ABSTRACT
Purpose
Modern vitreo-retinal surgery has scaled new frontiers with the advent of better instrumentation. However, physiological tremors, intraocular dexterity and difficulty in visualization hamper minimally invasive retinal surgery. Robotics has the potential to overcome these limitations and improve surgical outcomes. This review aims to provide a comprehensive summary of the advances made in the field of robotics in vitreo-retinal surgery.
Methods
This review included 30 studies comprising randomized control trials, nonrandomized comparative studies and systematic reviews on the application of robotics in vitreo-retinal surgery.
Results
Robotic systems presently available in vitreo-retinal surgery can be broadly classified based on the extent of automation into five categories: robot-assisted, co-manipulated, tele-operated, partially/fully automated and magnetically controlled devices. Key features of individual devices are highlighted in this review. Robotic assistance in vitreo-retinal surgery can maximize performance for routine procedures, enable high-precision procedures such as targeted gene therapy and retinal vein cannulation, improve ergonomics, and revolutionize tele-surgery. Cost limitations and compatibility with available surgical systems are the barriers in implementation of robotics in retinal microsurgery.
Conclusion
This review provides a concise summary of the available robotic systems in vitreo-retinal surgery, advantages over conventional systems, current applications and future implications. Robotics is a rapidly evolving field, which holds great promise in the future of vitreo-retinal surgery.
INTRODUCTION
Vitreo-retinal surgery demands fine precision and accuracy within the limited constraints of the vitreous cavity. Since the advent of modern pars plana vitrectomy by Robert Machemer in 1970,Citation1 the field has evolved immensely. Sutureless surgery with smaller gauge instrumentation, greater efficiency of newer vitrectomy systems, wide-angled viewing systems and improved illumination, have drastically enhanced the outcomes in retinal surgery. Simultaneously, the gamut of indications for vitrectomy has also expanded, creating an increasing demand for better instrumentation. Optimizing visualization, spatial resolution, depth perception and manipulation dexterity are a few of the challenges that a retinal surgeon needs to overcome. Studies have shown that human tremor amplitude at the instrument tip during microsurgery averages at about 108 µm.Citation2 Accurate positioning of surgical tool tip during ILM peeling requires precision of within 10 µm. This exceeds physiological limits.Citation3 Lack of tactile or force feedback during vitreo-retinal surgery is another limitation. Difficulty in perception of force feedback during fine maneuvers occurs due to concomitant tool-trocar friction, increasing dependance on visual cues.Citation4 Ensuring best possible visualization, positional stability and overcoming baseline tremors are vital for further advancement in retinal microsurgery. Robotics with real-time OCT incorporation could hold the answer to circumvent these issues and improve surgical outcomes.
An ideal robotic system should be compact and lightweight, making it easy for the surgeon to transition between manual and robotic assistance for specific steps. Tremor filtering and force sensing feedback are essential requisites to establish superiority over routine manual minimally invasive surgery.Citation5 Sustained positioning of instruments at the desired site, or “freeze-functionality,” can enhance efficiency of static tasks such as drug delivery at a target site. Motion scaling is a feature by which the motion output at the surgical tool tip is only a fraction of the input force applied.Citation6 Improved visualization and concurrent real-time OCT guidance are key in an ideal robotic surgical system. Intuitive control, rapid entry and exit of instruments and ability to overcome inadvertent movements could perfect these systems for vitreo-retinal surgery under local anaesthesia.
METHOD OF LITERATURE REVIEW
Literature search for this review was done in September 2021 on PubMed using the following keywords and their iterations: “robotics in vitreo-retinal surgery”, “telemanipulation”, “comanipulation”, and “OCT guidance.” Additional articles were obtained from the bibliography of the literature retrieved using the aforementioned keywords. This resulted in a total of 38 articles. The articles were carefully screened to include the most relevant studies comprising randomized control trials, nonrandomized comparative studies and systematic reviews in the English language. This narrowed down the final number of articles to those subsequently included in this review.
Classification of robotic assistance in vitreo-retinal surgery:
Robotic systems can be broadly classified into robot-assisted tools, co-manipulation devices, tele-operated systems, magnetically controlled and fully automated devices.Citation7,Citation8 A brief overview of the individual systems and relevant examples is elucidated in .
Table 1. Classification of robotic systems in vitreo-retinal surgery.
AVAILABLE ROBOTIC SYSTEMS
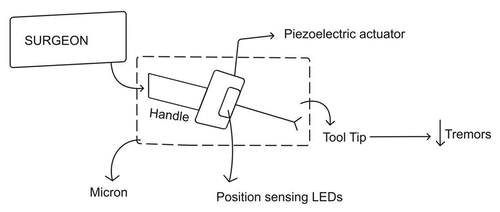
MICRON
Riviere et al. developed MICRON, which is a handheld active stabilizing instrument. It senses tremors and erroneous hand movements of the surgeon and deflects them by means of the self-actuating tip (.Citation3 The tip is composed of piezoelectric crystals, detects tremors by means of optical sensors and attenuates them fivefold. Other benefits include minimal workspace intrusion, intuitive interface and motion scaling (.Citation9
Table 2. Robotic systems in vitreoretinal surgery.
“Smart” Forceps
These are routine forceps into which force sensors have been incorporated which provides auditory feedback to the surgeon. “Smart” forceps would alert the surgeon when the force utilized during a crucial step exceeds the ideal limit, thereby reducing intraoperative complications.Citation15
Steady-Hand Eye Robot
The steady hand instrument is a co-manipulator developed by Johns Hopkins University. It aids combined control of the surgical tool by the robotic system and the surgeon. The mechanics of the instrument include an XYZ system permitting free movement in all directions, a roll mechanism that is a rotating platform favoring intraocular access of the tool, and a tilt mechanism that allows adjustment of tool angulation. The tool holder allows attachment of a variety of surgical instruments. Negation of tremors, force sensing, force scaling and quick-release mechanism in case of unexpected eye movements are advantages of this system.Citation10,Citation16
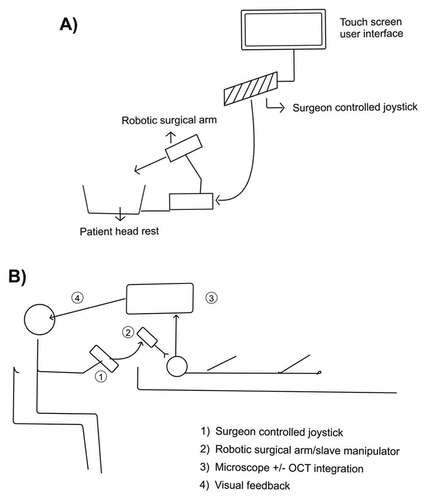
Preceyes Surgical System
The Preceyes Surgical system (Preceyes BV, Eindhoven, the Netherlands) is a highly precise tele-manipulation robotic device that has been developed for vitreo-retinal surgery (.This system has been validated in clinical research trials and has a CE mark, available for use in Europe for vitreo-retinal surgical interventions.Citation11 The Preceyes system operates on a ‘master–slave’ design wherein the surgeon handles the motion controller or input joystick, which in turn controls the movements of the surgical robotic arm (slave operator). Relatively larger movements on the input joystick by the surgeon translate into much finer and precise movements at the tool tip, making it a highly efficient and accurate system. The standby function, whereby a particular intraocular instrument can be held stationary, while the surgeon adjusts position, makes it ergonomically advantageous. Motion scaling, tremor filtering and virtual z-boundary are other useful features of this system. The z-boundary is a pre-defined distance from the retina beyond which further movement of the surgical tool tip will be restricted. Visual feedback to the surgeon is provided through the standard surgical microscope, and real-time OCT guidance could potentially be incorporated. The Preceyes system is compact and non-obtrusive and can be fitted on to the temporal aspect of the operation table. The surgeon can also perform the operation from a remote location using the Preceyes system, enhancing its role in telesurgery.Citation6,Citation17
Edwards et al. conducted the first double-armed randomized clinical trial to study the viability of Preceyes system in human eyes. In the first arm, patients requiring ERM peeling or ILM peeling for macular hole were randomized to either robotic or manual surgery groups. Outcomes were equally successful in both groups, and differences in retinal microtrauma were insignificant. Robot-assisted surgery however required a longer duration for completion. In the second arm, patients requiring subretinal rtPA for submacular hemorrhage were randomized to receive either manual or robot-assisted injection. Sub-retinal injection was performed successfully using the robot-assisted system under local anaesthesia. The standby function and the ability to slowly inject the drug while maintaining positional stability, make robot-assisted subretinal injections superior in terms of safety. This study therefore provided proof-of-concept for the use of robotic systems in vitreo-retinal surgery.Citation11
Jacobsen et al. conducted a trial to compare manual with robot-assisted surgery using the Eyesi virtual-reality simulator. A randomized control crossover study was done to compare outcomes among experienced and novice surgeons. Duration of surgery using robotic assistance was uniformly longer. However, improved precision with a lower rate of tissue damage, particularly in the case of novice surgeons, was the advantage noted with robot-assisted surgery.Citation6
Maberley et al. conducted a study to compare manual vs robot-assisted ILM peeling (Preceyes system) on the Eyesi virtual surgical simulator platform. The vitreo-retinal surgeons recruited in the study were performing robot-assisted surgery for the first time. The study demonstrated relative ease in mastering the new technique. The robot-assisted surgery, however, took significantly longer when compared to the manual approach. Intraocular movements were lower, and safety of the surgical procedure with fewer simulated macular hemorrhages was demonstrated in robot-assisted surgery.Citation18
RAM!S
This is a hybrid parallel-serial tele-manipulation device with piezoelectric actuators developed by the Technical University of Munich. Higher stability, increased precision, adjustable remote center of motion, and less workspace intrusion due to its compact structure are potential benefits of the RAM!S surgical system.Citation12 OCT-guided depth-tracking of the subretinal needle during injection has also been experimentally demonstrated ex-vivo in pig eyes, using this system.Citation19
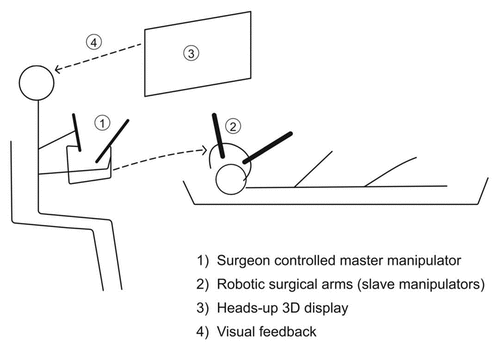
Intraocular Robotic Interventional Surgical System (IRISS)
The IRISS is a novel surgical system with combined automation and augmented reality tele-manipulation (. This system has the advantage of two surgical arms to simultaneously control more than one instrument, bringing it a step closer to manually performed procedures. The dual arms are manipulated by the surgeon at a distance, using heads-up display, thereby improving ergonomics. Added benefits include tremor filtering and motion scaling. The IRISS system has potential benefits and utility in both anterior segment and vitreo-retinal surgery.Citation13
OctoMag
OctoMag utilizes an extraocular magnetic field to control intraocular procedures such as retinal vein cannulation and localized drug delivery. High precision and dexterity can be achieved with this system for intraocular surgery. The limitation is the relatively large area occupied by the extraocular magnetic field generators utilized by such systems.Citation14,Citation20 Charreyron et al. demonstrated subretinal injections ex vivo in pig eyes by utilizing magnetic microcannulas under both microscope visualization and OCT guidance.Citation21
SURGICAL APPLICATIONS OF ROBOTIC SYSTEMS
Membrane peeling:
ILM and ERM peeling, which require high surgical precision, have been demonstrated with robotic systems. Improved safety with tremor negation and fewer intra-operative complications are potential advantages, at the expense of slightly longer surgical duration.Citation6,Citation11 Incorporation of OCT guidance and force feedback enhances accuracy of robotic systems in membrane peeling.Citation22
(2) Retinal vein cannulation:
A potential treatment modality for retinal vein occlusions is cannulation of the narrowed vein with a micropipette and concomitant release of an anticoagulant to aid in clot lysis. This requires precision and surgical skill that exceed human limits.Citation23 Gijbels et al utilized a co-manipulation device and successfully performed the first in-human retinal vein cannulation. Robot-assistance aided tremor free insertion of the device, ensuring stability for a period of up to 10 minutes, during which Ocriplasmin was injected into the cannulated vein, following which the device was safely removed.Citation24
(3) Drug delivery/Gene therapy:
Subretinal injection with robotic assistance, of drugs such as t-PA, has been demonstrated in humans. Robotic systems ensure ideal positioning and stable slow infusion of the drug, reducing associated surgical complications such as vitreous hemorrhage and retinal detachment.Citation11 Gene therapy requires prolonged deliberate infusion of the therapeutic agent at the target site, without breach of Bruch’s membrane. Robotic assistance could optimize accuracy and enhance safety in implementation of targeted gene therapy.Citation25,Citation26
SUMMARY
Robotics confers immense advantage over conventional minimally invasive surgical systems, to transcend human physiological limits.Citation27 Substantial economic investment for equipment in robotic surgical systems, which at present have not demonstrated superior outcomes, is a barrier to its widespread adoption in vitreo-retinal surgery.Citation28 Workspace intrusion by equipment and longer surgical duration are other areas that need refinement for implementation of robotic surgery in routine practice. However, robotics may benefit in specific tasks such as targeted gene therapy and drug delivery that require higher precision and accuracy. Preceyes surgical system and similar devices, which enable the surgeon to control equipment from a distant site, could revolutionize telesurgery. Haptic feedback, that would improve tactile sensation, could be incorporated into robotic systems to improve surgical accuracy.Citation29 A hybrid system that allows the surgeon to freely switch between fully automated surgery and manual control, would be ideal to adopt into surgical practice in the near future.Citation17 Given the promising trends and rapid advances made in the field over the past two decades, further research and investment are warranted to realize the full potential of robotics in retinal microsurgery.
DISCLOSURE STATEMENT
No potential conflict of interest was reported by the author(s).
Additional information
Funding
REFERENCES
- Machemer R, Buettner H, Norton EW, Parel JM. Vitrectomy: a pars plana approach. Trans - Am Acad Ophthalmol Otolaryngol Am Acad Ophthalmol Otolaryngol. Aug 1971;75(4):813–820.
- Singh SPN, Riviere CN. Physiological tremor amplitude during retinal microsurgery. In: Proceedings of the IEEE 28th Annual Northeast Bioengineering Conference (IEEE Cat No02CH37342) [Internet]. Philadelphia, PA, USA: IEEE; 2002 [cited 2021 Oct 9]. p. 171–172. Available from: https://ieeexplore.ieee.org/document/999520/
- Riviere CN, Tech Ang W, Khosla PK. Toward active tremor canceling in handheld microsurgical instruments. IEEE Trans Robot Autom. 2003 Oct;19(5):793–800. doi:10.1109/TRA.2003.817506.
- Jagtap AS, Riviere CN. Applied force during vitreoretinal microsurgery with handheld instruments. Conf Proc Annu Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Annu Conf September 1-5, San Francisco, California, USA. 2004;2004:2771–2773.
- Bergeles C, Yang G-Z. From passive tool holders to microsurgeons: safer, smaller, smarter surgical robots. IEEE Trans Biomed Eng. 2014 May;61(5):1565–1576. doi:10.1109/TBME.2013.2293815.
- Forslund Jacobsen M, Konge L, Alberti M, la Cour M, Park YS, Thomsen ASS. Robot-assisted vitreoretinal surgery improves surgical accuracy compared with manual surgery: a randomized trial in a simulated setting. RETINA. 2020 Nov;40(11):2091–2098. doi:10.1097/IAE.0000000000002720.
- Ahronovich EZ, Simaan N, Joos KM. A review of robotic and OCT-aided systems for vitreoretinal surgery. Adv Ther. 2021 May;38(5):2114–2129. doi:10.1007/s12325-021-01692-z.
- Gerber MJ, Pettenkofer M, Hubschman J-P. Advanced robotic surgical systems in ophthalmology. Eye Lond Engl. Sep 2020;34(9):1554–1562.
- MacLachlan RA, Becker BC, Tabares JC, Podnar GW, Lobes LA, Riviere CN. Micron: an actively stabilized handheld tool for microsurgery. IEEE Trans Robot. 2012 Feb;28(1):195–212. doi:10.1109/TRO.2011.2169634.
- He X, Roppenecker D, Gierlach D, et al. . . Houston, Texas, USA:American Society of Mechanical Engineers; 2012 International Mechanical Engineering Congress and Exposition Houston, Texas, USA:cited 2021 Oct 10. 145–153. . https://asmedigitalcollection.asme.org/IMECE/proceedings/IMECE2012/45189/145/260572
- Edwards TL, Xue K, Meenink HCM, et al. First-in-human study of the safety and viability of intraocular robotic surgery. Nat Biomed Eng. Jun 18 2018;2(9):649–656. doi:10.1038/s41551-018-0248-4.
- Nasseri MA, Eder M, Nair S, et al. The introduction of a new robot for assistance in ophthalmic surgery. Annu Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Annu Int Conf 3-7 July 2013 Osaka, Japan. 2013;2013:5682–5685.
- Wilson JT, Gerber MJ, Prince SW, et al. Intraocular robotic interventional surgical system (IRISS): mechanical design, evaluation, and master-slave manipulation. Int J Med Robot Comput Assist Surg MRCAS. 2018 Feb;14((1) 2–4).
- Kummer MP, Abbott JJ, Kratochvil BE, Borer R, Sengul A, Nelson BJ. OctoMag: an electromagnetic system for 5-DOF wireless micromanipulation. IEEE Trans Robot. 2010 Dec;26(6):1006–1017. doi:10.1109/TRO.2010.2073030.
- Cutler N, Balicki M, Finkelstein M, et al. Auditory force feedback substitution improves surgical precision during simulated ophthalmic surgery. Invest Ophthalmol Vis Sci. Feb 15 2013;54(2):1316–1324. doi:10.1167/iovs.12-11136.
- Mitchell B, Koo J, Iordachita I, et al. Development and application of a new steady-hand manipulator for retinal surgery. In: Proceedings 2007 IEEE International Conference on Robotics and Automation [Internet]. Roma: IEEE; 2007 [cited 2021 Oct 10]. p. 623–629. Available from: https://ieeexplore.ieee.org/document/4209160/
- de Smet MD, Naus GJL, Faridpooya K, Mura M. Robotic-assisted surgery in ophthalmology. Curr Opin Ophthalmol. 2018 May;29(3):248–253. doi:10.1097/ICU.0000000000000476.
- Maberley DAL, Beelen M, Smit J, et al. A comparison of robotic and manual surgery for internal limiting membrane peeling. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol. Apr 2020;258(4):773–778. doi:10.1007/s00417-020-04613-y.
- Zhou M, Yu Q, Huang K, et al. Towards robotic-assisted subretinal injection: a hybrid parallel–serial robot system design and preliminary evaluation. IEEE Trans Ind Electron. Aug 2020;67(8):6617–6628. doi:10.1109/TIE.2019.2937041.
- Ullrich F, Bergeles C, Pokki J, et al. Mobility experiments with microrobots for minimally invasive intraocular surgery. Invest Ophthalmol Vis Sci. Apr 23 2013;54(4):2853–2863. doi:10.1167/iovs.13-11825.
- Charreyron SL, Boehler Q, Danun AN, Mesot A, Becker M, Nelson BJ. A magnetically navigated microcannula for subretinal injections. IEEE Trans Biomed Eng. 2021 Jan;68(1):119–129. doi:10.1109/TBME.2020.2996013.
- Channa R, Iordachita I, Handa JT. Robotic vitreoretinal surgery. Retina Phila Pa. 2017 Jul;37(7):1220–1228. doi:10.1097/IAE.0000000000001398.
- de Smet MD, Meenink TCM, Janssens T, et al. Robotic assisted cannulation of occluded retinal veins. PLoS ONE. Sep 27 2016;11(9):e0162037. doi:10.1371/journal.pone.0162037.
- Gijbels A, Smits J, Schoevaerdts L, et al. In-human robot-assisted retinal vein cannulation, a world first. Ann Biomed Eng. Oct 2018;46(10):1676–1685. doi:10.1007/s10439-018-2053-3.
- de Smet MD. Robotic assistance and its impact on vitreoretinal surgery. Expert Rev Ophthalmol. 2020 May 3;15(3):127–128. doi:10.1080/17469899.2020.1764351.
- Fusco S, Ullrich F, Pokki J, et al. Microrobots: a new era in ocular drug delivery. Expert Opin Drug Deliv. Nov 2014;11(11):1815–1826. doi:10.1517/17425247.2014.938633.
- Verma Y. Can robotics be the future of ophthalmic surgery? J Robot Surg. 2021 Apr 30;15(6):975–976. doi:10.1007/s11701-021-01247-y.
- Barbash GI, Glied SA. New technology and health care costs–the case of robot-assisted surgery. N Engl J Med. 2010 Aug 19;363(8):701–704. doi:10.1056/NEJMp1006602.
- Francone A, Huang JM, Ma J, Tsao T-C, Rosen J, Hubschman J-P. The effect of haptic feedback on efficiency and safety during preretinal membrane peeling simulation. Transl Vis Sci Technol. 2019 Jul 3;8(4):2. doi:10.1167/tvst.8.4.2.