ABSTRACT
Purpose
Congenital hereditary endothelial dystrophy (CHED) is a rare, autosomal recessive, monogenic corneal condition with variable expressivity. Often presents in bilateral symmetrical progressive corneal cloudiness that starts in the early infancy. It is characterized by increased corneal thickness, profound corneal edema, and thickening of the Descemet membrane due to endothelial dysfunction. The published literature lacks uniform guidelines for grading corneal cloudiness and management algorithm for CHED cases. This article focuses on applying newer investigational modalities to fine-tune surgical outcomes and more recent CHED management strategies.
Methods
This comprehensive literature review was performed based on a search on the PubMed database of relevant CHED articles focusing on those published in the last 7 years. A total of around 70 articles were reviewed, and 17 of them were included in this review. These include systemic reviews, randomized controlled clinical trials, cohort studies, case-controlled studies, and case series.
Results
Corneal cloudiness grading in CHED using subjective and objective methods using Anterior Segment Optical Coherence Tomography (AS-OCT) and densitometry using Scheimpflug imaging can help select appropriate management plan for CHED cases. DSAEK outscores penetrating keratoplasty with much fewer complications and expedites visual recovery, which helps mitigate amblyopia.
Conclusion
Managing cases of CHED has been a challenge due to the dilemma in timing and appropriate surgical method selection and lack of definitive medical or other conservative approaches. Currently, DSAEK has shown favorable results in cases of CHED. However, appropriate staging of CHED and selecting the appropriate management approach appears to play a critical role in managing such cases. Besides these, novel treatment modalities such as nonsteroidal anti-inflammatory agents (NSAIDS) that target restoring water-flux activity in subtype of CHED and gene editing using CRISPR-Cas9 are promising paradigm treatment modalities.
Introduction
Autosomal recessive congenital hereditary endothelial dystrophy (CHED)Citation1 is a rare corneal endothelial dystrophy that is more common in seen in the Asian and Middle Eastern countries due to higher rates of parental consanguinity. Premature corneal endothelial senescence is critical in declining visual functions due to early-onset cloudy cornea in childhood endothelial dystrophies. At the same time, late-onset Fuch’s endothelial corneal dystrophy (FECD) is more prevalent worldwide with varying ethnicity and geographic distribution. CHED is characterized by bilateral, diffuse, non-inflammatory, and ground glass cloudy corneal consequences. Mutations of SLC4A11,Citation2 an abundant corneal solute transporter, have been attributed to the causation of CHED, additional post-lingual sensorineural deafness in Harboyan syndrome,Citation3 and the early-onset variant of FECD.
The clinical spectrum in CHED can be variable even within a family. The disease is often bilaterally symmetrical but can vary in terms of age at onset of corneal clouding, the density of corneal clouding, the severity of stromal architectural changes, and presence of secondary changes like degenerative pannus, spheroidal degeneration or band-shaped keratopathy.
Several childhood corneal disorders manifest with early-onset diffuse corneal cloudiness, and few have sight-threatening (primary congenital glaucoma) or life-threatening (mucopolysaccharidosis) consequences as well. Therefore, it is imperative to unravel underlying conditions accurately, predict the natural course, and pre-empt ocular and systemic associations. Such a systematic approach would help provide timely genetic counseling, and expedite appropriate medical or surgical intervention. Current published literature lacks uniform guidelines regarding the aid of investigational modalities in grading the corneal cloudiness and a management algorithm including both surgical and medical management of a case of CHED.
This review undertakes, a comprehensive evaluation combined with investigational modalities to ascertain the effect of factors like the age of onset, the density of opacity, secondary stromal changes, and try and unravel a management algorithm for effectively managing childhood corneal clouding especially in CHED.
Method of literature search
PubMed search was performed using search terms: Congenital hereditary endothelial dystrophy, investigations, and management of CHED. All articles published in the English language and relevant to the topic published from 2015 to 2021 were reviewed and included in this review.
Investigations
Investigation modalities have been aiding in diagnosing conditions, knowing the extent of involvement, appropriate staging of the disease, and possible prognosis. Some of the modalities that help in CHED have been discussed below.
Pachymetry
CHED cases generally show a corneal thickness reading of two to three times more than age-related normal corneas. Grading of the corneal cloudiness has been very subjective, and there have not been many methods to quantify the amount of corneal opacity accurately. Some of the strategies to aid in assessing the grade of the cloudiness are as follows:
Slit-lamp Evaluation
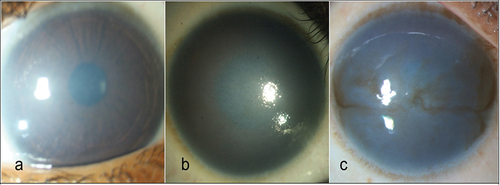
One subjective method of grading corneal clouding is using slit-lamp biomicroscopy or slit-lamp photographs. A study by Ramappa et al.Citation4 graded corneal clouding in CHED based on visibility of iris structures and presence or absence of anterior stromal scaring. as mild-minimal corneal cloudiness not obscuring the iris details (), moderate-obscured iris details, but visible pupillary silhouette () and severe-iris and pupillary details obscured (). The above grading was exclusively used in CHED cases.
Figure 1. Grading of corneal haze. (a) Mild disease with minimal corneal cloudiness not obscuring the iris details. (b) Moderate corneal haze obscures the iris details, but the pupillary silhouette could be seen. (c) Severe disease wherein the iris, and pupillary details both are obscured (Ramappa et al.Citation4).
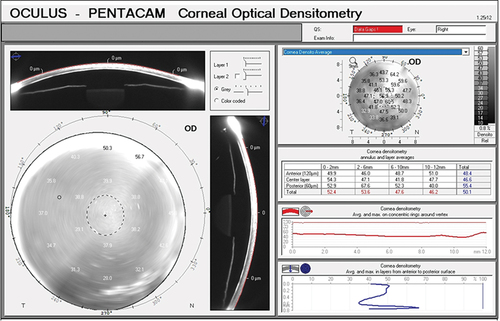
Densitometry Using Scheimpflug Imaging
Corneal densitometry measures the reflectance pattern using a Scheimpflug imaging device, i.e., quantum of light being reflected from individual corneal layers. The densitometry readings are standardized from a scale of 0 to 100 grayscale units (GSU). Value of 0 denotes minimum light scatter, i.e., maximum transparency and 100 denotes maximum light scatters, i.e., minimum transparency.Citation5,Citation6 Based on these readings, one can get a quantitative estimation of the amount of corneal opacification that the patient is having at individual layers ().
AS-OCT (Anterior Segment Optical Coherence Tomography)
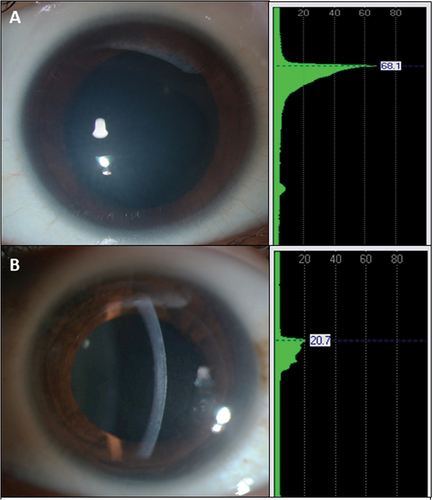
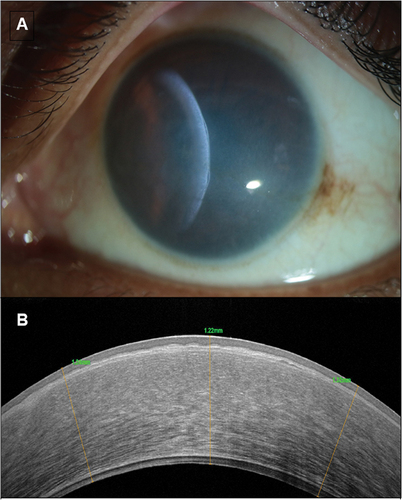
AS-OCT in CHED shows a generalized increase in corneal thickness (). There is thickened epithelial layer with underlying irregular Bowman’s membrane, increased stromal thickness, and abnormally thickened and hyporeflective Descemet’s membrane. Light backscattering can be studied using AS-OCT for objective estimation of corneal transparency.Citation7 Long-standing CHED cases on OCT can also show the presence of secondary corneal changes like Descemet’s level scaring ().
Figure 4. Shows the slit-lamp biomicroscopy photographs of- congenital hereditary endothelial dystrophy, with the ground-glass appearance of the cornea with the slit beam suggestive of increased corneal thickness (a). Anterior segment OCT shows CHED with a thickened epithelial layer with underlying irregular bowman’s membrane, increased stromal thickness and abnormally thickened Descemet membrane (b).
Management
Managing corneal opacification in CHED has been a challenge due to the lack of a conservative approach. Currently, the only definitive treatment modality available is various forms of corneal transplantation, which have several limitations considering the longevity of these children. The options for providing a clear optical axis in these children include conventional full-thickness penetrating keratoplasty (PK),Citation8 rotational auto-keratoplasty (RAK),Citation9 deep anterior lamellar keratoplasty (DALK),Citation10 Descemet’s stripping automated endothelial keratoplasty (DSAEK),Citation11–13 and Descemet membrane endothelial keratoplasty (DMEK).Citation14–16
Penetrating keratoplasty
Penetrating keratoplasty (PK) was the standard of treatment for children with CHED. Still, the decision to proceed with PK is complex, owing to the challenges of pediatric PK and the threat of amblyopia. Paediatric PK presents unique preoperative, intraoperative, and postoperative issues that need to be addressed. Preoperatively, the timing and risk–benefit ratio of surgery must be considered. Assessment of the degree of visual impairment is challenging in the preverbal child, and some children with mild CHED may have reasonably good vision even without undergoing a surgical intervention.
The correct time for surgical intervention is still unclear. While the surgical timing lacks uniform consensus, there is a trend toward early intervention to circumvent irreversible stromal changes, particularly when it comes to endothelial grafting. Pediatric PK poses several unique challenges that are not encountered in adults. Intraoperative challengesCitation17 in PK for CHED in younger age groups include managing the low scleral rigidity and tendency for anterior rotation of the lens-iris diaphragm that predispose to shallowing of the anterior chamber, peripheral anterior synechiae (PAS) formation, iris incarceration in the wound, endothelial trauma, glaucoma, and expulsion of intraocular contents due to an “open to sky’ procedure. Also, there is a high demand for postoperative care. Pediatric patients tend to a rapid and severe inflammatory response, predisposing them to graft rejection, PAS formation, glaucoma, and cataract. Robust wound healing may result in corneal neovascularization, uneven contraction of tissue, early loosening of sutures, irregular astigmatism, overriding of graft tissue with poor epithelialization, and ulceration. Frequent, often monthly, examinations under anesthesia and vigilant parental or caregiver observation for signs of rejection are important for early detection of graft rejection and suture-related complications. Along with these, a corneal wound that is of full-thickness is at a higher risk of traumatic rupture especially in children. Considering these, corneal surgeries have often been delayed for as long as possible, if not avoided in this age group.
A recent retrospective study comparing PK data done between 1978 and 2013 at the Royal Victoria Eye and Ear Hospital (RVEEH) reported a poor outcome in this Irish cohort with a mean graft survival rate of 36% after a follow-up of 101 months.Citation18 However, earlier studies have shown better graft survival rates in pediatric PK cases in CHED. Despite the disadvantages mentioned above, PKP provides a pristine clear corneal grafting and can be the treatment of choice in patients with long-standing corneal edema that have developed significant stromal scarring.
Descemet-stripping automated endothelial keratoplasty (dsaek)
Descemet-stripping automated endothelial keratoplasty (DSAEK) is currently one of the treatments of choice in adults with a various corneal endothelial disease. Endothelial transplantation procedures such as DSAEK have a notable edge over conventional penetrating keratoplasty, in having a faster visual rehabilitation period, less sutures-and-suture-related complications, and a preserved structural integrity of the globe. These benefits are key in planning surgery in pediatric age group. DSAEK, being a “closed-system” technique, precludes to a great extent, sight-threatening intraoperative complications, particularly suprachoroidal hemorrhage, which not only become infrequent but also more easily manageable. The structural integrity of the globe is well maintained to its anatomical state and so, postoperative wound dehiscence is infrequent.
A recent study (Ramappa et alCitation4) evaluated the long-term outcomes of DSAEK in corneal endothelial disorders in children less than 14 years of age. Out of 167 eyes that were operated, 86.2% maintained a clear graft at a median follow-up of 2.5 years. The paediatric DSAEK survival probability at 1, 3, 5, and 7 years was 92%, 87%, 78%, and 78%, superior to the results reported for pediatric Penetrating Keratoplasty (PK). In this series, CHED was the most common indication for pediatric DSAEK (116 eyes that is 69.5%), which had a long-term graft survival probability of 97%, 95%, 90%, and 90% at 1, 3, 5, and 7 years, respectively. This study also suggested that CHED with no previous interventions influenced long-term graft survival in a positive way. Visual acuity was noted to improve up to 3 years in CHED. In cases of previously failed grafts, also DSAEK gave excellent visual outcomes and could be a superior alternative to performing a repeat PK in a pediatric population. This study also highlighted that while DSAEK effectively accomplished excellent graft survival with a quick visual recovery, it was limited in attaining the pristine graft clarity in specific subset of cases like congenital hereditary endothelial dystrophy (CHED), unlike a PK due to presence of interface irregularity.Citation11,Citation12,Citation15,Citation19
Another technical challenge in endothelial keratoplasty is the difficulty of peeling off the Descemet membrane due to impaired visibility, thickened Descemet endothelial complex, more robust stromal adhesions, and difficulty getting the right separation plane stripping Descemet membrane in a single piece. Also, in knowing orientation and attachment of lenticule intraoperatively. Therefore, EK in children merits several modifications to overcome technical difficulties not encountered in adult cases.
The double ring sign has been used to estimate the DSAEK graft orientation, intraoperative OCT also can aid in visualizing the real-time orientation of such graft as is published with “acute angled bevel sign” in these cases.Citation20,Citation21 Another concern is cataracts following endothelial keratoplasty in a child possible due to a low corneoscleral rigidity resulting in anterior chamber fluctuations, and inadvertent lens injury, immature blood-aqueous-barrier resulting in extensive postoperative inflammation, air tamponade, secondary glaucoma, possible resurgeries, and long-term use of steroids in the postoperative period. Cataract development post DSAEK can be prevented by preoperative use of miotics, minimizing intraoperative manipulations, and judiciously tapering steroids.Citation22
During the immediate postoperative recovery period, extended sedation to ensure the child’s strict supine positioning and avoidance of eye rubbing help further minimize the complication of lenticule dislocation. Besides, recently, it has been shown that DSAEK has lower rates of IOP elevation when compared to PK. Also, some authors believe that DSAEK is associated with lower rates of acute graft rejection. This would translate into reduced postoperative visits with fewer examinations under anesthesia and a less frequent steroid dosage with an early tapering of steroids, thus avoiding the side effects of steroid use. A higher rate of infections associated with pediatric PK sutures is another disadvantage that DSAEK could overcome.Citation23
In conclusion, DSAEK would provide a stable, more predictable refraction with faster visual recovery that can help address the amblyopia issue in CHED patients.
The superior long-term clinical outcomes with low complication rates suggest that DSAEK is a safe and effective surgical alternative in corneal endothelial patients ().
Table 1. Comparative summary of the results of DSAEK in patients with congenital hereditary endothelial dystrophy or corneal endothelial dysfunction (studies with sample size >10 from 2011 to 2021).
Descemet’s membrane endothelial keratoplasty (dmek)
Descemet’s membrane endothelial keratoplasty (DMEK) further refinement of posterior Lamellar approaches. The graft is prepared by manually stripping the Descemet’s membrane from the donor cornea and is transplanted into the host. The graft is extremely fragile and thin, has a propensity to roll up, and can be inserted via a smaller incision than used in DSAEK. Contrary to DSAEK, the DMEK graft has no stromal layer, which avoids the incompatibility of the host and donor stromal fibers.Citation28 However, the procedure is much more demanding and challenging to manage among infants and leads to more endothelial cell loss and dislocation than other EK techniques.
Few studies have reported DMEK to provide better results and less long-term graft rejection than DSAEK and PK.Citation29 There are very few reports of outcomes of DMEK in cases of CHED. A retrospective study compared DMEK performed in 14 eyes of CHED, showing that 13/14 eyes maintained a clear cornea at final follow-up (mean, 16.9 ± 8.1 months). Following surgery, corrected distance visual acuity improved from 0.9 ± 0.3 logMAR (Snellen 20/158) to 0.4 ± 0.2 (20/50). DMEK has been noted to offer the most rapid visual rehabilitation among all endothelial keratoplasty techniques; however, more long-term studies are required for outcomes of DMEK in CHED cases.Citation14
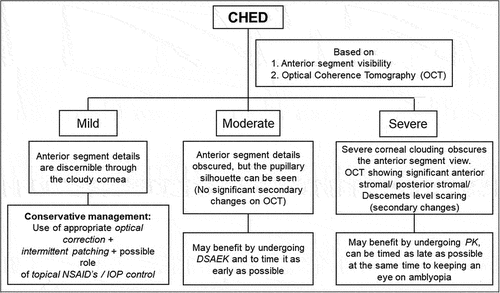
Based on the outcomes from studies conducted using various surgical modalities, the cases of CHED with significant secondary corneal changes like stromal and Descemet’s level scaring, band-shaped keratopathy, etc., benefit more from undergoing a full-thickness keratoplasty than undergoing a DSAEK. DSAEK can be the treatment of choice, especially CHED cases with a relatively clearer cornea and minimal to nil secondary corneal changes. There are also those cases of CHED with a very mild haze and near-normal visual acuity that do not warrant any surgical management. All these factors can help enable us decide the modality for management of such a case ().
Even though keratoplasty is the mainstay of treatment for patients with CHED, amblyopia management, and genetic counseling are crucial in the visual rehabilitation of children with CHED. Amblyopia management should be as aggressive as possible, with prompt refractive correction and patching therapy directed under the expert guidance of a pediatric ophthalmologist. Without successful treatment of amblyopia, the visual outcome is poor despite a clear medium. It is also necessary for genetic counseling of the patient and to explain that the risk of having a sibling with CHED is 25%. The risk of the patient having affected offspring is very low, provided the patient avoids consanguineous relationships.
Future Possibilities in Management
Current therapeutic interventions penetrating keratoplasty, Descemet’s stripping automated endothelial keratoplasty (DSAEK), and Descemet’s membrane endothelial keratoplasty (DMEK) face problems such as shortage of donor corneas, complex surgical procedure, the incidence of graft failure in acute and chronic phases,Citation30,Citation31 and the reluctance of parents to be willing to have their child operated at such a young age. Thus, a noninvasive modality of managing such cases might tide over such issues in the future.
We know that CHED is due to a mutation in the SLC4A11 gene leading to a dysfunctional BTR1 membrane protein and unable to express this protein on the cell surface. Further, the retained protein in the endoplasmic reticulum and the effects of various drugs on its expression to the surface were taken up by studies conducted by Alka et al.Citation32 on HEK293 cells expressing CHED and FECD mutation. They showed the efficacy of NSAIDs in moving the endoplasmic reticulum-retained missense mutant SLC4A11 in 20 out of 30 test cells, which showed a potential role of NSAIDs in these conditions.
Another potential role could be using antiglaucoma drops to reduce intraocular pressure (IOP) and thus the imbibition pressure (IP), thus reducing the amount of corneal edema in cases of CHED.
Such modalities may have a possible role in reducing the amount of corneal opacity, tiding over the time between presentation and surgery and thus may help delay surgery till the child reaches an appropriate age where the benefit outweighs the risk of surgery.
CRISPR-cas9 is another method of gene editing that can enable in-vivo reversal of the mutation in CHED cases and could have a potential role in the future of management of CHED.
Based on current evidence, the pediatric corneal surgeon can adopt an approach () to manage CHED cases based on critical period, presence or absence of ocular or systemic comorbidities, the density of amblyopia, and severity of corneal clouding.
Conclusion
CHED is a primarily endothelial dysfunction characterized by bilateral, progressive corneal clouding due to premature endothelial senescence. The mainstay treatment is early surgical keratoplasty to clear the visual axis during neuroplasticity. DSAEK provides superior long-term clinical outcomes with low complication rates than the conventional penetrating keratoplasty for CHED, especially in corneas with minimal secondary stromal changes. For those with significant stromal scarring, PKP can outscore EK by providing a pristine graft clarity but has the additional surgical risks involved that need to be titrated for each case. Besides these, novel, less invasive treatment modalities are being studied, including NSAIDs, IOP lowering agents, and gene editing using the CRISPR-Cas9.
Contributors
All the authors fulfill the criteria of authorship.
Ethics approval
The Institutional Review Board of LV Prasad Eye Institute, KAR campus, Hyderabad, Telangana, approved the study
Source(s) of support
Hyderabad Eye Institute and Hyderabad Eye Research Foundation, Hyderabad, India.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Weiss JS, Møller HU, Aldave AJ, et al. IC3D classification of corneal dystrophies–edition 2. Cornea. 2015 Feb;34(2):117–159. doi:10.1097/ICO.0000000000000307.
- Roy S, Praneetha DC, Vendra VP. Mutations in the corneal endothelial dystrophy-associated Gene SLC4A11 render the cells more vulnerable to oxidative insults. Cornea. 2015 Jun;34(6):668–674. doi:10.1097/ICO.0000000000000421.
- Desir J, Abramowicz M. Congenital hereditary endothelial dystrophy with progressive sensorineural deafness (Harboyan syndrome). Orphanet J Rare Dis. Published 2008 Oct 15 2008;3(1):28. doi:10.1186/1750-1172-3-28.
- Ramappa M, Mohamed A, Achanta DSR, Tumati CSK, Chaurasia S, Edward DP. Descemet stripping automated endothelial keratoplasty in pediatric age group: a decade of our experience. Cornea. 2021;40(12):1571–1580. doi:10.1097/ICO.0000000000002811.
- Lopes B, Ramos I, Ambrósio R. Jr Corneal densitometry in keratoconus. Cornea. 2014;33(12):1282–1286. doi:10.1097/ICO.0000000000000266.
- Ni Dhubhghaill S, Rozema JJ, Jongenelen S, et al. Normative values for corneal densitometry analysis by Scheimpflug optical assessment. Invest Ophthalmol Vis Sci. 2014;55(1):162–168. doi:10.1167/iovs.13-13236.
- Wang J, Simpson TL, Fonn D. Objective measurements of corneal light-backscatter during corneal swelling, by optical coherence tomography. Invest Ophthalmol Vis Sci. 2004 Oct;45(10):3493–3498. doi:10.1167/iovs.04-0096.
- Aasuri MK, Garg P, Gokhle N, et al. Penetrating keratoplasty in children. Cornea. 2000;19(2):140–144. doi:10.1097/00003226-200003000-00004.
- Ramappa M, Pehere NK, Murthy SI, et al. Rotational autokeratoplasty in pediatric patients for nonprogressive paracentral corneal scars. Ophthalmology. 2012;119(12):2458–2462. doi:10.1016/j.ophtha.2012.06.045.
- Ashar JN, Pahuja S, Ramappa M, et al. Deep anterior lamellar keratoplasty in children. Am J Ophthalmol. 2013;155(3):570–574 e1. doi:10.1016/j.ajo.2012.09.029.
- Ramappa M, Ashar J, Vaddavalli PK, et al. Endothelial keratoplasty in children: surgical challenges and early outcomes. Br J Ophthalmol. 2012;96(8):1149–1151. doi:10.1136/bjophthalmol-2011-300941.
- Ashar JN, Ramappa M, Chaurasia S. Endothelial keratoplasty without Descemet’s stripping in congenital hereditary endothelial dystrophy. J AAPOS. 2013;17(1): 22–24. doi:10.1016/j.jaapos.2012.09.013
- Ashar JN, Ramappa M, Vaddavalli PK. Paired-eye comparison of Descemet’s stripping endothelial Keratoplasty and penetrating Keratoplasty in children with congenital hereditary endothelial dystrophy. Br J Ophthalmol. 2013;97(10):1247–1249. doi:10.1136/bjophthalmol-2012-302602.
- Saad A, Ghazzal W, Keaik M, Indumathy TR, Fogla R. Outcomes of Descemet’s membrane endothelial keratoplasty for congenital hereditary endothelial dystrophy. J AAPOS. 2020 Dec;24(6):358.e1–358.e6. doi:10.1016/j.jaapos.2020.07.018. Epub 2020 Nov 27
- Sharma N, Agarwal R, Jhanji V, Bhaskar S, Kamalakkannan P, Nischal KK. Lamellar keratoplasty in children. Surv Ophthalmol. 2020 Nov-Dec;65(6):675–690. doi:10.1016/j.survophthal.2020.04.002
- Mittal V, Sehdev N, Mittal R. Descemet membrane endothelial keratoplasty in congenital hereditary endothelial dystrophy: initial experiences. Cornea. 2021 Aug 1;40(8):972–976. doi:10.1097/ICO.0000000000002701.
- Vanathi M, Panda A, Vengayil S, et al. Pediatric keratoplasty. Surv Ophthalmol. 2009;54(2):245–271. doi:10.1016/j.survophthal.2008.12.011.
- AlArrayedh H, Collum L, Murphy CC. Outcomes of penetrating keratoplasty in congenital hereditary endothelial dystrophy. Br J Ophthalmol. 2018 Jan;102(1):19–25. doi:10.1136/bjophthalmol-2016-309565.
- Medsinge A, Nischal KK. Paediatric keratoplasty: choices and conundrums. Br J Ophthalmol. 2013;97(10):1225–1227. doi:10.1136/bjophthalmol-2013-303469.
- Titiyal JS, Kaur M, Shaikh F, Bari A. “Acute-angled bevel” sign to assess donor lenticule orientation in ultra-thin Descemet stripping automated endothelial keratoplasty. BMJ Case Rep. 2019;12(2):e227927. doi:10.1136/bcr-2018-227927.
- Asif MI, Bafna RK, Sharma N, et al. Microscope integrated optical coherence tomography guided Descemet stripping automated endothelial keratoplasty in congenital hereditary endothelial dystrophy. Clin Ophthalmol. 2021 Jul 27;15:3173–3181. doi:10.2147/OPTH.S300286.
- Sati A, Ramappa M, Chaurasia S. Cataract following endothelial keratoplasty (EK) in a child. Med J Armed Forces India. 2013;69(4):398–399. doi:10.1016/j.mjafi.2012.08.027.
- Anshu A, Price MO, Price FW Jr. Risk of corneal transplant rejection significantly 464 reduced with Descemet’s membrane endothelial Keratoplasty. Ophthalmology. 2012: 465(119): 536–540. doi:10.1016/j.ophtha.2011.09.019
- Mohebbi M, Nabavi A, Fadakar K, Hashemi H. Outcomes of Descemet-stripping automated endothelial keratoplasty in congenital hereditary endothelial dystrophy. Eye Contact Lens. 2020 Jan;46(1):57–62. doi:10.1097/ICL.0000000000000604.
- Yang F, Hong J, Xiao G, et al. Descemet stripping endothelial keratoplasty in pediatric patients with congenital hereditary endothelial dystrophy. Am J Ophthalmol. Jan 2020;209:132–140. doi:10.1016/j.ajo.2019.08.010.
- Madi S, Santorum P, Busin M. Descemet stripping automated endothelial keratoplasty in pediatric age group. Saudi J Ophthalmol. 2012 Jul;26(3):309–313. doi:10.1016/j.sjopt.2012.04.006.
- Busin M, Beltz J, Scorcia V. Descemet-stripping automated endothelial keratoplasty for congenital hereditary endothelial dystrophy. Arch Ophthalmol. Sep 2011;129(9):1140–1146.
- Melles GR, Ong TS, Ververs B, and van der Wees J . Descemet Membrane Endothelial Keratoplasty (DMEK). Cornea. 09 2006;25: 987-90. PubMed: 17102683. doi: 10.1097/01.ico.0000248385.16896.34.
- Anshu A, Price MO, Price FW. Risk of corneal transplant rejection significantly reduced with Descemet’s membrane endothelial keratoplasty. Ophthalmology. 2012 Mar;119(3):536–540. doi: 10.1016/j.ophtha.2011.09.019
- Stuart AJ, Romano V, Virgili G, and Shortt AJ. Descemet’s membrane endothelial keratoplasty (DMEK) versus Descemet’s stripping automated endothelial keratoplasty (DSAEK) for corneal endothelial failure. Cochrane Database Syst Rev. Jun, 2018;6(6):CD012097. doi:10.1002/14651858.CD012097.pub2.
- Trindade B, Eliazar GC. Descemet membrane endothelial keratoplasty (DMEK): an update on safety, efficacy and patient selection. Clin Ophthalmol. 2019;13:1549–1557. Auckland, N.Z. doi:10.2147/OPTH.S178473.
- Alka K, Casey JR. Ophthalmic nonsteroidal anti-inflammatory drugs as a therapy for corneal dystrophies caused by SLC4A11 mutation. Invest Ophthalmol Vis Sci. 2018;59(10):4258. doi:10.1167/iovs.18-24301.