NHMRC #1174385). Guide Dogs NSW/ACT provides support for the Centre for Eye Health (the clinic from which case studies were obtained), and authors LN-S, AL, PK and MK. The authors would also like to thank Judy Nam and Rebecca Milston for their technical assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
SUPPLEMENTARY MATERIAL
Supplemental data for this article can be accessed on the publisher’s website
Additional information
Funding
References
- Flaxel CJ, Adelman RA, Bailey ST, et al. Posterior Vitreous Detachment, Retinal Breaks, and Lattice Degeneration Preferred Practice Pattern(R). Ophthalmology. 2020;127:146–181. doi:10.1016/j.ophtha.2019.09.027.
- Choi J, Moon JW, Shin HJ. Chronic kidney disease, early age-related macular degeneration, and peripheral retinal drusen. Ophthalmic Epidemiol. 2011;18:259–263. doi:10.3109/09286586.2011.602509.
- Csincsik L, MacGillivray TJ, Flynn E, et al. Peripheral Retinal Imaging Biomarkers for Alzheimer’s Disease: a Pilot Study. Ophthalmic Res. 2018;59:182–192. doi:10.1159/000487053.
- Droz I, Mantel I, Ambresin A, et al. Genotype-phenotype correlation of age-related macular degeneration: influence of complement factor H polymorphism. Br J Ophthalmol. 2008;92:513–517. doi:10.1136/bjo.2007.127811.
- Nivison-Smith L, Milston R, Chiang J, et al. Peripheral retinal findings in populations with macular disease are similar to healthy eyes. Ophthalmic Physiol Opt. 2018;38:584–595. doi:10.1111/opo.12589.
- Ritchie CW, Peto T, Barzegar-Befroei N, et al. Peripheral Retinal Drusen as a Potential Surrogate Marker for Alzheimer’s Dementia: a Pilot Study Using Ultra-Wide Angle Imaging. Invest Ophthalmol Vis Sci. 2011;52:6683.
- Seddon JM, Cote J, Page WF, et al. The US twin study of age-related macular degeneration: relative roles of genetic and environmental influences. Arch Ophthalmol. 2005;123:321–327. doi:10.1001/archopht.123.3.321.
- Seddon JM, Reynolds R, Rosner B. Peripheral retinal drusen and reticular pigment: association with CFHY402H and CFHrs1410996 genotypes in family and twin studies. Invest Ophthalmol Vis Sci. 2009;50:586–591. doi:10.1167/iovs.08-2514.
- Guymer R, Wu Z. Age-related macular degeneration (AMD): more than meets the eye. The role of multimodal imaging in today’s management of AMD-A review. Clin Exp Ophthalmol. 2020;48:983–995. doi:10.1111/ceo.13837.
- Ly A, Nivison-Smith L, Hennessy M, Kalloniatis M. The advantages of intermediate-tier, inter-optometric referral of low risk pigmented lesions. Ophthalmic Physiol Opt. 2017;37:661–668. doi:10.1111/opo.12413.
- Ly A, Nivison-Smith L, Hennessy MP, Kalloniatis M. Collaborative care of non-urgent macular disease: a study of inter-optometric referrals. Ophthalmic Physiol Opt. 2016;36:632–642. doi:10.1111/opo.12322.
- Lens A, Nemeth SC, Ledford JK. Ocular Anatomy and Physiology. Thorofare, NJ, SLACK Incorporated: 2008.
- Bastek JV, Siegel EB, Straatsma BR, Foos RY. Chorioretinal juncture. Pigmentary patterns of the peripheral fundus. Ophthalmology. 1982;89:1455–1463. doi:10.1016/S0161-6420(82)34617-5.
- Lewis H, Straatsma BR, Foos RY, Lightfoot DO. Reticular degeneration of the pigment epithelium. Ophthalmology. 1985;92:1485–1495. doi:10.1016/S0161-6420(85)33829-0.
- Choi W, Mohler KJ, Potsaid B, et al. Choriocapillaris and choroidal microvasculature imaging with ultrahigh speed OCT angiography. PLoS One. 2013;8:e81499. doi:10.1371/journal.pone.0081499.
- Tan CS, Heussen F, Sadda SR. Peripheral autofluorescence and clinical findings in neovascular and non-neovascular age-related macular degeneration. Ophthalmology. 2013;120:1271–1277. doi:10.1016/j.ophtha.2012.12.002.
- Lengyel I, Csutak A, Florea D, et al. A Population-Based Ultra-Widefield Digital Image Grading Study for Age-Related Macular Degeneration-Like Lesions at the Peripheral Retina. Ophthalmology. 2015;122:1340–1347. doi:10.1016/j.ophtha.2015.03.005.
- Domalpally A, Clemons TE. Writing Committee for the OPRs. Peripheral Retinal Changes Associated with Age-Related Macular Degeneration in the Age-Related Eye Disease Study 2: age-Related Eye Disease Study 2 Report Number 12 by the Age-Related Eye Disease Study 2 Optos PEripheral RetinA (OPERA) Study Research Group. Ophthalmology. 2017;124:479–487. doi:10.1016/j.ophtha.2016.12.004.
- American Academy of Ophthalmology: Comprehensive Adult Medical Eye Evaluation Preferred Practice Pattern. American Academy of Ophthalmology Preferred Practice Patterns Committee, Hoskins Center for Quality Eye Care; 2020.
- Quinn N, Csincsik L, Flynn E, et al. The clinical relevance of visualising the peripheral retina. Prog Retin Eye Res. 2019;68:83–109. doi:10.1016/j.preteyeres.2018.10.001.
- Nagiel A, Lalane RA, Sadda SR, Schwartz SD. ULTRA-WIDEFIELD FUNDUS IMAGING: a Review of Clinical Applications and Future Trends. Retina. 2016;36:660–678. doi:10.1097/IAE.0000000000000937.
- Brown K, Sewell JM, Trempe C, et al. Comparison of image-assisted versus traditional fundus examination. Eye Brain. 2013;5:1–8. doi:10.2147/EB.S37646.
- Croft DE, van Hemert J, Wykoff CC, et al. Precise montaging and metric quantification of retinal surface area from ultra-widefield fundus photography and fluorescein angiography. Ophthalmic Surg Lasers Imaging Retina. 2014;45:312–317. doi:10.3928/23258160-20140709-07.
- Sagong M, van Hemert J, Olmos de Koo LC, et al. Assessment of accuracy and precision of quantification of ultra-widefield images. Ophthalmology. 2015;122:864–866. doi:10.1016/j.ophtha.2014.11.016.
- McNabb RP, Grewal DS, Mehta R, et al. Wide field of view swept-source optical coherence tomography for peripheral retinal disease. Br J Ophthalmol. 2016;100:1377–1382. doi:10.1136/bjophthalmol-2015-307480.
- Kolb JP, Klein T, Kufner CL, et al. Ultra-widefield retinal MHz-OCT imaging with up to 100 degrees viewing angle. Biomed Opt Express. 2015;6:1534–1552. doi:10.1364/BOE.6.001534.
- Reznicek L, Klein T, Wieser W, et al. Megahertz ultra-wide-field swept-source retina optical coherence tomography compared to current existing imaging devices. Graefes Arch Clin Exp Ophthalmol. 2014;252:1009–1016. doi:10.1007/s00417-014-2640-4.
- Carrai P, Pichi F, Bonsignore F, et al. Wide-field spectral domain-optical coherence tomography in central serous chorioretinopathy. Int Ophthalmol. 2015;35:167–171. doi:10.1007/s10792-014-0034-6.
- Choudhry N, Golding J, Manry MW, Rao RC. Ultra-Widefield Steering-Based Spectral-Domain Optical Coherence Tomography Imaging of the Retinal Periphery. Ophthalmology. 2016;123:1368–1374. doi:10.1016/j.ophtha.2016.01.045.
- Pichi F, Smith SD, Abboud EB, et al. Wide-field optical coherence tomography angiography for the detection of proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2020;258:1901–1909. doi:10.1007/s00417-020-04773-x.
- Straatsma BR, Landers MB, Kreiger AE. The ora serrata in the adult human eye. Arch Ophthalmol. 1968;80:3–20. doi:10.1001/archopht.1968.00980050005002.
- Shukla M, OP A. Developmental variations of peripheral retina. Indian J Ophthalmol. 1980;28:189–193.
- Campagnoli TR, Smiddy WE. Peripheral Retinal Abnormalities. In: Medina CA, Townsend JH, Singh AD, eds. Manual of Retinal Diseases: A Guide to Diagnosis and Management. Miami: Springer International Publishing Switzerland; 2016: 243-247
- Spencer LM, Foos RY, Straatsma BR. Enclosed Bays of the Ora Serrata: relationship to Retina Tears. Arch Ophthalmol. 1970;83:421–425. doi:10.1001/archopht.1970.00990030421006.
- Lonn LI, Smith TR. Ora Serrata Pearls: clinical and Histological Correlation. Arch Ophthalmol. 1967;77:809–813. doi:10.1001/archopht.1967.00980020811020.
- Adams ST. Pars Plana Cysts. A.M.A. Arch Ophthalmol. 1957;58:328–330. doi:10.1001/archopht.1957.00940010340002.
- Jones WL, Reidy RW. Atlas of the Peripheral Ocular Fundus. Boston, Butterworth-Heinemann Medical; 1985.
- Mannino G, Malagola R, Abdolrahimzadeh S, et al. Ultrasound biomicroscopy of the peripheral retina and the ciliary body in degenerative retinoschisis associated with pars plana cysts. Br J Ophthalmol. 2001;85:976–982. doi:10.1136/bjo.85.8.976.
- Allen RA, Miller DH, Straatsma BR. Cysts of the posterior ciliary body (pars plana). Arch Ophthalmol. 1961;66:302–313. doi:10.1001/archopht.1961.00960010304003.
- Schepens CL. Clinical aspects of pathologic changes in the vitreous body. Am J Ophthalmol. 1954;38:8–21. doi:10.1016/0002-9394(54)90004-5.
- Rutnin U, Schepens CL. Fundus appearance in normal eyes. II. The standard peripheral fundus and developmental variations. Am J Ophthalmol. 1967;64:840–852. doi:10.1016/0002-9394(67)93056-5.
- Demaine ED, Demaine ML, Iacono J, Langerman S. Wrapping spheres with flat paper. Comput Geom. 2009;42:748–757. doi:10.1016/j.comgeo.2008.10.006.
- Rutnin U, Schepens CL. Fundus appearance in normal eyes. 3. Peripheral degenerations. Am J Ophthalmol. 1967;64:1040–1062.
- O’Malley PF, Allen RA. Peripheral cystoid degeneration of the retina. Incidence and distribution in 1,000 autopsy eyes. Arch Ophthalmol. 1967;77:769–776. doi:10.1001/archopht.1967.00980020771010.
- Tolentino FI, Lapus JV, Novalis G, et al. Fluorescein angiography of degenerative lesions of the peripheral fundus and rhegmatogenous retinal detachment. Int Ophthalmol Clin. 1976;16:13–29. doi:10.1097/00004397-197601610-00005.
- Foos RY, Feman SS. Reticular cystoid degeneration of the peripheral retina. Am J Ophthalmol. 1970;69:392–403. doi:10.1016/0002-9394(70)92272-5.
- Streeten BW. Development of the human retinal pigment epithelium and the posterior segment. Arch Ophthalmol. 1969;81:383–394. doi:10.1001/archopht.1969.00990010385017.
- Teng CC, Katzin HM. An anatomic study of the peripheral retina. II. Peripheral cystoid degeneration of the retina; formation of cysts and holes. Am J Ophthalmol. 1953;36:29–39. doi:10.1016/0002-9394(53)91505-0.
- Straatsma BR. Clinical features of degenerative retinoschisis. Aust J Ophthalmol. 1980;8:201–206. doi:10.1111/j.1442-9071.1980.tb00339.x.
- O’Malley P, Allen RA, Straatsma BR. O’Malley CC: paving-Stone Degeneration of the Retina. Arch Ophthalmol. 1965;73:169–182. doi:10.1001/archopht.1965.00970030171006.
- Corbelli E, Borrelli E, Parravano M, et al. Multimodal imaging characterization of peripheral drusen. Graefes Arch Clin Exp Ophthalmol. 2020;258:543–549. doi:10.1007/s00417-019-04586-7.
- Suetsugu T, Kato A, Yoshida M, et al. Evaluation of peripheral fundus autofluorescence in eyes with wet age-related macular degeneration. Clin Ophthalmol. 2016;10:2497–2503. doi:10.2147/OPTH.S120402.
- Witmer MT, Kozbial A, Daniel S, Kiss S. Peripheral autofluorescence findings in age-related macular degeneration. Acta Ophthalmol. 2012;90:e428–433. doi:10.1111/j.1755-3768.2012.02434.x.
- Quinn N, Wright D, Peto T, et al. Prevalence and characteristics of peripheral retinal lesions in an ageing population. Invest Ophthalmol Vis Sci. 2017;58:1497.
- Lengyel I, Tufail A, Hosaini HA, et al. Association of drusen deposition with choroidal intercapillary pillars in the aging human eye. Invest Ophthalmol Vis Sci. 2004;45:2886–2892. doi:10.1167/iovs.03-1083.
- Rudolf M, Clark ME, Chimento MF, et al. Prevalence and morphology of druse types in the macula and periphery of eyes with age-related maculopathy. Invest Ophthalmol Vis Sci. 2008;49:1200–1209. doi:10.1167/iovs.07-1466.
- Postel EA, Agarwal A, Schmidt S, et al. Comparing age-related macular degeneration phenotype in probands from singleton and multiplex families. Am J Ophthalmol. 2005;139:820–825. doi:10.1016/j.ajo.2004.12.029.
- Bae K, Cho K, Kang SW, et al. Peripheral Reticular Pigmentary Degeneration and Choroidal Vascular Insufficiency, Studied by Ultra Wide-Field Fluorescein Angiography. PLoS One. 2017;12:e0170526. doi:10.1371/journal.pone.0170526.
- Straatsma BR, Lewis H, Foos RY, Evans R. Fluorescein angiography in reticular degeneration of the pigment epithelium. American Journal of Ophthalmology. 1985;100:202–208. doi:10.1016/s0002-9394(14)75007-x.
- Humphrey WT, Carlson RE, Valone JA Jr. Senile reticular pigmentary degeneration. Am J Ophthalmol. 1984;98:717–722. doi:10.1016/0002-9394(84)90686-X.
- Friberg VJ, Derk BA, Friberg TR, et al. The Croatian Opera Study II: further study of the peripheral retinal features in AMD subjects and controls. Investigative Ophthalmology & Visual Science ARVO Annual Meeting Abstract 56, Denver, Colorado, 2015.
- Seo T, Iwabuchi K, Kato M, Watanabe I. Reticular degeneration of retinal pigment epithelium. Nippon Ganka Gakkai Zasshi. 1992;96:1161–1166.
- Feke GT, Tagawa H, Deupree DM, et al. Blood flow in the normal human retina. Invest Ophthalmol Vis Sci. 1989;30:58–65.
- Takahashi K, Muraoka K, Kishi S, Shimizu K. Watershed zone in the human peripheral choroid. Ophthalmology. 1996;103:336–342. doi:10.1016/S0161-6420(96)30695-7.
- Lains I, Park DH, Mukai R, et al. Peripheral Changes Associated With Delayed Dark Adaptation in Age-related Macular Degeneration. Am J Ophthalmol. 2018;190:113–124. doi:10.1016/j.ajo.2018.03.035.
- Hunter JE. Retinal white without pressure: review and relative incidence. Am J Optom Physiol Opt. 1982;59:293–296.
- Karlin DB, Curtin BJ. Peripheral chorioretinal lesions and axial length of the myopic eye. Am J Ophthalmol. 1976;81:625–635. doi:10.1016/0002-9394(76)90129-X.
- Nagpal KC, Huamonte F, Constantaras A, et al. Migratory white-without-pressure retinal lesions. Arch Ophthalmol. 1976;94:576–579. doi:10.1001/archopht.1976.03910030270003.
- Orlin A, Fatoo A, Ehrlich J, et al. Ultra-widefield fluorescein angiography of white without pressure. Clin Ophthalmol. 2013;7:959–964. doi:10.2147/OPTH.S43450.
- Fawzi AA, Nielsen JS, Mateo-Montoya A, et al. Multimodal imaging of white and dark without pressure fundus lesions. Retina. 2014;34:2376–2387. doi:10.1097/IAE.0000000000000388.
- Diaz RI, Sigler EJ, Randolph JC, et al. Spectral domain optical coherence tomography characteristics of white-without-pressure. Retina. 2014;34:1020–1021. doi:10.1097/IAE.0000000000000012.
- Shukla M, Ahuja OP. White with pressure (WWP) and white without pressure (WWOP) lesions. Indian J Ophthalmol. 1982;30:129–132.
- Akbani MI, Reddy KRK, Vishwanath K, Saleem M. Prevalence of Peripheral Retinal Degenerations in the Cases of Myopia- A Prospective Study. Indian J Public Health ResDev. 2014;5:58/63. doi:10.5958/j.0976-5506.5.2.074.
- Eisner G. Biomicroscopy of the Peripheral Fundus: An Atlas and Textbook. Berlin, New York: Springer; 1973.
- Daicker B. Are the symptoms “white with pressure” and “white without pressure” related to peripheral retinal sclerosis? Mod Probl Ophthalmol. 1975;15:82–90.
- Tassman W, Annesley W Jr. Retinal detachment in the retinopathy of prematurity. Arch Ophthalmol. 1966;75:608–614. doi:10.1001/archopht.1966.00970050610005.
- Brockhurst RJ, Albert DM, Zakov ZN. Pathologic findings in familial exudative vitreoretinopathy. Arch Ophthalmol. 1981;99:2143–2146. doi:10.1001/archopht.1981.03930021019006.
- Pemberton JW, Freeman HM, Schepens CL. Familial retinal detachment and the Ehlers-Danlos syndrome. Arch Ophthalmol. 1966;76:817–824. doi:10.1001/archopht.1966.03850010819007.
- Gilmour DF. Familial exudative vitreoretinopathy and related retinopathies. Eye (Lond). 2015;29:1–14. doi:10.1038/eye.2014.70.
- Gozum N, Cakir M, Gucukoglu A, Sezen F. Relationship between retinal lesions and axial length, age and sex in high myopia. Eur J Ophthalmol. 1997;7:277–282. doi:10.1177/112067219700700313.
- Talbot JF, Bird AC, Maude GH, et al. Sickle cell retinopathy in Jamaican children: further observations from a cohort study. Br J Ophthalmol. 1988;72:727–732. doi:10.1136/bjo.72.10.727.
- Chang MY, McBeath JB, McCannel CA, McCannel TA. ‘Shadow sign’ in congenital hypertrophy of the retinal pigment epithelium of young myopic pigmented patients. Eye (Lond). 2016;30:160–163. doi:10.1038/eye.2015.187.
- Nagpal KC, Goldberg MF, Asdourian G, et al. Dark-without-pressure fundus lesions. Br J Ophthalmol. 1975;59:476–479. doi:10.1136/bjo.59.9.476.
- Moysidis SN, Koulisis N, Ameri H, et al. Multimodal Imaging of Geographic Areas of Retinal Darkening. Retin Cases Brief Rep. 2015;9:347–351. doi:10.1097/ICB.0000000000000231.
- Sherman T, Palileo BM, Adam CR, Abrams GW. Dark Without Pressure In A Case Of Choroidal Osteoma. Retin Cases Brief Rep. 2020. doi:10.1097/ICB.0000000000001026.
- Flores Pimentel MA, Duncan JL, de Alba Campomanes AG, Moore A. Dark without pressure retinal changes in a paediatric age group. Eye (Lond). 2020; 35: 1221-1227. doi: 10.1038/s41433-020-1088-5.
- Chen X, Liang M. Changes in Dark without Pressure. Ophthalmol Retina. 2018;2:1077. doi:10.1016/j.oret.2018.07.010.
- Byer NE. Clinical study of lattice degeneration of the retina. Trans Am Acad Ophthalmol Otolaryngol. 1965;69:1065–1081.
- Straatsma BR, Zeegen PD, Foos RY, et al. Lattice degeneration of the retina. XXX Edward Jackson Memorial Lecture. Am J Ophthalmol. 1974;77:619–649. doi:10.1016/0002-9394(74)90525-X.
- Kothari A, Narendran V, Saravanan VR. In vivo sectional imaging of the retinal periphery using conventional optical coherence tomography systems. Indian J Ophthalmol. 2012;60:235–239. doi:10.4103/0301-4738.95885.
- Manjunath V, Taha M, Fujimoto JG, Duker JS. Posterior lattice degeneration characterized by spectral domain optical coherence tomography. Retina. 2011;31:492–496. doi:10.1097/IAE.0b013e3181ed8dc9.
- Byer NE. Rethinking Prophylactic Therapy of Retinal Detachment. New York: Ophthalmic Communications Society; 1992.
- Lang GK. Daumann FJ: [Peripheral fundus changes in subjects with healthy eyes (pilots)]. Klin Monbl Augenheilkd. 1982;181:493–495. doi:10.1055/s-2008-1055279.
- Mitry D, Charteris DG, Fleck BW, et al. The epidemiology of rhegmatogenous retinal detachment: geographical variation and clinical associations. Br J Ophthalmol. 2010;94:678–684. doi:10.1136/bjo.2009.157727.
- Semes LP, Holland WC, Likens EG. Prevalence and laterality of lattice retinal degeneration within a primary eye care population. Optometry. 2001;72:247–250.
- Shiomi Y. Study of lattice degeneration of the retina. Part I. Clinical features of lattice degeneration of the retina without retinal detachment (author’s transl). Nippon Ganka Gakkai Zasshi. 1980;84:2036–2042.
- Shiomi Y. Study of lattice degeneration of the retina. Part 2. Clinical features of lattice degeneration of the retina (author’s transl). Nippon Ganka Gakkai Zasshi. 1981;85:269–275.
- Zhang T, Zuo Y, Wei Y, et al. The Prevalence and Associations of Peripheral Retinopathy: baseline Study of Guangzhou Office Computer Workers. J Ophthalmol. 2018:2358690. doi:10.1155/2018/2358690.
- Sato K, Tsunakawa N, Inaba K, Yanagisawa Y. Fluorescein angiography on retinal detachment and lattice degeneration. I. Equatorial degeneration with idiopathic retinal detachment. Nippon Ganka Gakkai Zasshi. 1971;75:635–642.
- Meguro A, Ideta H, Ideta R, et al. In-depth analysis of the COL2A1 gene in lattice degeneration of the retina. Invest Ophthalmol Vis Sci. 2014;55:6435.
- Okazaki S, Meguro A, Ideta R, et al. Common variants in the COL2A1 gene are associated with lattice degeneration of the retina in a Japanese population. Mol Vis. 2019;25:843–850.
- Yamane T, Meguro A, and Takeuchi M, et al. Association study of RHBDD1-COL4A4 gene polymorphisms with susceptibility to lattice degeneration of the retina in a Japanese population. Investigative Ophthalmology & Visual Science . 2015;56: 1095-1095.
- Tsukahara I. A study of lattice degeneration of the retina with slit-lamp microscope. Nippon Ganka Gakkai Zasshi. 1967;71:247–250.
- Tillery WV, Lucier AC. Round atrophic holes in lattice degeneration–an important cause of phakic retinal detachment. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1976;81:509–518.
- Byer NE. Long-term natural history of lattice degeneration of the retina. Ophthalmology. 1989;96:1396–1401. doi:10.1016/S0161-6420(89)32713-8. discussion 1401-1392.
- Byer NE. Changes in and prognosis of lattice degeneration of the retina. Trans Am Acad Ophthalmol Otolaryngol. 1974;78:114–125.
- Foos RY, Simons KB. Vitreous in lattice degeneration of retina. Ophthalmology. 1984;91:452–457. doi:10.1016/S0161-6420(84)34266-X.
- Folk JC, Arrindell EL, Klugman MR. The fellow eye of patients with phakic lattice retinal detachment. Ophthalmology. 1989;96:72–79. doi:10.1016/S0161-6420(89)32926-5.
- Ho T-C, Ho A. Long-term natural course of lattice degeneration of the retina in high myopic eyes - A ten-year long term study. Invest Ophthalmol Vis Sci. 2015;56:2965.
- Hyams SW, Neumann E. Peripheral retina in myopia. With particular reference to retinal breaks. Br J Ophthalmol. 1969;53:300–306. doi:10.1136/bjo.53.5.300.
- Friedman Z, Neumann E, Hyams S. Vitreous and peripheral retina in aphakia. A study of 200 non-myopic aphakic eyes. Br J Ophthalmol. 1973;57:52–57. doi:10.1136/bjo.57.1.52.
- Hyams SW, Neumann E, Friedman Z:, Myopia-aphakia II. Vitreous and peripheral retina. Br J Ophthalmol. 1975;59:483–485. doi:10.1136/bjo.59.9.483.
- Kottow M. Peripheral retinal degenerations and breaks. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1980;214:53–60. doi:10.1007/BF00414537.
- Aaberg TM, Stevens TR. Snail track degeneration of the retina. Am J Ophthalmol. 1972;73:370–376. doi:10.1016/0002-9394(72)90065-7.
- Shukla M, Ahuja OP. A possible relationship between lattice and snail track degenerations of the retina. Am J Ophthalmol. 1981;92:482–485. doi:10.1016/0002-9394(81)90639-5.
- Bec P, Malecaze F, Arne JL, Mathis A. Lattice degeneration of the peripheral retina: ultrastructural study. Ophthalmologica. 1985;191:107–113. doi:10.1159/000309568.
- Hyams SW, Meir E, Ivry M, et al. Chorioretinal Lesions Predisposing to Retinal Detachment. Am J Ophthalmol. 1974;78:429–437. doi:10.1016/0002-9394(74)90230-X.
- Robertson DM, Link TP, Rostvold JA. Snowflake degeneration of the retina. Ophthalmology. 1982;89:1513–1517. doi:10.1016/S0161-6420(82)34609-6.
- Straatsma BR, Foss RY. Typical and Reticular Degenerative Retinoschisis: XXVI Francis I. Proctor Memorial Lecture. Am JOphthalmol. 1973;75:551–575. doi:10.1016/0002-9394(73)90809-X.
- Byer NE. Long-term natural history study of senile retinoschisis with implications for management. Ophthalmology. 1986;93:1127–1137. doi:10.1016/S0161-6420(86)33601-7.
- Byer NE. Clinical Study of Senile Retinoschisis. Arch Ophthalmol. 1968;79:36–44. doi:10.1001/archopht.1968.03850040038012.
- Regillo CD, Custis PH. Surgical management of retinoschisis. Curr Opin Ophthalmol. 1997;8:80–86. doi:10.1097/00055735-199706000-00014.
- Xue K, Muqit MMK, Ezra E, et al. Incidence, mechanism and outcomes of schisis retinal detachments revealed through a prospective population-based study. Br J Ophthalmol. 2017;101:1022–1026. doi:10.1136/bjophthalmol-2016-309750.
- Yeoh J, Rahman W, Chen FK, da Cruz L. Use of spectral-domain optical coherence tomography to differentiate acquired retinoschisis from retinal detachment in difficult cases. Retina. 2012;32:1574–1580. doi:10.1097/IAE.0b013e3182411d90.
- Gottinger W. Retinoschisis und Ablatio. Klin Mbl Augenheilkd. 1976;169:14–21.
- Yassur Y, Feldberg R, Axer-Siegel R, et al. Argon laser treatment of senile retinoschisis. Br J Ophthalmol. 1983;67:381–384. doi:10.1136/bjo.67.6.381.
- Foos RY. Senile retinoschisis. Relationship to cystoid degeneration. Trans Am Acad Ophthalmol Otolaryngol. 1970;74:33–51.
- Rachitskaya AV, Yuan A, Singh RP, et al. Optical coherence tomography of outer retinal holes in senile retinoschisis and schisis-detachment. Br J Ophthalmol. 2017;101:445–448. doi:10.1136/bjophthalmol-2016-308551.
- Mitry D, Singh J, Yorston D, et al. The predisposing pathology and clinical characteristics in the Scottish retinal detachment study. Ophthalmology. 2011;118:1429–1434. doi:10.1016/j.ophtha.2010.11.031.
- Byer NE. Spontaneous Regression of Senile Retinoschisis. Arch Ophthalmol. 1972;88:207–209. doi:10.1001/archopht.1972.01000030209016.
- Clare G, Sullivan P, Gregor Z. Spontaneous regression of degenerative retinoschisis associated with outer leaf retinal breaks. Retina. 2005;25:1116–1117. doi:10.1097/00006982-200512000-00029.
- Watzke RC, Folk JC, Lauer AK. Foveal involvement by acquired retinoschisis: long-term visual outcomes. Retina. 2013;33:606–612. doi:10.1097/IAE.0b013e3182695a6f.
- Foos R. Retinal Holes. Am J Ophthalmol. 1978;86:354–358. doi:10.1016/0002-9394(78)90239-8.
- Kuhn F, Aylward B. Rhegmatogenous retinal detachment: a reappraisal of its pathophysiology and treatment. Ophthalmic Res. 2014;51:15–31. doi:10.1159/000355077.
- Neumann E, Hyams S. Conservative management of retinal breaks. A follow-up study of subsequent retinal detachment. Br J Ophthalmol. 1972;56:482–486. doi:10.1136/bjo.56.6.482.
- Casswell EJ, Abou Ltaif S, Carr T, et al. Widefield Spectral-Domain Optical Coherence Tomography Imaging of Peripheral Round Retinal Holes with or without Retinal Detachment. Retina. 2019;39:1047–1053. doi:10.1097/IAE.0000000000002133.
- Wilkinson CP. Interventions for asymptomatic retinal breaks and lattice degeneration for preventing retinal detachment. Cochrane Database Syst Rev. 2014. doi:10.1002/14651858.CD003170.pub4:CD003170.
- Byer NE. What happens to untreated asymptomatic retinal breaks, and are they affected by posterior vitreous detachment? Ophthalmology. 1998;105:1045–1049. doi:10.1016/S0161-6420(98)96006-7. discussion 1049-1050.
- Davis MD. Natural history of retinal breaks without detachment. Arch Ophthalmol. 1974;92:183–194. doi:10.1001/archopht.1974.01010010191001.
- Murakami-Nagasako F, Ohba N. Phakic retinal detachment associated with atrophic hole of lattice degeneration of the retina. Graefes Arch Clin Exp Ophthalmol. 1983;220:175–178. doi:10.1007/BF02186664.
- Chou SC, Yang CH, Lee CH, et al. Characteristics of primary rhegmatogenous retinal detachment in Taiwan. Eye (Lond). 2007;21:1056–1061. doi:10.1038/sj.eye.6702397.
- Laatikainen L, Tolppanen EM. Characteristics of rhegmatogenous retinal detachment. Acta Ophthalmol (Copenh). 1985;63:146–154. doi:10.1111/j.1755-3768.1985.tb01527.x.
- Byer NE. Cystic retinal tufts and their relationship to retinal detachment. Arch Ophthalmol. 1981;99:1788–1790. doi:10.1001/archopht.1981.03930020662007.
- Byer NE. Relationship of cystic retinal tufts to retinal detachment. Dev Ophthalmol. 1981;2:36–42.
- Taney LS, Baumal CR. Optical coherence tomography of a cystic retinal tuft. JAMA Ophthalmol. 2014;132:1191. doi:10.1001/jamaophthalmol.2014.190.
- Foos RY. Vitreous base, retinal tufts and retinal tears: pathogenic relationships. In: Pruett RC, Regan CDJ, eds. Retinal Congress. New York: Apple-Century-Crofts; 1974:259.
- Straatsma BR, Foos RY, Feman SS. Degenerative disease of the peripheral retina. In: Duane DD, ed. Clinical Ophthalmology. Vol. 3. Philadelphia Harper &: Row; 1986:1.
- Foos RY, Allen RA. Retinal tears and lesser lesions of the peripheral retina in autopsy eyes. Am J Ophthalmol. 1967;64:643–655. doi:10.1016/0002-9394(67)90571-5.
- Cahill M, Gallagher P, Whitehead A, Acheson R. Autosomal dominant peripheral cystic retinal patches and non-cystic retinal tufts associated with peripapillary crescents, retinal breaks and uveitis. Graefes Arch Clin Exp Ophthalmol. 2001;239:102–108. doi:10.1007/s004170000239.
- Chan CC, Koch CA, Kaiser-Kupfer MI, et al. Loss of heterozygosity for the NF2 gene in retinal and optic nerve lesions of patients with neurofibromatosis 2. J Pathol. 2002;198:14–20. doi:10.1002/path.1174.
- Waisberg V, Rodrigues LO, Nehemy MB, et al. Spectral-Domain Optical Coherence Tomography Findings in Neurofibromatosis Type 2. Invest Ophthalmol Vis Sci. 2016;57:OCT262–267. doi:10.1167/iovs.15-18919.
- Murakami-Nagasako F, Ohba N. Phakic retinal detachment associated with cystic retinal tuft. Graefes Arch Clin Exp Ophthalmol. 1982;219:188–192. doi:10.1007/BF02156845.
- Hirose T, Lee KY, Schepens CL. Snowflake degeneration in hereditary vitreoretinal degeneration. Am J Ophthalmol. 1974;77:143–153. doi:10.1016/0002-9394(74)90665-5.
- Edwards AO. Clinical features of the congenital vitreoretinopathies. Eye (Lond). 2008;22:1233–1242. doi:10.1038/eye.2008.38.
- Chen CJ, Everett TK, Marascalco D. Snowflake degeneration: an independent entity or a variant of retinitis pigmentosa? South Med J. 1986;79:1216–1223. doi:10.1097/00007611-198610000-00006.
- Jiao X, Ritter R 3rd, Hejtmancik JF, Wards AO. Genetic linkage of snowflake vitreoretinal degeneration to chromosome 2q36. Invest Ophthalmol Vis Sci. 2004;45:4498–4503. doi:10.1167/iovs.04-0722.
- Lee MM, Ritter R 3rd, Hirose T, et al. Snowflake vitreoretinal degeneration: follow-up of the original family. Ophthalmology. 2003;110:2418–2426. doi:10.1016/S0161-6420(03)00828-5.
- Hejtmancik JF, Jiao X, Li A, et al. Mutations in KCNJ13 cause autosomal-dominant snowflake vitreoretinal degeneration. Am J Hum Genet. 2008;82:174–180. doi:10.1016/j.ajhg.2007.08.002.
- Pattnaik BR, Tokarz S, Asuma MP, et al. Snowflake vitreoretinal degeneration (SVD) mutation R162W provides new insights into Kir7.1 ion channel structure and function. PLoS One. 2013;8:e71744. doi:10.1371/journal.pone.0071744.
- Hirose T, Wolf E, Schepens CL. Retinal functions in snowflake degeneration. Ann Ophthalmol. 1980;12:1135–1146.
- Pollack A, Uchenik D, Chemke J, Oliver M. Prophylactic laser photocoagulation in hereditary snowflake vitreoretinal degeneration. A family report. Arch Ophthalmol. 1983;101:1536–1539. doi:10.1001/archopht.1983.01040020538005.
- Kucukiba K, Erol N, Bilgin M. Evaluation of Peripheral Retinal Changes on Ultra-Widefield Fundus Autofluorescence Images of Patients with Age-Related Macular Degeneration. Turk J Ophthalmol. 2020;50:6–14. doi:10.4274/tjo.galenos.2019.00359.
- Ukalovic K, Cao S, Lee S, et al. Drusen in the Peripheral Retina of the Alzheimer’s Eye. Curr Alzheimer Res. 2018;15:743–750. doi:10.2174/1567205015666180123122637.
- Merijohn GK, Bader JD, Frantsve-Hawley J, Aravamudhan K. Clinical decision support chairside tools for evidence-based dental practice. J Evid Based Dent Pract. 2008;8:119–132. doi:10.1016/j.jebdp.2008.05.016.
- FDI World Dental Federation. New chairside guide focuses on caries prevention. Br Dent J. 2017;223:398. doi:10.1038/sj.bdj.2017.801.
- Royal College of Ophthalmologists: rCOphth Clinical Guidelines. https://www.rcophth.ac.uk/standards-publications-research/clinical-guidelines/
- Hart KM, Abbott C, Ly A, et al. Optometry Australia’s chairside reference for the diagnosis and management of age-related macular degeneration. Clin Exp Optom. 2020;103:254–264. doi:10.1111/cxo.12964.
- Centre for Eye. Health: CFEH Chairside References. https://www.centreforeyehealth.com.au/education/cfeh-educational-resources/ Accessed July 22, 2020
- Ly A, Nivison-Smith L, Hennessy M, Kalloniatis M. Pigmented Lesions of the Retinal Pigment Epithelium. Optom Vis Sci. 2015;92:844–857. doi:10.1097/OPX.0000000000000640.
- Chu RL, Pannullo NA, Adam CR, et al. Morphology of Peripheral Vitreoretinal Interface Abnormalities Imaged with Spectral Domain Optical Coherence Tomography. J Ophthalmol. 2019;2019:3839168. doi:10.1155/2019/3839168.