ABSTRACT
Background
Aqueous deficiency dry eye disease is a chronic and potentially sight-threatening condition, that occurs due to the dysfunction of the lacrimal glands. The aim of this review was to describe the various recent developments in the understanding, diagnosis and treatment of lacrimal gland insufficiency in aqueous deficiency dry eye disease.
Methods
A MEDLINE database search using PubMed was performed using the keywords: “dry eye disease/syndrome”, “aqueous deficient/deficiency dry eye disease”, “lacrimal gland” and “Sjogren’s syndrome”. After scanning through 750 relevant abstracts, 73 eligible articles published in the English language from 2016 to 2021 were included in the review.
Results
Histopathological and ultrastructural studies have revealed new insights into the pathogenesis of cicatrising conjunctivitis-induced aqueous deficiency, where the lacrimal gland acini remain uninvolved and retain their secretory property, while significant ultrastructural changes in the gland have been observed. Recent advances in diagnosis include the techniques of direct clinical assessment of the lacrimal gland morphology and secretion, tear film osmolarity, tear film lysozyme and lactoferrin levels, tear film interferometry and lacrimal gland confocal microscopy. Developments in the treatment of aqueous deficiency dry eye disease, apart from the nanoparticle-based tear substitutes, include secretagogues like diquafosol tetrasodium and rebamipide, anti-inflammatory topical agents like nanomicellar form of cyclosporine and lifitegrast, scleral contact lenses, neurostimulation, and acupuncture for increasing the amount of tear production, minor salivary gland transplantation, faecal microbial transplantation, lacrimal gland regeneration and mesenchymal stem cell therapy.
Conclusions
Significant advances in the understanding, diagnosis and management of lacrimal gland insufficiency and its role in aqueous deficiency dry eye disease have taken place within the second half of the last decade. Of which, translational breakthroughs in terms of newer drug formulations and regenerative medicine are most promising.
Introduction
Dry eye disease (DED) is a major cause of chronic ocular morbidity, affecting millions of individuals worldwide.1,Citation2 It is estimated that about 40% of the Indian urban population or close to 270 million people would be affected by DED in the next decade.Citation1 The second Tear Film and Ocular Surface Society dry eye workshop (TFOS DEWS II) has defined DED as “a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage and neurosensory abnormalities play an etiological role”.Citation2 Old age, female sex, medication use, dry environment or extended screen time on electronic devices are the known risk factors which lead to the development of DED.Citation1,Citation3 DED has been classified into evaporative dry eye (EDE), and aqueous-deficient dry eye (ADDE) disease, based on Meibomian or lacrimal gland involvement, respectively, although the DEWS II guidelines suggest that these variants may lie along the continuum of the disease.Citation2 Irrespective of the underlying cause, instability of the tear film leads to ocular surface inflammation which is responsible for the clinical features characteristic of the disease.Citation4 Among the two broad categories, ADDE due to lacrimal gland insufficiency accounts for about a third of all cases of DEDCitation1 but is the more serious and potentially blinding condition, since it can cause severe ocular surface inflammation and chronic keratopathy. This review summarizes the significant advances in the understanding, diagnosis and management of lacrimal gland insufficiency and its role in ADDE that have taken place within the second half of the last decade.
Methodology
A MEDILINE search using PubMed was performed with the keywords: “dry eye”, “dry eye disease”, “dry eye syndrome”, “aqueous deficient dry eye”, “aqueous deficiency dry eye”, “Sjögren’s syndrome” and “lacrimal gland” for articles published between 2016 and 2021. The search revealed a total of 20,945 articles. Articles not having abstracts, not in the English language, not relevant to the topic of interest and repetitive articles were excluded. A total of 750 abstracts were scanned, and 73 relevant articles were included in the review.
Etiology and pathogenesis of lacrimal gland insufficiency in ADDE
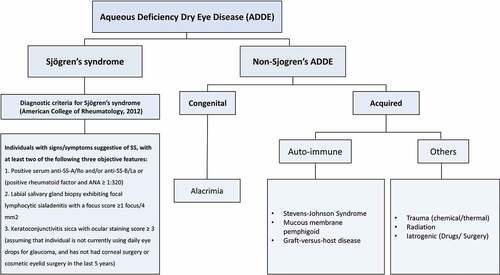
Aqueous deficiency dry eye disease can occur if the lacrimal gland is congenitally absent, developed incompletely or there is an acquired damage to the gland and its periductular tissue due to the ongoing inflammation. Depending on the underlying etiology, ADDE is classified into Sjögren’s and non-Sjögren’s type ().Citation2
Figure 1. Classification of aqueous deficiency dry eye disease into Sjögren’s and non-Sjögren’s dry eye disease. Non-Sjögren’s ADDE can further be classified into congenital form such as in cases of alacrimia and acquired form seen in various auto-immune diseases and other conditions such as chemical/thermal trauma, radiation and iatrogenic damage.
Sjögren’s Syndrome-Associated ADDE
Sjögren’s syndrome (SS) is a systemic autoimmune condition wherein lymphocytic infiltration of the exocrine glands including the lacrimal gland occurs, leading to persisting inflammation and symptoms of dry eye and dry mouth, besides various other systemic manifestations such as arthritis. For its diagnosis, the American-European Consensus Group 2012-revised classification criteria are the most commonly used ().Citation5–7 The pathogenesis of SS is multifactorial. Genetic susceptibility, female sex, viral infections, overactivation of B cells, role of Th1 and Th17 cells and increase in the pro-inflammatory cytokines such as matrix metalloproteinases (MMPs) are known to play a role in its pathogenesis.Citation8 Role of ocular and gut microbiome in the pathophysiology of SS has been recently reported. The gut microbiota is closely related to the immune system during development.Citation9 It maintains a balance between the immune responses and is said to play a crucial role of immune regulation. Any dysbiosis in the gut microbiome may lead to auto-immune diseases such as SS.Citation10–12
Non-Sjögren’s Type of ADDE
This can further be classified into congenital and acquired types (). Congenital alacrima is a rare condition of absence of tear production from birth. There is abnormal or deficient development of the lacrimal gland or its ductal tissues. It is associated with a multitude of systemic disorders and is an important and rare cause of paediatric dry eye disease.Citation13 Presentation can vary from congenital absence or hyposecretion of tears, developmental delay, to severe multi-systemic involvement.Citation14–16
Acquired causes of non-Sjögren’s-type ADDE include immune-mediated conditions such as Stevens–Johnson syndrome (SJS), mucous membrane pemphigoid (MMP) and graft versus host disease (GVHD).Citation3,Citation17,Citation18 Stevens-Johnson syndrome is a chronic mucocutaneous blistering disease, which is triggered by drugs or viral infections.Citation19–22 It is said to occur as a result of dysregulated immune response, mainly pertaining to innate immunity.Citation23,Citation24 It is a cytokine-mediated response, which leads to the deposition of neutrophils in the conjunctiva of these patients.Citation25 In both SJS and MMP, there is chronic inflammation of the ocular surface, which if not treated in time, leads to cicatrising conjunctivitis, ultimately causing ADDE. Graft versus host disease (GVHD) is an adverse immunological event following allogeneic hematopoietic stem cell transplantation. It includes a T cell-mediated immune response which causes infiltration and inflammation of ocular structures such as the lacrimal glands and conjunctiva, leading to ADDE.Citation26,Citation27 Other acquired causes of non-Sjögren’s-type ADDE include ocular chemical burns, iatrogenic causes such as kerato-refractive surgeries, long-term use of topical and systemic medications and radiation therapy for head and neck cancers. Some of these conditions, like radiation therapy, cause direct damage to the lacrimal gland and the ocular surface, while others, like topical anti-glaucoma medications, cause chronic inflammation and fibrosis of the ocular surface leading to cicatrising changes.
Normal anatomy of healthy lacrimal glands
Gross and Applied Anatomy
The main lacrimal gland is located in the supero-temporal orbit, behind the orbital rim, in the lacrimal gland fossa. It extends from the lateral edge of superior rectus muscle to the frontozygomatic suture and posteriorly upto the posterior aspect of the globe.Citation28,Citation29 The lacrimal gland is seen as an almond-shaped, concavo-convex bilobed structure, divided into two lobes by the lateral horn of levator aponeurosis.Citation29–32 The larger lobe being the orbital lobe constitutes about 60–70% of the gland, weighs about 600–700 g and is located behind the orbital septum.Citation28,Citation31–34 Hence, the orbital lobe cannot be clinically visualized. The smaller palpebral lobe, located beneath the levator aponeurosis, is relatively flat and can be examined clinically in the superior fornix by elevation or eversion of the lateral part of the upper eyelid. It appears as a pale, pinkish, lobulated structure.Citation28,Citation32,Citation35 Although lacrimal gland secretions are thought to mainly contribute to reflex tearing, recent reports suggest that the gland has an important role in basal tear secretion as well.Citation36–38
There are two types of accessory lacrimal glands, the glands of Wolfring (along the tarsal border) and Krause (in the substantia propria of fornices). Glands of Wolfring are more in number, present mainly in the upper eyelid, while the Gland of Krause are larger in size. These glands, like the main lacrimal gland, are serous in nature and of eccrine variety. Parasympathetic nerves and hormones stimulate the accessory lacrimal glands, which contribute to both basal and reflex secretions.Citation39
Microscopic Structure
Microscopically, the lacrimal gland is formed of acinar epithelial cells forming lobules draining into intralobular ducts. The ducts are lined by tubular columnar cell, surrounded by myoepithelial cells.Citation39,Citation40 The intralobular ducts further merge into interlobular ducts which are lined by cuboidal cells and open in the superior and inferior fornix as 3–12 excretory ductules.Citation39–42 Recently, the role of columnar cells of ducts in the secretion of tears have also been found; however, this still requires further studies for confirmation.Citation43 The intervening interstitial connective tissue contains lymphocytes, plasma cells, mucosa-associated lymphoid tissue, fibroblasts, blood vessels and nerve fibres.Citation29,Citation32,Citation39 Accessory lacrimal glands are similar to the main lacrimal gland microscopically.
Pathological and ultrastructural changes in lacrimal gland insufficiency
Sjögren’s-Associated ADDE
In SS, the lacrimal gland undergoes progressive fibrosis and hence gradually loses its secretory function. The histological examination of the lacrimal gland has revealed acinar dilation, interstitial fibrosis, with inflammatory aggregates and atrophy with fibrosis of the gland.Citation44
Cicatrising Conjunctivitis-Associated ADDE
In chronic cicatrising conjunctivitis such as SJS, MMP, and chemical burns, there is an ongoing sub-conjunctival fibrosis, leading to the destruction of the lacrimal ductules, both in the palpebral and orbital lobes, which on histological examination is seen as peri-ductular, interlobar and interlobular lymphocytic infiltration and fibrosis. The acini, particularly in the orbital lobe, may remain uninvolved and retain their secretory property.Citation44,Citation45 These changes are in stark contrast to the diffuse glandular atrophy and inflammatory aggregation that is seen in SS. However, despite the relative preservation of acinar structure on histopathology, significant ultrastructural changes have also been observed, showing disturbance in productivity and secretory activity of the glands in cicatrising disease like SJS.Citation46
Diagnostic modalities of lacrimal gland insufficiency in ADDE
The traditional standard basic tests for the diagnosis of DED include ocular surface disease index score (OSDI), Schirmer’s test, tear film height and tear film break up time (TBUT). Since this review focuses mainly on ADDE due to the lacrimal gland insufficiency, for the simplicity of discussion, the recent advances in diagnostic modalities have been classified into direct assessment of the lacrimal gland and indirect assessment of lacrimal gland insufficiency by tests based on tear film parameters.
Direct Clinical Assessment of Lacrimal Gland and Its Function
Morphometry
Clinically, on slit lamp bio-microscopy, one can assess the lacrimal gland by asking the patient to look inferonasally and lifting the upper eyelid to observe the contour, size and appearance of the palpebral lobe of the lacrimal gland. Recently a study by Singh et al. showed that in normal individuals and those with EDE, the palpebral lobe is bilaterally symmetrical, having a convex pinkish appearance. However, in cases of ADDE, the palpebral lobe size is bilaterally asymmetrical, appears flatter than normal and loses its convexity. Further, in SJS, the palpebral lobe appears smaller and there is a presence of symblepharon along with scarring.Citation35 Therefore, clinical assessment of the palpebral lobe morphology can not only help us in diagnosing ADDE but can also help us in differentiating between the underlying etiological causes of SS and SJS.
Tear Flow Rate or Tear Secretion
Direct assessment of tear secretion (DATS) from the excretory ducts on the surface of the palpebral lobe of lacrimal lobe was first described in 1986.Citation41 It was developed on the concept of the Seidel’s test, wherein a point of leak appears as a stained flow of fluid from the focal source. Similarly, simply touching the surface of the palpebral lobe with a fluorescein strip shows the appearance of the fluid at the duct openings and its rate of flow and washout.Citation45 Recent studies have shown its utility in diagnosing the subtype of ADDE. Singh et al. showed the difference in the number of ductular opening and flow rate between patients of cicatrising conjunctivitis, SS and normal healthy individuals. The patients of cicatrising conjunctivitis had reduced number of ductular opening and tear flow rate as compared to the patients of SS.Citation35 Also the latency for the initiation of the tear flow and the rate of flow can be assessed. The time lag is maximum in the cicatrising group followed by the SS group. The advantage of both lacrimal gland morphometry and DATS is that they can be performed in the clinic and can aid clinicians to diagnose the subtype of DED.
Indirect Assessment of Lacrimal Gland Function (Tear Film-Based Tests)
Tear Film Breakup (TBFU) Patterns
Due to the ongoing inflammation and subsequent injury to the epithelial cells and microvilli, ocular surface shows positive staining after the topical application of fluorescein. In 2017, Yokoi et al. described the characteristic tear film breakup patterns in the various subtypes of DED, thereby proving an aid for diagnosis. They described five main patterns. As per their report, an area break of ocular surface staining points towards severe grade of ADDE, a line and dimple break indicate mild-to-moderate grade of ADDE, while a spot break determines decreased wettability of the surface.Citation47 Based on this pattern, DED can be classified into three independent subtypes, ADDE, decreased wettability DE (DWDE), and increased evaporation. Hence, based on the pattern, it is possible to identify which component of the tear film should be supplemented. This novel concept of a layer-by-layer diagnosis and therapy is known as “tear film-oriented diagnosis” (TFOD) and “tear film-oriented therapy” (TFOT).Citation48
Non-Invasive Tear Film Breakup Time (NIBUT)
Instillation of fluorescein dye is considered invasive in nature since it is associated with chances of transmission of infection; hence, a newer method known as non-invasive tear film break up time (NITBUT) was introduced.Citation49 It is a placido disc-based assessment of the tear film, which determines the tear film stability based on the reflection of mires from the ocular surface. The reflection gets altered when the tear film stability is compromised.Citation50,Citation51 However, there appears to be significant difference in the values measured by TBUT and NITBUT. Average value of 3–4 NIBUT measurements provides a more accurate result, and hence the average value should be preferred over a single measurement.Citation52
Tear Meniscus Height
An objective way of assessing the tear film volume is by using the anterior segment optical coherence tomography (OCT)-based measurement of tear meniscus height (TMH). Time domain (TD-OCT) was the first OCT used for this purpose. Recent advances in spectral domain and swept source OCT have proven to be more useful, since it has the added benefit of helping in distinguishing the subgroup of DED.Citation53–56 The TMH is lowest in Sjogren’s syndrome, followed by non-Sjogrens ADDE.Citation57
Tear Strip Meniscometry
This device uses a water absorbing material similar to the Schirmer’s test. It has the added benefit that it is quick, providing results within 5 seconds, and is a non-invasive method to quantify the tear volume.Citation58,Citation59
Tear Osmolarity
In case of ADDE and EDE, due to the insufficient aqueous secretion and normal or excessive evaporation, the ocular surface remains in a chronic state of inflammation. This inflammation of ocular surface results in outpouring of inflammatory products and cytokines into the tear film. This results in increasing the tear film osmolarity. The TearLab Osmolarity System (TearLab Corporation, San Diego, California, USA) is currently the only FDA-approved commercially available in-office device for measuring tear film osmolarity.Citation60 It collects a sample of 50 nL of tear film and gives results within just a few seconds.Citation61 The normal tear osmolarity has a value of 302 mOsm/L with an inter-eye difference of 8 mOsm/L.Citation60,Citation62 It is worth noting that tear osmolarity is variable depending on the time of the day, or food intake, and hence must never be considered as a static value.Citation61,Citation63
MMP-9
Due to the evidence of increase in the pro-inflammatory cytokines such as MMP-9 in tear film of patients with DED, MMP-9 assessment has turned a useful aid for diagnosis. MMP-9 is an important endopeptidase released in the tear film during the extracellular matrix remodelling and facilitating leukocyte migration and chronic state of inflammation.Citation64–66 Currently, InflammaDry test (RPS, Inc., Sarasota, Florida, USA) is the only FDA-approved device for the detection of MMP-9 in patients. However, the test is non-specific for DED since the MMP-9 levels can be elevated even in other inflammatory ocular conditions such as keratitis.Citation67
Lactoferrin and Lysozyme
These are the normal constituents of the tear film, which are said to have a protective mechanism in the form of antibacterial, antitumor and immunomodulatory property. Lactoferrin levels in tear fluid are mainly reduced in auto-immune conditions such as SS.Citation68–70
Lacrimal Gland Imaging
CT scan, MRI scan and PET scan are some of the known modalities used for lacrimal gland imaging. Recently, the role of in vivo confocal microscopy of the lacrimal gland has been demonstrated in DED patients.Citation71 It can assess the acinar unit density, acinar unit diameter and inflammatory cell density in both the Meibomian and lacrimal glands.Citation72 This is a relatively unexplored area and there is enormous scope for further research in the field of lacrimal gland imaging.
Treatment of lacrimal gland insufficiency
Pharmacological Treatment
Lacrimal gland insufficiency manifests mainly as a reduction in the tear volume which further leads to increased tear hyperosmolarity, resulting in chronic ocular surface inflammation and disease sequelae. (). Hence, the pharmacological treatment of ADDE includes topical tear supplements, punctal plugs, secretagogues and anti-inflammatory agents such as corticosteroids, cyclosporine and tacrolimus. There is also an important role of controlling the underlying systemic disease, particularly in immune-mediated conditions like SS and GVHD.
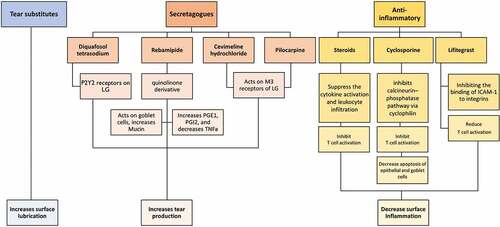
Figure 2. Classification, mechanism of action and the effect on ocular surface of various topical drugs used in the treatment of ADDE. LG, lacrimal gland; PG, prostaglandins; TNF, tumor necrosis factor; M3, muscarinic receptor and ICAM-1, intercellular adhesion molecule.
Tear Supplements
These have been the first line of management in patients with ADDE, regardless of the disease severity. They are known to improve the ocular surface lubrication, tear retention, tear osmolarity and TBUT and reduce the ocular surface staining. However, there are various types of artificial tears available which differ in its constituents.Citation73,Citation74 Preservatives such as benzalkonium chloride should be avoided as it causes stinging sensation and ocular surface toxicity with long-term use. The pH of the human tear is between 6.9 and 7.5. Any compound having a higher pH causes burning sensation upon instillation. These factors further worsen the pre-existing symptoms of a patient with DED. Current recommendations are that a preservative-free compound having a lower pH should be preferred. Due to the varied compositions, the drops need to be individualized in every patient.
Secretagogues
This is a new class of drugs, which mainly acts on the receptors of the lacrimal gland and helps in increasing the tear production. This class of drugs include diquafosol tetrasodium, rebamipide, cevimeline and oral pilocarpine. Diquafosol tetrasodium is available as a 3% ophthalmic solution. It acts on P2Y2 receptors, which are found on the conjunctiva, meibomian and lacrimal gland. It increases the intracellular calcium and thereby helps in increasing the tear secretion.Citation75–82 Rebamipide is a quinolinone derivative, which has a dual effect. It acts on goblet cells and increases the mucin production, thereby improving the tear film stability and epithelial regeneration. It also has an added benefit in increasing the levels of PGE2 and PGI2 and reducing the level of TNF-A, thereby preventing macrophage infiltration in the conjunctiva and reducing the amount of ocular surface inflammation and improving the tear film stability.Citation82–86 Cevimeline hydrochloride is a cholinergic agonist that binds to muscarinic acetylcholine receptors, especially to the M3 receptor, producing an increase in glandular secretions. Oral pilocarpine is also known to be having similar mechanism and helps in increasing both the salivary and aqueous tear production in patients of SS.Citation87–90
Anti-inflammatory Agents
The pathogenesis of DEDCitation90 and molecular interaction of various anti-inflammatory agents have been illustrated in . Topical corticosteroids help in reducing the ocular surface inflammation by interrupting the inflammatory cycle. They mainly suppress the cytokine activation and leukocyte infiltration, thereby controlling the ongoing inflammation. However, in view of the various side effects, their continued long-term use is not recommended. Topical cyclosporine acts by inhibiting the calcineurin–phosphatase pathway via cyclophilin. It is also said to increase the goblet cell density and increase tear production. It is said to inhibit T cell activation and decrease apoptosis of epithelial and goblet cells.Citation91 FDA has approved 0.05% cyclosporine emulsion (Restasis, Allergan Inc. Irvine, CA, USA), which has anti-inflammatory property and in addition is also known to increase the tear production.Citation92–94 It is said to give better results than the use of lubricants alone in the treatment of DED.Citation95
Figure 3. Mechanism of action of the anti-inflammatory agents such as steroids, cyclosporine, and Lifitegrast used in ADED. TNF-α, tumor necrosis factor alpha; IL-, interleukin; JNK, c-Jun N-terminal kinase; ERK, extracellular signal–related kinase; APC, antigen presenting cells; MMP, matrix metalloproteinase; ICAM-1, intercellular adhesion molecule 1; LFA-1, lymphocyte function-associated antigen 1; MHCII, major histocompatibility complex II; TCR, T cell receptor; IFN-γ, interferon gamma. Adapted from Periman et al.Citation90
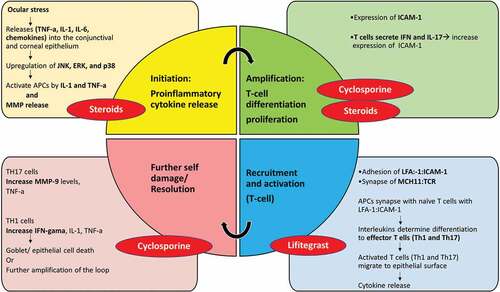
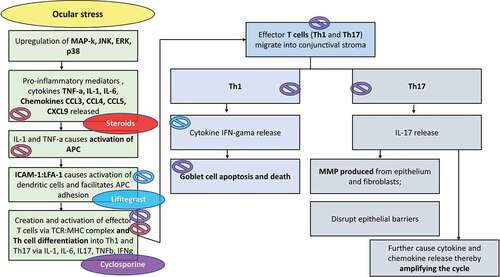
Figure 4. Site of action during the pathophysiology of ADED-induced inflammatory cascade and the molecular interaction of the various anti-inflammatory agents used in ADED. MAP-K, mitogen-activated protein kinase; JNK, c-Jun N-terminal kinase; ERK, extracellular signal–related kinase; TNF-α, tumor necrosis factor alpha; IL-, interleukin; CCL,CXCL- chemokines; ICAM-1, intercellular adhesion molecule 1; LFA-1, lymphocyte function-associated antigen 1; TGF-β, transforming growth factor beta; IFN-γ, interferon gamma and MMP, matrix metalloproteinase. Adapted from Periman et al.Citation90
Cequa (CEQUA, Sun Pharmaceutical Industries, Inc., Cranbury, NJ), a preservative-free nanomicellar form of cyclosporine, has also recently been approved by FDA. It has the advantage of better drug penetration and rapid onset of action.Citation96 Lifitegrast (XiidraTM, Novartis, USA) was approved in 2016 to treat DED.Citation97 It is a nanoparticle, which prevents the inflammatory cascade by inhibiting the binding of ICAM-1 to any integrins. It inhibits binding of leukocyte-associated antigen-1 (LFA-1) on T cells to its ligand intercellular adhesion molecule-1 (ICAM1) on antigen presenting, epithelial and vascular endothelial cells and prevents the formation of the immunological synapse that is required for full T cell activation. It is available for topical use as 5% ophthalmic solution.Citation98–102 Metformin, a first-line drug used in type 2 diabetes, has also emerged as an effective anti-inflammatory drug useful in patients of SS.Citation103 Multiple other treatment modalities to promote body’s natural anti-inflammatory mechanism are described which include oral supplementation with Omega-3 polyunsaturated fatty acids (PUFAs), curcumin and anti-oxidants, which suppress interleukins and the inflammatory cascade.Citation104,Citation105
Contact Lenses
Soft bandage contact lenses are known to improve the symptoms of patients with DED. Many recent studies have shown the role of scleral and PROSE (Prosthetic Replacement of Ocular Surface Ecosystem) lenses in terms of improving symptoms and visual acuity, particularly in cases with severe ADDE in patients with chronic ocular sequelae of SJS.Citation106,Citation107 These lenses are filled with fluid, which provide lubrication to the ocular surface. It improves the quality of vision as well as improved the symptoms of patients. However, the limitation is its availability, expense and need of training for its application and removal.Citation108–110
Neuronal Stimulation Therapy
Acupuncture is a form of Chinese traditional medical therapy, wherein needles are applied at specific points on the body for neuronal stimulation. It has shown to be effective in treating DED. However, further studies are needed to gauge its efficacy.Citation111–113 Role of anterior ethmoidal nerve stimulation being better than lacrimal nerve stimulation for increasing the aqueous production is also known.Citation114 Intranasal Tear Neurostimulator (TrueTear, Allergan plc) is a similar device designed to deliver microcurrents to the nasal cavity, stimulating the nasolacrimal pathway. It has recently received FDA approval to temporarily increase tear production. It has shown significant improvements in ocular dryness and discomfort compared along with a good safety profile and hence appears to be a promising new management strategy for these patients.Citation115 The iTEAR ®100 device is a similar device; however, unlike TrueTear, this stimulates the anterior ethmoidal nerves at the tip of the nose. Further clinical trials for its efficacy and safety are awaited.
Surgical Management
Minor Salivary Gland Transplantation (MSGT)
This surgical option has shown to be useful and effective in patients with certain types of severe ADDE. The surgery was first described in 2008.Citation116 The graft tissue is obtained from under the oral labial mucosa and transplanted onto the posterior eyelid lamella. It increases the tear secretions in patients of non-Sjögren’s type of ADDE.Citation117–119 Recently, a modified technique has also been described, wherein the graft is taken en-bloc, retaining the overlying mucosa and submucosal tissue to act as a barrier against post-operative sub-conjunctival fibrosis. In the modified technique the graft is placed over the superior bulbar surface of the eye.Citation119 Certain clinical tests have been described to help selecting the donor site and in assessing the functionality of the transplanted glands post-operatively.Citation120–122 The test is similar to the DATS test, wherein a fluorescein strip is applied onto the labial mucosa, and based on the number of excretory duct openings, the donor site can be selected.Citation120,Citation121,Citation123,Citation124 Similarly, the test can be used to objectively assess the number of functional openings and secretory rate in the transplanted graft on the ocular surface.Citation122 It is important to remember that MSGT is not recommended in Sjögren’s type of DED because the underlying disease process affects both the salivary and the lacrimal glands.
Mesenchymal Stem Cell Transplantation
Mesenchymal/stromal stem cells (MSCs) possess immunomodulatory properties and multilineage differentiation abilities. It acts by suppressing both local and systemic inflammation and inhibiting the activation and proliferation of T cells.Citation125 It has a potential role in treating patients with severe or refractory DED. The literature also shows its role in treating patients with steroid-resistant GVHD-induced DED.Citation126 Injection of MSCs derived from bone marrow or adipose tissue into the lacrimal gland has also shown efficacy.Citation127 Further results of randomized control trials from the same group is still awaited.
Lacrimal Gland Regeneration
Lacrimal gland regeneration using MSC, in addition to immunomodulation, is one of the main focuses of current research and is gaining popularity.Citation128–131 Further advances include the development of bio-engineered lacrimal glands, which can produce tears having the protein content similar to human tears.Citation132,Citation133 Researchers have also described the establishment of 3D organoids of the human lacrimal gland, which can engraft and produce mature tear products after orthotopic transplantation in mice.Citation134 Organoids can be expanded over multiple months and recapitulate morphological and transcriptional features of lacrimal ducts. These exciting and novel approaches are awaiting clinical translation.
Faecal Microbial Transplant
The role and interaction of ocular-gut-microbiome have been found to have a major role to play in the pathophysiology of ocular surface diseases, especially in auto-immune conditions such as SS. Hence, supplementation with probiotics or metabolites of commensal bacteria could have a major role in future treatment directives for dry eye diseases.Citation135,Citation136 Faecal microbial transplant showed efficacy in patients of immune-mediated DED; however, further trials would be needed to prove its efficacy and safety.Citation137
Summary
Aqueous deficiency dry eye disease, although the less common form of DED as compared to EDE, is the more serious clinical sub-type that causes chronic ocular morbidity and is potentially blinding. The underlying cause of ADDE is the dysfunction of the lacrimal glands, which is mostly acquired and due to auto-immune diseases that either directly target the gland, like in SS, or the periglandular tissue and the secretory ductules, like in SJS. Recent advancements have improved our understanding of the pathological processes affecting the lacrimal gland, particularly in cicatrising non-Sjögren’s type of ADDE. Developments in diagnostics include both simple clinical diagnostic techniques like direct assessment of lacrimal gland morphology and secretion, which can be performed in office settings without any specialized equipment and sophisticated, yet elegant and objective tear-film-based tests like osmolarity measurements. Introduction of newer therapeutic agents, such as secretagogues like diquafosol, anti-inflammatory agents like preservative free nanomicellar form of cyclosporine and lifitegrast, have expanded the treatment options and are likely to bring more relief to patients. Lastly, the role of the gut microbiome is being explored through faecal microbial transplantation in SS-associated ADDE, while the final frontier of lacrimal gland rejuvenation, repair or replacement is also being challenged through MSC therapy and bioengineered glands and organoids.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Donthineni PR, Kammari P, Shanbhag SS, Singh V, Das AV, Basu S. Incidence, demographics, types and risk factors of dry eye disease in India: electronic medical records driven big data analytics report I. Ocul Surf. 2019;17(2):250–256. doi:10.1016/j.jtos.2019.02.007.
- Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–283. doi:10.1016/j.jtos.2017.05.008.
- Craig JP, Nelson JD, Azar DT, et al. TFOS DEWS II report executive summary. Ocul Surf. 2017;15(4):802–812. doi:10.1016/j.jtos.2017.08.003.
- Hessen M, Akpek EK. Dry eye: an inflammatory ocular disease. J Ophthalmic Vis Res. 2014;9:240–250.
- Brito-Zerón P, Ramos-Casals M. Advances in the understanding and treatment of systemic complications in Sjögren’s syndrome. Curr Opin Rheumatol. 2014;26(5):520–527. doi:10.1097/BOR.0000000000000096.
- Wang J, Zhou L, Liu B. Update on disease pathogenesis, diagnosis, and management of primary Sjögren’s syndrome. Int J Rheum Dis. 2020;23(6):723–727. doi:10.1111/1756-185X.13839.
- Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European consensus group. Ann Rheum Dis. 2002;61(6):554–558. doi:10.1136/ard.61.6.554.
- Tian Y, Yang H, Liu N, Li Y, Chen J. Advances in pathogenesis of Sjögren’s syndrome. J Immunol Res. 2021;2021:5928232. doi:10.1155/2021/5928232.
- Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. 2016;375(24):2369–2379. doi:10.1056/NEJMra1600266.
- Zhong D, Wu C, Zeng X, Wang Q. The role of gut microbiota in the pathogenesis of rheumatic diseases. Clin Rheumatol. 2018;37(1):25–34. doi:10.1007/s10067-017-3821-4.
- de Paiva CS, Jones DB, Stern ME, et al. Altered mucosal microbiome diversity and disease severity in Sjögren syndrome. Sci Rep. 2016;6(1):23561. doi:10.1038/srep23561.
- Mandl T, Marsal J, Olsson P, Ohlsson B, Andréasson K. Severe intestinal dysbiosis is prevalent in primary Sjögren’s syndrome and is associated with systemic disease activity. Arthritis Res Ther. 2017;19(1):237. doi:10.1186/s13075-017-1446-2.
- Donthineni PR, Das AV, Basu S. Dry eye disease in children and adolescents in India. Ocul Surf. 2020 Oct;18(4):777–782. doi:10.1016/j.jtos.2020.07.019.
- Adams J, Schaaf CP. Diagnosis and genetics of alacrima. Clin Genet. 2018;94(1):54–60. doi:10.1111/cge.13173.
- Zhao Z, Allen RC. Congenital alacrima. Orbit. 2021;41(2):162–169. doi:10.1080/01676830.2021.1974057.
- Gupta N, Farooqui JH, Agni M, Kumar A, Sharma M, Mathur U. Alacrima, a rare cause of pediatric dry eye. J Aapos. 2018;22(3):233–235. doi:10.1016/j.jaapos.2017.11.003.
- Fraunfelder FT, Sciubba JJ, Mathers WD. The role of medications in causing dry eye. J Ophthalmol. 2012;2012:285851. doi:10.1155/2012/285851.
- Singh S. Congenital alacrimia with lacrimal gland hypoplasia. Orbit. 2021;1. doi:10.1080/01676830.2021.1942503.
- Bianchine JR, Macaraeg PV Jr., Lasagna L, et al. Drugs as etiologic factors in the Stevens-Johnson syndrome. Am J Med. 1968;44(3):390–405. doi:10.1016/0002-9343(68)90110-1.
- Patel TK, Barvaliya MJ, Sharma D, Tripathi C. A systematic review of the drug-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Indian population. Indian J Dermatol Venereol Leprol. 2013;79(3):389–398. doi:10.4103/0378-6323.110749.
- Mockenhaupt M, Viboud C, Dunant A, et al. Stevens- Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Invest Dermatol. 2008;128(1):35–44. doi:10.1038/sj.jid.5701033.
- McCormack JG. Mycoplasma pneumoniae and the erythema multiforme–Stevens Johnson syndrome. J Infect. 1981;3(1):32–36. doi:10.1016/S0163-4453(81)92236-2.
- Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: part I. Introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013;69(2):173.e1-13; quiz 85–6. doi:10.1016/j.jaad.2013.05.003.
- Williams GP, Tomlins PJ, Denniston AK, et al. Elevation of conjunctival epithelial CD45INTCD11b⁺CD16⁺CD14⁻ neutrophils in ocular Stevens- Johnson syndrome and toxic epidermal necrolysis. Invest Ophthalmol Vis Sci. 2013;54(7):4578–4585. doi:10.1167/iovs.13-11859.
- Pavlos R, Mallal S, Phillips E. HLA and pharmacogenetics of drug hypersensitivity. Pharmacogenomics. 2012;13(11):1285–1306. doi:10.2217/pgs.12.108.
- Carreno-Galeano JT, Dohlman TH, Kim S, Yin J, Dana R. A review of ocular graft-versus-host disease: pathophysiology, clinical presentation and management. Ocul Immunol Inflammation. 2021;29(6):1190–1199. doi:10.1080/09273948.2021.1939390.
- Tung CI. Current approaches to treatment of ocular graft-versus-host disease. Int Ophthalmol Clin. 2017;57(2):65–88. doi:10.1097/IIO.0000000000000167.
- Lorber M. Gross characteristics of normal human lacrimal glands. Ocul Surf. 2007;5(1):13–22. doi:10.1016/S1542-0124(12)70049-6.
- Eugene WR. Wolff’s Anatomy of the Eye and Orbit. 7 ed. Philadelphia PA: WB Saunders; 1976. 222.
- Mafee MF, Haik BG. Lacrimal gland and fossa lesions: role of computed tomography. Radiol Clin North Am. 1987;25:767–779.
- Forrester JVDA, McMenamin P, Lee WR. The Eye: Basic Sciences in Practice. 1. Philadelphia PA: WB Saunders; 1996.
- Snell RSLM. Clinical Anatomy of the Eye. 2. Malden MA: Blackwell Science; 1998.
- S T. Ocular Anatomy and physiology. Boston MA: Blackwell scientific; 1993.
- Forrester JVDA, McMenamin PG, Lee WR. The Eye. Basic Sciences in Practice. 2. Edinburgh: WB Saunders,; 2002.
- Singh S, Shanbhag SS, Basu S. Palpebral lobe of the human lacrimal gland: morphometric analysis in normal versus dry eyes. Br J Ophthalmol. 2021;105(10):1352–1357. doi:10.1136/bjophthalmol-2020-316929.
- Jones LT. The lacrimal secretory system and its treatment. Am J Ophthalmol. 1966;62(1):47–60. doi:10.1016/0002-9394(66)91676-X.
- Jordan A, Baum J. Basic tear flow Does it exist? Ophthalmol. 1980;87(9):920–930.doi:10.1016/S0161-6420(80)35143-9.
- Singh S, Basu S. Effect of topical anesthesia on the secretory activity of the main lacrimal Gland. Cornea. 2020;39(10):e24–e5. doi:10.1097/ICO.0000000000002410.
- Singh S, Basu S. The human lacrimal gland: historical perspectives, current understanding, and recent advances. Curr Eye Res. 2020;45(10):1188–1198. doi:10.1080/02713683.2020.1774065.
- Kim EC, Doh SH, Chung SY, et al. Direct visualization of aqueous tear secretion from lacrimal gland. Acta Ophthalmol. 2017;95(4):e314–e22. doi:10.1111/aos.13335.
- Bron AJ. Lacrimal streams: the demonstration of human lacrimal fluid secretion and the lacrimal ductules. Br J Ophthalmol. 1986;70(4):241–245. doi:10.1136/bjo.70.4.241.
- Obata H. Anatomy and histopathology of the human lacrimal gland. Cornea. 2006;25(10 Suppl 1):S82–9. doi:10.1097/01.ico.0000247220.18295.d3.
- Tóth-Molnár E, Ding C. New insight into lacrimal gland function: role of the duct epithelium in tear secretion. Ocul Surf. 2020;18(4):595–603. doi:10.1016/j.jtos.2020.07.002.
- Singh S, Narang P, Vashist U, Mittal V. Histomorphological changes in lachrymal glands of patients with chronic Stevens-Johnson Syndrome. Cornea. 2019;38(9):e39–e40. doi:10.1097/ICO.0000000000002037.
- Singh S, Mishra DK, Shanbhag S, et al. Lacrimal gland involvement in severe dry eyes after Stevens-Johnson Syndrome. Ophthalmology. 2021;128(4):621–624. doi:10.1016/j.ophtha.2020.08.016.
- Singh S, Basu S. Ultrastructural study of the lacrimal glands in severe dry eye disease following Stevens-Johnson syndrome. Ocul Surf. 2022;23:204–206. doi:10.1016/j.jtos.2021.10.005.
- Yokoi N, Georgiev GA, Kato H, et al. Classification of fluorescein breakup patterns: a Novel method of differential diagnosis for dry eye. Am J Ophthalmol. 2017;180:72–85. doi:10.1016/j.ajo.2017.05.022.
- Yokoi N, Georgiev GA. Tear film–oriented diagnosis and tear film–oriented therapy for dry eye based on tear film dynamics. Invest Ophthalmol Vis Sci. 2018;59(14):DES13–DES22. doi:10.1167/iovs.17-23700.
- Hong J, Sun X, Wei A, et al. Assessment of tear film stability in dry eye with a newly developed keratograph. Cornea. 2013;32(5):716–721. doi:10.1097/ICO.0b013e3182714425.
- Fuller DG, Potts K, Kim J. Noninvasive tear breakup times and ocular surface disease. Optom Vis Sci. 2013;90(10):1086–1091. doi:10.1097/OPX.0000000000000023.
- Lee R, Yeo S, Aung HT, Tong L. Agreement of noninvasive tear break-up time measurement between Tomey RT-7000 Auto refractor-keratometer and oculus keratograph 5M. Clin Ophthalmol. 2016;10:1785–1790. doi:10.2147/OPTH.S110180.
- Cho P, Douthwaite W. The relation between invasive and noninvasive tear break-up time. Optom Vis Sci. 1995;72(1):17–22. doi:10.1097/00006324-199501000-00004.
- Chan HH, Zhao Y, Tun TA, Tong L. Repeatability of tear meniscus evaluation using spectral- domain Cirrus® HD-OCT and time-domain Visante® OCT. Cont Lens Anterior Eye. 2015;38(5):368–372. doi:10.1016/j.clae.2015.04.002.
- Qiu X, Gong L, Sun X, Jin H. Age-related variations of human tear meniscus and diagnosis of dry eye with Fourier-domain anterior segment optical coherence tomography. Cornea. 2011;30(5):543–549. doi:10.1097/ICO.0b013e3181fb84ea.
- Fukuda R, Usui T, Miyai T, Yamagami S, Amano S. Tear meniscus evaluation by anterior segment swept-source optical coherence tomography. Am J Ophthalmol. 2013;155(4):620–4, 4.e1–2. doi:10.1016/j.ajo.2012.11.009.
- Akiyama R, Usui T, Yamagami S. Diagnosis of dry eye by tear meniscus measurements using anterior segment swept source optical coherence tomography. Cornea. 2015;34(Supplement 11):S115–20. doi:10.1097/ICO.0000000000000583.
- Qiu X, Gong L, Lu Y, Jin H, Robitaille M. The diagnostic significance of Fourier-domain optical coherence tomography in Sjögren syndrome, aqueous tear deficiency and lipid tear deficiency patients. Acta Ophthalmol. 2012;90(5):e359–66. doi:10.1111/j.1755-3768.2012.02413.x.
- Dogru M, Ishida K, Matsumoto Y, et al. Strip meniscometry: a new and simple method of tear meniscus evaluation. Invest Ophthalmol Vis Sci. 2006;47(5):901–1895. doi:10.1167/iovs.05-0802.
- Ibrahim OM, Dogru M, Ward SK, et al. The efficacy, sensitivity, and specificity of strip meniscometry in conjunction with tear function tests in the assessment of tear meniscus. Invest Ophthalmol Vis Sci. 2011;52(5):2194–2198. doi:10.1167/iovs.10-5986.
- Szalai E, Berta A, Szekanecz Z, Szûcs G, Módis L Jr. Evaluation of tear osmolarity in non- Sjögren and Sjögren syndrome dry eye patients with the TearLab system. Cornea. 2012;31(8):867–871. doi:10.1097/ICO.0b013e3182532047.
- Versura P, Profazio V, Campos EC. Performance of tear osmolarity compared to previous diagnostic tests for dry eye diseases. Curr Eye Res. 2010;35(7):553–564. doi:10.3109/02713683.2010.484557.
- Lemp MA, Bron AJ, Baudouin C, et al. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol. 2011;151(5):792–8.e1. doi:10.1016/j.ajo.2010.10.032.
- Thulasi P, Djalilian AR. Update in current diagnostics and therapeutics of dry eye disease. Ophthalmology. 2017;124(11s):S27–s33. doi:10.1016/j.ophtha.2017.07.022.
- Lanza NL, McClellan AL, Batawi H, et al. Dry Eye Profiles in patients with a positive elevated surface matrix metalloproteinase 9 point-of-care test versus negative patients. Ocul Surf. 2016;14(2):216–223. doi:10.1016/j.jtos.2015.12.007.
- Messmer EM, von Lindenfels V, Garbe A, Kampik A. Matrix Metalloproteinase 9 testing in dry eye disease using a commercially available point-of-care immunoassay. Ophthalmology. 2016;123(11):2300–2308. doi:10.1016/j.ophtha.2016.07.028.
- Sivak JM, Fini ME. MMPs in the eye: emerging roles for matrix metalloproteinases in ocular physiology. Prog Retin Eye Res. 2002;21(1):1–14. doi:10.1016/S1350-9462(01)00015-5.
- Pinto-Fraga J, Enríquez-de-Salamanca A, Calonge M, et al. Severity, therapeutic, and activity tear biomarkers in dry eye disease: an analysis from a phase III clinical trial. Ocul Surf. 2018;16(3):368–376. doi:10.1016/j.jtos.2018.05.001.
- Flanagan JL, Willcox MD. Role of lactoferrin in the tear film. Biochimie. 2009;91(1):35–43. doi:10.1016/j.biochi.2008.07.007.
- Danjo Y, Lee M, Horimoto K, Hamano T. Ocular surface damage and tear lactoferrin in dry eye syndrome. Acta Ophthalmol (Copenh). 1994;72(4):433–437. doi:10.1111/j.1755-3768.1994.tb02791.x.
- Sonobe H, Ogawa Y, Yamada K, et al. A novel and innovative paper-based analytical device for assessing tear lactoferrin of dry eye patients. Ocul Surf. 2019;17(1):160–166. doi:10.1016/j.jtos.2018.11.001.
- Kojima T, Dogru M, Kawashima M, Nakamura S, Tsubota K. Advances in the diagnosis and treatment of dry eye. Prog Retin Eye Res. 2020;78:100842. doi:10.1016/j.preteyeres.2020.100842.
- Matsumoto Y, Ibrahim OMA. Application of in vivo confocal microscopy in dry eye disease. Invest Ophthalmol Vis Sci. 2018;59(14):Des41–des7. doi:10.1167/iovs.17-23602.
- Lemp MA. Management of dry eye disease. Am J Manag Care. 2008;14:S88–101.
- Pucker AD, Ng SM, Nichols JJ. Over the counter (OTC) artificial tear drops for dry eye syndrome. Cochrane Database Syst Rev. 2016;2:Cd009729. doi:10.1002/14651858.CD009729.pub2.
- Kamiya K, Nakanishi M, Ishii R, et al. Clinical evaluation of the additive effect of diquafosol tetrasodium on sodium hyaluronate monotherapy in patients with dry eye syndrome: a prospective, randomized, multicenter study. Eye (Lond). 2012;26(10):1363–1368. doi:10.1038/eye.2012.166.
- Keating GM. Diquafosol ophthalmic solution 3 %: a review of its use in dry eye. Drugs. 2015;75(8):911–922. doi:10.1007/s40265-015-0409-7.
- Bremond-Gignac D, Gicquel JJ, Chiambaretta F. Pharmacokinetic evaluation of diquafosol tetrasodium for the treatment of Sjögren’s syndrome. Expert Opin Drug Metab Toxicol. 2014;10(6):905–913. doi:10.1517/17425255.2014.915026.
- Nakamura M, Imanaka T, Sakamoto A. Diquafosol ophthalmic solution for dry eye treatment. Adv Ther. 2012;29(7):579–589. doi:10.1007/s12325-012-0033-9.
- Takamura E, Tsubota K, Watanabe H, Ohashi Y. A randomised, double-masked comparison study of diquafosol versus sodium hyaluronate ophthalmic solutions in dry eye patients. Br J Ophthalmol. 2012;96(10):1310–1315. doi:10.1136/bjophthalmol-2011-301448.
- Koh S, Ikeda C, Takai Y, Watanabe H, Maeda N, Nishida K. Long-term results of treatment with diquafosol ophthalmic solution for aqueous-deficient dry eye. Jpn J Ophthalmol. 2013;57(5):440–446. doi:10.1007/s10384-013-0251-y.
- Carracedo G, Crooke A, Guzman-Aranguez A, de Lara Mj P, Martin-Gil A, Pintor J. The role of dinucleoside polyphosphates on the ocular surface and other eye structures. Prog Retin Eye Res. 2016;55:182–205. doi:10.1016/j.preteyeres.2016.07.001.
- Fujihara T, Murakami T, Fujita H, Nakamura M, Nakata K. Improvement of corneal barrier function by the P2Y(2) agonist INS365 in a rat dry eye model. Invest Ophthalmol Vis Sci. 2001;42:96–100.
- Kashima T, Itakura H, Akiyama H, Kishi S. Rebamipide ophthalmic suspension for the treatment of dry eye syndrome: a critical appraisal. Clin Ophthalmol (Auckland, NZ). 2014;8:1003–1010. doi:10.2147/OPTH.S40798.
- Kinoshita S, Awamura S, Oshiden K, Nakamichi N, Suzuki H, Yokoi N. Rebamipide (OPC- 12759) in the treatment of dry eye: a randomized, double-masked, multicenter, placebo-controlled phase II study. Ophthalmology. 2012;119(12):2471–2478. doi:10.1016/j.ophtha.2012.06.052.
- Tajima K, Hattori T, Takahashi H, et al. Rebamipide suppresses TNF-α production and macrophage infiltration in the conjunctiva. Vet Ophthalmol. 2018;21(4):347–352. doi:10.1111/vop.12510.
- Itoh S, Itoh K, Shinohara H. Regulation of human corneal epithelial mucins by rebamipide. Curr Eye Res. 2014;39(2):133–141. doi:10.3109/02713683.2013.834939.
- Fox RI. Use of cevimeline, a muscarinic M1 and M3 agonist, in the treatment of Sjögren’s syndrome. Adv Exp Med Biol. 2002;506:1107–1116.
- Ono M, Takamura E, Shinozaki K, et al. Therapeutic effect of cevimeline on dry eye in patients with Sjögren’s syndrome: a randomized, double-blind clinical study. Am J Ophthalmol. 2004;138(1):6–17. doi:10.1016/j.ajo.2004.02.010.
- Petrone D, Condemi JJ, Fife R, Gluck O, Cohen S, Dalgin P. A double-blind, randomized, placebo-controlled study of cevimeline in Sjögren’s syndrome patients with xerostomia and keratoconjunctivitis sicca. Arthritis Rheumatism. 2002;46(3):748–754. doi:10.1002/art.510.
- Vivino FB, Al-Hashimi I, Khan Z, et al. Pilocarpine tablets for the treatment of dry mouth and dry eye symptoms in patients with Sjögren syndrome: a randomized, placebo-controlled, fixed-dose, multicenter trial. P92-01 study group. Arch int med. 1999;159(2):174–181. doi:10.1001/archinte.159.2.174.
- Periman LM, Perez VL, Saban DR, Lin MC, Neri P. The immunological basis of dry eye disease and current topical treatment options. J Ocul Pharmacol Ther. 2020;36(3):137–146. doi:10.1089/jop.2019.0060.
- Leonardi A, Van Setten G, Amrane M, et al. Efficacy and safety of 0.1% cyclosporine A cationic emulsion in the treatment of severe dry eye disease: a multicenter randomized trial. Eur J Ophthalmol. 2016;26(4):287–296. doi:10.5301/ejo.5000779.
- Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000;107(4):631–639. doi:10.1016/S0161-6420(99)00176-1.
- Stevenson D, Tauber J, Reis BL. Efficacy and safety of cyclosporin A ophthalmic emulsion in the treatment of moderate-to-severe dry eye disease: a dose-ranging, randomized trial. The cyclosporin A Phase 2 study group. Ophthalmology. 2000;107(5):967–974. doi:10.1016/S0161-6420(00)00035-X.
- Tuan HI, Chi SC, Kang YN. An updated systematic review with meta-analysis of randomized trials on topical cyclosporin A for dry-eye disease. Drug Des Devel Ther. 2020;14:265–274. doi:10.2147/DDDT.S207743.
- Mandal A, Gote V, Pal D, Ogundele A, Mitra AK. Ocular pharmacokinetics of a topical ophthalmic nanomicellar solution of cyclosporine (Cequa®) for dry eye disease. Pharm Res. 2019;36(2):36. doi:10.1007/s11095-018-2556-5.
- Abidi A, Shukla P, Ahmad A. Lifitegrast: a novel drug for treatment of dry eye disease. J Pharmacol Pharmacother. 2016;7(4):194–198.doi:10.4103/0976-500X.195920.
- Hsueh PY, Ju Y, Vega A, Edman MC, MacKay JA, Hamm-Alvarez SF. A multivalent ICAM-1 binding nanoparticle which inhibits ICAM-1 and LFA-1 interaction represents a new tool for the investigation of autoimmune-mediated dry eye. Int J Mol Sci. 2020;21(8):2758. doi:10.3390/ijms21082758.
- Stem ME, Gao J, Morgan GA, et al. The role of ICAM-1 as a signal protein for predisposition of ocular surface inflammation. Adv Exp Med Biol. 2002;506(Pt B):753–759.
- Semba CP, Gadek TR. Development of lifitegrast: a novel T-cell inhibitor for the treatment of dry eye disease. Clin Ophthalmol (Auckland, NZ). 2016;10:1083–1094. doi:10.2147/OPTH.S110557.
- Donnenfeld ED, Karpecki PM, Majmudar PA, et al. Safety of lifitegrast ophthalmic solution 5.0% in patients with dry eye disease: a 1-Year, multicenter, randomized, placebo-controlled study. Cornea. 2016;35(6):741–748. doi:10.1097/ICO.0000000000000803.
- Sheppard JD, Torkildsen GL, Lonsdale JD, et al. Lifitegrast ophthalmic solution 5.0% for treatment of dry eye disease: results of the OPUS-1 phase 3 study. Ophthalmology. 2014;121(2):475–483. doi:10.1016/j.ophtha.2013.09.015.
- Kim J, Kim YS, Park SH. Metformin as a Treatment Strategy for Sjögren’s syndrome. Int J Mol Sci. 2021;22:(13).
- Sheppard JD Jr., Singh R, McClellan AJ, et al. Long-term supplementation with n-6 and n-3 PUFAs improves moderate-to-severe keratoconjunctivitis sicca: a randomized double-blind clinical trial. Cornea. 2013;32(10):1297–1304. doi:10.1097/+ICO.0b013e318299549c.
- Rogers NM, Kireta S, Coates PT. Curcumin induces maturation-arrested dendritic cells that expand regulatory T cells in vitro and in vivo. Clin Exp Immunol. 2010;162(3):460–473. doi:10.1111/j.1365-2249.2010.04232.x.
- Basu S, Shanbhag SS, Gokani A, Kedar R, Bahuguna C, Sangwan VS. Chronic ocular sequelae of Stevens-Johnson syndrome in children: long-term impact of appropriate therapy on natural history of disease. Am J Ophthalmol. May 2018;189:17–28. doi:10.1016/j.ajo.2018.01.028.
- Shanbhag SS, Shah S, Singh M, Bahuguna C, Donthineni PR, Basu S. Lid-related keratopathy in Stevens-Johnson syndrome: natural course and impact of therapeutic interventions in children and adults. Am J Ophthalmol. Nov 2020;219:357–365. doi:10.1016/j.ajo.2020.07.006.
- Alipour F, Kheirkhah A, Jabarvand Behrouz M. Use of mini scleral contact lenses in moderate to severe dry eye. Cont Lens Anterior Eye. 2012;35(6):272–276. doi:10.1016/j.clae.2012.07.006.
- La Porta Weber S, Becco de Souza R, Gomes J Á P, Hofling-Lima AL. The use of the esclera scleral contact lens in the treatment of moderate to severe dry eye disease. Am J Ophthalmol. 2016;163:167–73.e1. doi:10.1016/j.ajo.2015.11.034.
- Bavinger JC, DeLoss K, Mian SI. Scleral lens use in dry eye syndrome. Curr Opin Ophthalmol. 2015;26(4):319–324. doi:10.1097/ICU.0000000000000171.
- Na JH, Jung JH, Park JG, Song PH, Song CH. Therapeutic effects of acupuncture in typical dry eye: a systematic review and meta-analysis. Acta Ophthalmol. 2021;99(5):489–498. doi:10.1111/aos.14651.
- Kim BH, Kim MH, Kang SH, Nam HJ. Optimizing acupuncture treatment for dry eye syndrome: a systematic review. BMC Complement Altern Med. 2018;18(1):145. doi:10.1186/s12906-018-2202-0.
- Yang L, Yang Z, Yu H, Song H. Acupuncture therapy is more effective than artificial tears for dry eye syndrome: evidence based on a meta-analysis. Evidence-based complementary and alternative medicine: eCAM. 2015;2015:143858.
- Kossler AL, Brinton M, Patel ZM, Dalal R, Ta CN, Palanker D. Chronic electrical stimulation for tear secretion: lacrimal vs. anterior ethmoid nerve. Ocul Surf. 2019;17(4):822–827. doi:10.1016/j.jtos.2019.08.012.
- Senchyna M, Ousler GW, Jerkins G, et al. Effect of TrueTear™ on dry eye symptoms during exposure to a controlled adverse environment. Invest Ophthalmol Vis Sci. 2018;59(9):918.
- Geerling G, Raus P, Murube J. Minor salivary gland transplantation. Dev Ophthalmol. 2008;41:243–254.
- Su JZ, Wang Z, Liu XJ, Lv L, Yu GY. Use of saliva flow rate measurement in minor salivary glands autotransplantation for treatment of severe dry eye disease. Br J Ophthalmol. 2021;2020–317552. doi:10.1136/bjophthalmol-2020-317552.
- Qin Y, Zhang Y, Liang Q, et al. Labial salivary gland transplantation for severe dry eye in a rhesus monkey model. Invest Ophthalmol Vis Sci. 2018;59(6):2478–2486. doi:10.1167/iovs.18-23966.
- Wakamatsu TH, Sant’Anna A, Cristovam PC, Alves VAF, Wakamatsu A, Gomes JAP. Minor salivary gland transplantation for severe dry eyes. Cornea. 2017;36(Suppl 1):S26–s33. doi:10.1097/ICO.0000000000001358.
- Singh S, Basu S. A novel diagnostic technique of measuring labial minor salivary gland secretions using sodium fluorescein dye: implications for patients with dry eyes. Semin Ophthalmol. 2022 Jan 2;37(1):111–116. doi:10.1080/08820538.2021.1926518.
- Singh S, Basu S. Preoperative labial mucosa evaluation as a deciding tool for minor salivary gland transplantation. Ophthalmic Plast Reconstr Surg. 2021;37(3s):S121–s2. doi:10.1097/IOP.0000000000001842.
- Singh S, Basu S. Functional assessment of transplanted minor salivary glands. Cornea. 2020;39(8):e21–e2. doi:10.1097/ICO.0000000000002359.
- Marinho DR, Burmann TG, Kwitko S. Labial salivary gland transplantation for severe dry eye due to chemical burns and Stevens-Johnson syndrome. Ophthalmic Plast Reconstr Surg. 2010;26(3):182–184. doi:10.1097/IOP.0b013e3181b8c3ad.
- Vazirani J, Bhalekar S, Amescua G, Singh S, Basu S. Minor salivary gland transplantation for severe dry eye disease due to cicatrising conjunctivitis: multicentre long-term outcomes of a modified technique. Br J Ophthalmol. 2020;105(11):1485–1490. doi:10.1136/bjophthalmol-2020-316611.
- Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110(10):3499–3506. doi:10.1182/blood-2007-02-069716.
- Weng J, He C, Lai P, et al. Mesenchymal stromal cells treatment attenuates dry eye in patients with chronic graft-versus-host disease. Mol Ther: J Am Soci Gene Ther. 2012;20(12):2347–2354. doi:10.1038/mt.2012.208.
- Møller-Hansen M, Larsen AC, Toft PB, et al. Safety and feasibility of mesenchymal stem cell therapy in patients with aqueous deficient dry eye disease. Ocul Surf. 2021;19:43–52. doi:10.1016/j.jtos.2020.11.013.
- Dietrich J, Schrader S. Towards lacrimal gland regeneration: current concepts and experimental approaches. Curr Eye Res. 2020;45(3):230–240. doi:10.1080/02713683.2019.1637438.
- Kawakita T. Regeneration of lacrimal gland function to maintain the health of the ocular surface. Invest Ophthalmol Vis Sci. 2018;59(14):Des169–des73. doi:10.1167/iovs.17-23576.
- Yao Y, Zhang Y. The lacrimal gland: development, wound repair and regeneration. Biotechnol Lett. 2017;39(7):939–949. doi:10.1007/s10529-017-2326-1.
- Liu CY, Hirayama M, Ali M, Shah D, Aakalu VK. Strategies for Regenerating the Lacrimal Gland. Current Ophthalmol Rep. 2017;5(3):193–198.doi:10.1007/s40135-017-0142-3.
- Hirayama M, Tsubota K, Tsuji T. Bioengineered Lacrimal Gland Organ Regeneration in Vivo. J Funct Biomater. 2015;6(3):634–649. doi:10.3390/jfb6030634.
- Hirayama M, Tsubota K, Tsuji T. Generation of a bioengineered lacrimal gland by using the organ germ method. Methods Mol Biol. 2017;1597:153–165.
- Bannier-Hélaouët M, Post Y, Korving J, et al. Exploring the human lacrimal gland using organoids and single-cell sequencing. Cell Stem Cell. 2021 Jul 1;28(7):1221–1232.e7. doi:10.1016/j.stem.2021.02.024.
- Pflugfelder SC, de Paiva CS. The pathophysiology of dry eye disease: what we know and future directions for research. Ophthalmology. 2017;124(11s):S4–s13. doi:10.1016/j.ophtha.2017.07.010.
- Trujillo-Vargas CM, Schaefer L, Alam J, Pflugfelder SC, Britton RA, de Paiva CS. The gut-eye- lacrimal gland-microbiome axis in Sjögren Syndrome. Ocul Surf. 2020;18(2):335–344. doi:10.1016/j.jtos.2019.10.006.
- Watane A, Cavuoto KM, Rojas M, et al. Fecal microbial transplant in individuals with immune-mediated dry eye. Am J Ophthalmol. 2022;233:90–100. doi:10.1016/j.ajo.2021.06.022.
