ABSTRACT
Purpose
Assessment of ocular surface in patients using anti-glaucoma medications (AGM) is rarely a priority for clinicians since glaucoma management targets intraocular pressure and preserves vision. This review summarizes the various adverse effects of topical AGM on the ocular surface and highlights the importance of ocular surface assessment in these patients.
Methods
A literature search of articles (English only) on the subject matter was conducted focusing on recent articles published in the past 5 years.
Results
The use of multiple anti-glaucoma medications in glaucoma patients increases patients’ exposure to the drug and the preservatives present in these medications. Long-term use of these medications has deleterious effects on the conjunctiva, cornea, eyelids, and periocular tissues like trichiasis, entropion, symblepharon, forniceal shortening, punctate keratopathy, non-healing epithelial defects, and pannus. Treatment requires drug withdrawal or substitution by oral or topical non-preserved and less toxic preparations of AGMs. The ocular surface and symptoms can improve if the condition is diagnosed early and after drug withdrawal in over 90% of eyes. However, stopping or changing AGMs can often present with its own unique set of challenges in intra-ocular pressure control which may often need glaucoma surgery in close to 20% of eyes for IOP control.
Conclusion
Topical antiglaucoma medications (with their preservatives) can induce severe ocular surface and periorbital changes. Early identification and withdrawal of the offending drug/preservative can help to reverse the changes except in eyes with extensive cicatrization.
INTRODUCTION
Worldwide, glaucoma is the second leading cause of irreversible blindness.Citation1 The standard treatment of glaucoma is intraocular pressure (IOP) control with topical antiglaucoma medications (AGM). These topical medications need multiple dosing and long-term administration. Ocular surface toxicity and tear film abnormality with these medications is a serious problem, including the entire ocular surface the conjunctiva, cornea, and eyelids.Citation2–7 The consequences are serious with a potential risk of visual loss due to the poor ocular surface, non-compliance, and worsening of glaucoma. The prevalence of ocular surface disease (OSD) in subjects using antiglaucoma medications is as high as 49–59%.Citation8,Citation9 Severe drug-induced cicatricial conjunctivitis secondary to AGM is reported to be seen in close to 20% of eyes.Citation10 summarizes the adverse effects of various classes of topical antiglaucoma medications on ocular surface and adnexa.
Table 1. Table showing summary of adverse effects of various AGMs.
The symptoms of AGM-induced OSD can be nonspecific like dryness, stinging, burning, watering, foreign body sensation to specific like red eye, and blurred vision. The signs may range from tear film abnormalities, conjunctival congestion, follicular reaction, fibrosis, cicatrization, symblepharon formation, corneal superficial punctate keratitis, vascularization, and scarring.Citation3,Citation6,Citation11,Citation12 These side effects could be attributable to the active component of the drug or the preservative in the medication.Citation13
There are various commercially available AGMs that a clinician can select from based on their mechanism of action, dosing, efficacy, and affordability. The treatment of glaucoma is affected because of adverse effects of topical anti-glaucoma medications at two levels: first, the discomfort with medication affects the patient compliance to treatment and quality of life; and, second, the long-term medication-induced conjunctival toxicity has a higher failure rate of the filtration surgery.Citation13,Citation14 This review summarizes the various preservatives used in anti-glaucoma medications and their effects on ocular and periocular structures.
METHODS
A literature search was performed for published articles on ocular surface disease caused by topical antiglaucoma medications in PubMed.gov. The search used the combinations of the terms “drug allergy”, “ocular drug toxicity”, “ocular surface”, “antiglaucoma medications”, adverse effects of antiglaucoma medications. Of the articles published in the last 30 years, 65 abstracts were reviewed and 58 were included for the review. Articles that were non-specific/non-English were excluded.
PRESERVATIVES IN ANTI-GLAUCOMA MEDICATIONS
The need for sterility in multidose eyedrops necessitates the inclusion of preservatives in these solutions. Various preservatives are used in topical antiglaucoma medications and the commonly used ones are Benzalkonium chloride (BAK), Purite, Polyquaternium 1 as Polyquad, SofZia. A higher prevalence of OSD was observed in patients using topical AGM than in untreated healthy controls.Citation15 It is important to understand the preservatives used in antiglaucoma medications and their toxic effects.
Benzalkonium Chloride (BAK)
It is the most common preservative used in topical AGM. It is a quaternary ammonium compound with antimicrobial properties, it can break the intercellular junctions in the corneal epithelium thereby improving the drug penetration into the anterior chamber. However, BAK can also affect the human ocular surface cells with toxic effects similar to those seen in bacterial cells.Citation4 BAK affects several eye structures like the tear film, cornea, conjunctiva, and trabecular cells. This preservative can be retained in several ocular tissues up to 1 week after application thus predisposing to toxic effects on the conjunctival and corneal epithelium. BAK is known to cause activation of inflammatory mediators, delayed hypersensitivity, and allergic reactions.Citation5 It also affects the lipid layer of the tear film, decreases mucin production by its effect on conjunctival goblet cells thereby decreasing the tear film stability.Citation5,Citation16 Studies have shown that these long-term side effects do not decrease the IOP lowering ability of the glaucoma medication.Citation6
Purite (Stabilized Oxychloro Complex)
It is one of the newer generation preservatives used in topical ophthalmic preparations. It has both antibacterial and antiviral properties by its ability to cause oxidative injury through its oxychloro molecules. On instillation, this preservative breaks down into its components, water, oxygen, sodium, and chloride ions which are similar to the natural tear components hence lesser toxicity to the ocular surface.Citation6 Topical medications with this preservative are better tolerated, have lesser toxic effects, and hence better tolerated.Citation4
Polyquaternium-1 (Polyquad)
Polyquaternium-1 (Polyquad) is a polymeric quaternary ammonium molecule with antimicrobial properties, it is known to disrupt bacterial cell membranes.Citation17 It was initially used as a preservative in a multidose contact lens cleaning solution. It has a molecular size that is 27 times larger than BAK.Citation18 The larger size of the molecule is less likely to penetrate the corneal epithelial cells hence less cytotoxicity.Citation17 Studies have shown less apoptosis and oxidative stress compared to BAK when used as a preservative in antiglaucoma medications.Citation19 However, it has toxic effects on the conjunctival goblet cells, thereby decreasing aqueous tear film production.Citation4
Sofzia
It is an ionic-buffered preservative, composed of borate, propylene glycol, sorbitol, and zinc chloride. It is effective as an antimicrobial agent at low concentrations as it releases hydrogen peroxide. On topical installation, it converts to non-toxic components. Hence it induces less conjunctival inflammation and fewer corneal changes compared to BAK.Citation4
ADVERSE EFFECTS OF VARIOUS ANTI-GLAUCOMA MEDICATIONS ON THE OCULAR AND PERIOCULAR STRUCTURES
Eyelids
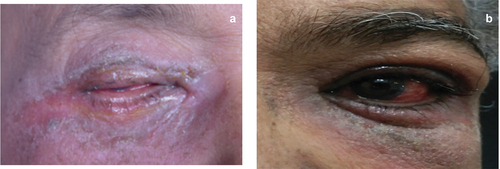
Topical beta-blockers can cause medication-induced adverse effects on the eyelids and conjunctiva. Apraclonidine can cause contact dermatitis of the periocular area and eyelids.Citation7 Contact dermatitis occurs due to a portion of the drug binds to the dermal protein to form a complex hapten, which sensitizes the individual. When the drug is re-instilled it induces a delayed hypersensitivity reaction, which is the cause for allergy. Apraclonidine can also cause ectropion of the eyelid which progressed to cicatricial ectropion in some patients.Citation20,Citation21 In individuals with preexisting lid laxity, tissue edema due to allergy can worsen the preexisting problem resulting in ectropion. Chronic allergy with skin excoriation can also cause fibrotic changes and tissue shortening that can lead to ectropion.
Topical brimonidine eye drops have been reported to cause reversible cicatricial ectropion and stopping the offending agent is the first measure in managing these patients. Brimonidine although an alpha2 agonist, is known to cause vasoconstrictive effects by its limited action on the alpha 1 receptor. These changes include vasoconstriction and reactive hyperemia of the conjunctiva, conjunctival follicular reaction, and causes vasoconstriction in the localized periocular and cheek areas (teardrop sign).Citation22
Dorzolamide is known to cause scaly in the periorbital area, periorbital dermatitis. This can lead to fibrosis of the skin with resultant ectropion and excess watering. The treatment includes stopping the offending drug and use of emollients/ tacrolimus in mild cases and may need a short course of topical steroid cream.Citation23,Citation24
Prostaglandin analogues can cause hyperpigmentation of eyelid skin. The pigmentation diminishes 1 month after cessation of the drug gradually and almost completely resolves in around 4 months.Citation25 These changes occur due to the up-regulation of tyrosinase activity in the melanocytes.
Although rare, Pilocarpine can cause contact dermatitis and eczema of the eyelids. Discontinuation of the drug and use of emollients or moisturizing agents can help.Citation26,Citation27 Eyelid side effects of anti-glaucoma medications. 1a) Eyelid skin ulceration 1b) Skin excoriation 2a) Entropion of the eyelid 2b) Lid margin keratinization 2c) Punctal stenosis.
Eyelashes
Prostaglandin analogues cause hypertrichosis and hyperpigmentation of the eyelashes. Increased lengthening of the eyelashes is often noted, which may need trimming. This may occur due to the stimulation of resting hair follicles. This may be reversed by stopping the medication. This adverse effect is used in the cosmetic industry to lengthen the eyelashes.Citation28,Citation29
Conjunctiva
Brimonidine causes conjunctival hyperemia and allergic conjunctivitis in 11.0–13.9% of subjects using this agent, often resulting in discontinuation of the drug by them.Citation30 Other common adverse effects include conjunctival follicular reaction and chemosis.Citation31 These adverse effects occur several months to years after initiation of the drug and stopping the offending drug helps to resolve the condition.
Apraclonidine is not a commonly used agent at present; however, it causes a severe allergic reaction by an immune response and is often discontinued. The features are severe hyperemia, follicular reaction, and excessive tearing.Citation7
Carbonic anhydrase inhibitors (CAIs) cause severe stinging and burning sensation on instillation, cause conjunctival hyperemia in up to 20.7% of patients. Allergic conjunctivitis, follicular conjunctivitis, limbal conjunctival follicles are reported several months after initiation of therapy.Citation32,Citation33
Pilocarpine also causes severe burning sensation on instillation of the drug. It causes allergic conjunctivitis, squamous metaplasia, keratinization of lid margins, a decrease in goblet cell density and scarring, and rarely cicatricial pemphigoid.Citation34
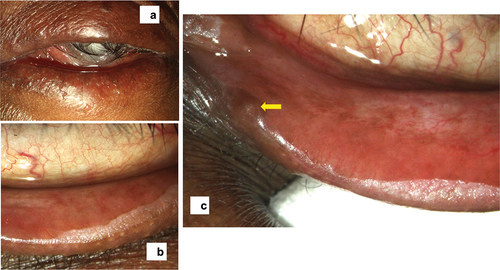
Topical beta-blockers can cause an increase in the fibroblasts and inflammatory cells in the conjunctiva, squamous metaplasia, and subconjunctival fibrosis. Chronic inflammation also results in subepithelial fibrosis, decrease in bulbar conjunctival goblet cell density results in ocular surface changes.Citation35 Conjunctival side effects of anti-glaucoma medications. a) Chemosis of the superior bulbar conjunctiva b) Follicular reaction c) Sub-conjunctival fibrosis d) Zipper like scarring e) Symblepharon with forniceal shortening.
Figure 3. The conjunctival changes showing chemosis and follicular reaction in the bulbar conjunctiva (a), a lacy follicular reaction in the tarsal conjunctiva (b), subconjunctival fibrosis of the palpebral conjunctiva (c), forniceal shortening (d), and symblepharon formation (e).
Studies have shown hyperemia and ocular surface inflammation to be more prevalent in subjects using topical AGM compared to controls and this seems to be more severe with prostaglandin analogues.Citation36
Cornea
Topical Beta-blockers cause toxic reactions on the cornea like superficial punctate keratitis, decreased corneal sensation, especially in elderly patients.Citation37,Citation38 Timolol disrupts epithelial cell stability and reepithelization.Citation7
An increase in central corneal thickness can be seen in patients using Dorzolamide and Brinzolamide, especially in the presence of preexisting corneal endothelium dysfunction. These drugs being carbonic anhydrase inhibitors, use of these medications can cause dysfunction of the already compromised endothelium with resultant corneal edema or decompensation.Citation39 CAIs can also cause corneal epithelial toxicity and decrease corneal sensation.Citation40
Miotics can cause epithelial erosions, punctate keratitis allergic marginal keratitis.Citation41,Citation42 Due to its pro-inflammatory nature, pilocarpine can precipitate graft rejection, which is shown to reverse on discontinuation of the drug.Citation43
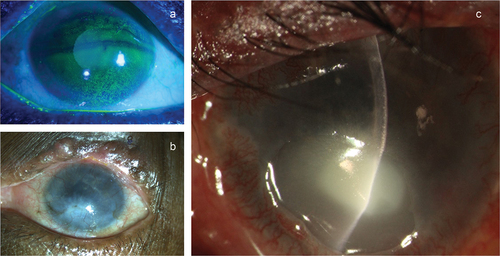
Prostaglandin analogues are known to cause recurrence of herpetic keratitis, reactivation of dormant disease and may also cause pseudodendrites or punctate keratitis.Citation44 The intraocular side effects are hyperpigmentation of the iris which occurs due to the up-regulation of tyrosinase activity in the stromal melanocytes.Citation45 Corneal side effects of anti-glaucoma medications. a) Superficial punctate keratitis b) Corneal scarring with vascularization c) Sterile corneal melt with a secondary infection.
Lacrimal System
Beta-Blockers and carbonic anhydrase inhibitors can cause lacrimal canalicular obstruction and can decrease tear production secondary to cicatricial scarring that occurs with long-term use of these medications.Citation46
DRY EYE DISEASE (DED)
can be described as a multifactorial disorder involving dysfunction of any lacrimal functional unit, the secretion of tears, the composition, distribution, and clearance.Citation47,Citation48 These can be affected by the complex interplay of the tears, conjunctival, and lid changes. There is excess evaporation of the aqueous layer of the tear film and an increase in the lipid layer due to tear film hyperosmolarity, conjunctival inflammation, and lid margin abnormality.Citation3,Citation49 All these changes cause severe dry eye and ocular surface disease.Citation49
The ocular surface inflammation can cause tear film instability, increased reflex tearing, and excess watering. The conjunctival inflammation also causes a decrease in goblet cells and mucin which further contributes to tear film instability.Citation3,Citation47
DRUG-INDUCED CICATRIZATION
Severe ocular surface disease produces clinical findings similar to mucous membrane pemphigoid and is usually associated with chronic use of AGM.Citation50
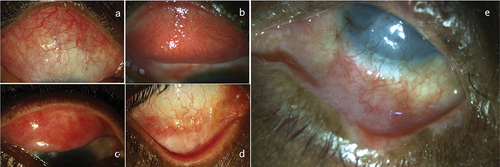
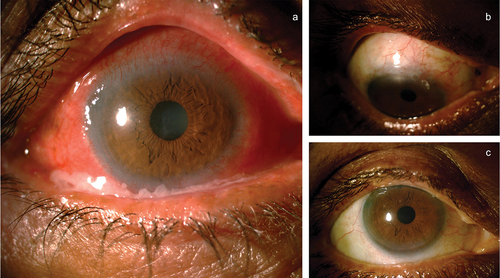
DICC presents as either a non-progressive, self-limiting “toxic” reaction to an offending topical drug or can be seen as a progressive, immunological process that affects the local immune system. When an immunological derangement occurs, IgG is found on the conjunctival epithelial basement membrane. The topical drugs can initiate a phenomenon called epitope spreading that determines the course of the autoimmune disease.Citation51 If the disease is a self-limiting one, stopping the offending drug can reverse the condition. Based on the IOP and the severity of glaucoma, oral CAI and or topical preservative-free AGM can be initiated. In case these do not help, may need to surgically manage glaucoma in these eyes. In the authors’ experience, close to 20% of eyes need surgical treatment for IOP control (our unpublished data). a) Representative case with severe OSD with AGM toxicity, b and c) Quiet eye post combined surgery with a good bleb. In eyes with “progressive” disease, the condition may not reverse even after stopping the offending agent. These eyes may need treatment with immunomodulating agents to control inflammation, and oral medications to control the IOP.
Figure 5. (a) A patient with antiglaucoma medication toxicity showing conjunctival congestion, discharge, and peripheral corneal vascularization, (b and c) Showing quiet eyes of the same patient with quiet ocular surface post combined cataract and trabeculectomy surgery and stopping all topical medications.
DIAGNOSIS
Conjunctival Impression Cytology
Conjunctival impression cytology is a technique to evaluate the conjunctival changes that develop with the use of topical anti-glaucoma medications. A variable loss of goblet cells, metaplasia of the conjunctival epithelial cells, an increase in inflammatory mediators is seen in DICC. There is a reduction in inflammatory mediators on impression cytology after withdrawal of the inciting agent in DICC. Nelson et al. proposed a 3-stage classification system to evaluate the conjunctival epithelium based on nuclear/cytoplasmic ratio and goblet cell density.Citation52
Hong et al.Citation53 studied impression cytology specimens of bulbar conjunctiva from patients receiving single medication like timolol, latanoprost, dorzolamide, or combination medications like timolol with latanoprost, and timolol with dorzolamide using Nelson’s method. After 6 months of topical AGM use, the scores were significantly higher in the fixed-combination therapy groups than in the monotherapy groups; however, there was no significant difference between the different types of medication.
Brandt et al. used impression cytology to evaluate conjunctival changes with long-term use of topical anti-glaucoma medications. The control group who were not receiving any topically administered medication had the lowest cumulative score that was consistent with normal healthy conjunctiva. The three of four treatment groups (beta-blockers, beta-blockers and pilocarpine, and maximal treatment) all demonstrated a statistically significant increase in cytologic grade compared to the control group. The largest difference between the treatment group and control is seen in those patients receiving maximal treatment, suggesting polypharmacy to affect the conjunctival cells.Citation54
Nuzzi et al. confirmed that long-term topical treatment for glaucoma induces changes in the conjunctival and subconjunctival tissues, suggesting a relationship between the duration of use of multiple topical medications (beta-blockers and pilocarpine in association) and the severity of the alterations of the ocular surface.Citation55
Evaluation of Biopsy Specimen
Use of any topical medication for 3 years or more was shown to be associated with significant subclinical inflammation.Citation12 Studies evaluated the effects of glaucoma eye drops on conjunctival morphology based on a biopsy of conjunctiva taken at the time of eye surgery. These studies were retrospective, and they compared biopsy findings in several groups of patients who used different regimens of eye drops at different times.
Sherwood et al. showed high concentrations of inflammatory cells, the macrophages, lymphocytes (both intraepithelial and subepithelial), mast cells, and fibroblast in subjects using at least 2 AGM over 1 year. These eyes were also associated with a decrease in goblet cell density.Citation56
Nuzzi et al. also showed, in conjunctival biopsy specimens from patients who were on long-term treatment for glaucoma, a significant increase in the thickness of the conjunctival epithelium, indirect fibroblastic stimulation, and indications of chronic inflammation.Citation55
Broadway et al. studied conjunctival biopsy specimens from glaucoma patients and showed that, as compared to the primary surgery group, patients treated with a beta-blocker alone showed more pale intraepithelial cells, presumed to be Langerhans cells, and mast cells. The multidose treated groups showed more pale cells, macrophages, lymphocytes, mast cells, and fibroblasts, and fewer goblet cells, both in comparison with patients undergoing short-term treatment and with those receiving a beta-blocker alone.Citation55
It is known that patients with the ocular surface disease exhibit inflammatory changes in the conjunctiva and cornea; biopsy studies of ocular tissues of patients using antiglaucoma eye drops have demonstrated a similar cellular reaction, suggesting a similar inflammatory response. Longer duration of treatment and more medications used are associated with more changes; however, it is not known when after initiation of antiglaucoma therapy, changes begin to occur.
Expression of Molecular Markers
Use of any topical medication preserved with benzalkonium chloride for 3 months or more was associated with a significant degree of subclinical inflammation as was shown by the presence of HLA-DR on conjunctival epithelial cells.Citation57
Baudouin et al. also showed expression of HLA-DR on conjunctival epithelial cells in patients taking AGM preserved with BAK for at least 6 months even without any clinical inflammation by indirect immunofluorescence technique done on impression cytology specimens, In the same study, all the healthy subjects were negative for HLA-DR on conjunctival epithelial cells.Citation58
Guglielminetti et al. also showed significant over-expression of HLA-DR in all patients using latanoprost with preservative compared to the control group that used unpreserved tear eye drops.Citation59
DIFFERENTIAL DIAGNOSIS
True Pemphigoid/Mucous Membrane Pemphigoid
Cicatricial conjunctivitis in eyes with true and psudopemohigoid may look similar with regard to clinical symptoms and certain signs. Certain features that distinguish the conditions are the presence of subepidermal autoantibodies on immunofluorescence in MMP which is absent in pseudopemphigoid. Early signs in DICC may be conjunctival cicatrization involving the lower fornix, in contrast to the conjunctival erosions in the pemphigoid. In late stages with cicatrization, both conditions may be indistinguishable and can be diagnosed based on the history of medication use, unilaterality, and biopsy.Citation60
Stevens-Johnson Syndrome
It is an acute, self-limiting condition that involves the skin and mucous membranes all over the body. Symblepharon formation is a major problem and ocular surface changes occur due to lid margin keratinization. The conjunctival scarring and fibrosis are not progressive unlike in DICC.Citation51
Ocular Pemphigus Vulgaris
This condition affects the mucous membranes with conjunctiva erosions and the medial aspect of the lower lid with lid margin erosions. They usually recover without much scarring. This condition needs to be differentiated from cicatrization including symblepharon, trichiasis, punctate keratitis, and entropion.Citation60
EFFECTS OF PREVIOUS TOPICAL THERAPY ON THE RESULTS OF GLAUCOMA FILTRATION SURGERY
Long-term use of anti-glaucoma medications has been reported to have a deleterious effect on surgical outcomes by altering postoperative wound healing.
Broadway et al.Citation12 and Lavin et al.Citation14 studied patients on different AGM and those who underwent primary surgery after a short course of AGM and found that long-term use of multiple AGM was a significant risk factor for failure of trabeculectomy. Failure is due to the relatively excessive healing response, due to excess fibrosis and scarring.
SUMMARY
Conclusion
It is well known that the chronic use of topical antiglaucoma medications induces ocular surface and periorbital changes. We have summarized the adverse effects that involve the tear film alterations, severe dry eye, lid margin keratinization, ulceration, scarring, conjunctival inflammation and scarring, corneal surface changes and some of them can affect the endothelial function as well.
While AGM is the mainstay of treatment in the management of glaucoma, one needs to thoroughly assess the ocular surface, tolerability, and likely adverse effects of these medications before prescribing them. Choosing combination medications, preservative-free medications can decrease the dosing as well as preservative exposure and their side effects. Periodic follow-up is necessary not just to assess the efficacy of the medications in terms of IOP control but to assess the compliance and the adverse effects of medications if any. In case adverse effects are noted, changing to a different class of medication, use of short course of anti-inflammatory agents like corticosteroids, lubricants, and reducing exposure to preservatives may help decrease the inflammation and subsequent scarring. This would also improve compliance and persistence with medication, a better quality of life, and preserve vision.
Recommendations in Clinical Practice
What can we do to prevent OSD and decrease medication toxicity?
1. Choosing medications with lower concentrations of preservative or non-BAK preservative.Citation3,Citation6
2 The use of preservative-free agents, as patients need lifelong therapy.Citation3
3 Using medications with better efficacy and once-daily dosing
4. Using a fixed combination of glaucoma drugs decreases preservative exposure in patients who need multiple medications.
5. A complete and good clinical evaluation of OSD should be periodically performed by addressing the tear film (Schirmer test, tear film breakup time). Corneal staining, conjunctival, and lid margin changes.Citation3
Prevention Strategies
It is very important to identify signs of drug toxicity and prompt withdrawal of the drug and appropriate management is needed.
Future Directions
Sustained Release Formulations
To decrease the cumulative toxic effect of preservatives on the ocular surface, once-daily dosing gel formulations are developed using Benzododecinium bromide (BDD).
Mild Less Toxic Preservatives
Almost all the topical anti-glaucoma medications that are used today contain BAK. Newer formulations are being developed with less toxic preservatives like SOC and sofZia which are proved to be less toxic to the corneal epithelium.
Preservative-free Formulations
These formulations are proved to be non-toxic to the epithelium. Hence, more preservative-free anti-glaucoma medications are to be developed as glaucoma patients need multiple dosing and lifelong use of these medications, however, it could be very expensive to patients.
DISCLOSURE STATEMENT
No potential conflict of interest was reported by the author(s).
Additional information
Funding
REFERENCES
- Tham Y-C, Li X, Wong TY, Quigley HA, Aung T, Cheng C-Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. doi:10.1016/j.ophtha.2014.05.013.
- Aydin Kurna S, Acikgoz S, Altun A, Ozbay N, Sengor T, Olcaysu OO. The effects of topical antiglaucoma drugs as monotherapy on the ocular surface: a prospective study. J Ophthalmol. 2014;2014:1–8. doi:10.1155/2014/460483.
- Actis AG, Rolle T. Ocular surface alterations and topical antiglaucomatous therapy: a review. Open Ophthalmol J. 2014;8:67. doi:10.2174/1874364101408010067.
- Kaur IP, Lal S, Rana C, Kakkar S, Singh H. Ocular preservatives: associated risks and newer options. Cutan Ocul Toxicol. 2009;28(3):93–103. doi:10.1080/15569520902995834.
- Saini M, Vanathi M, Dada T, Agarwal T, Dhiman R, Khokhar S. Ocular surface evaluation in eyes with chronic glaucoma on long term topical antiglaucoma therapy. Int J Ophthalmol. 2017;10(6):931. doi:10.18240/ijo.2017.06.16.
- Noecker R. Effects of common ophthalmic preservatives on ocular health. Adv Ther. 2001;18(5):205–215. doi:10.1007/BF02853166.
- Servat JJ, Bernardino CR. Effects of common topical antiglaucoma medications on the ocular surface, eyelids, and periorbital tissue. Drugs Aging. 2011;28(4):267–282. doi:10.2165/11588830-000000000-00000.
- Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17(5):350–355. doi:10.1097/IJG.0b013e31815c5f4f.
- Fechtner RD, Godfrey DG, Budenz D, Stewart JA, Stewart WC, Jasek MC. Prevalence of ocular surface complaints in patients with glaucoma using topical intraocular pressure-lowering medications. Cornea. 2010;29(6):618–621. doi:10.1097/ICO.0b013e3181c325b2.
- Thorne JE, Anhalt GJ, Jabs DA. Mucous membrane pemphigoid and pseudopemphigoid. Ophthalmology. 2004;111(1):45–52. doi:10.1016/j.ophtha.2003.03.001.
- Broadway DC, Grierson I, O’Brien C, Hitchings RA. Adverse effects of topical antiglaucoma medication: II. The outcome of filtration surgery. Arch Ophthalmol. 1994;112(11):1446–1454. doi:10.1001/archopht.1994.01090230060021.
- Broadway DC, Grierson I, O’Brien C, Hitchings RA. Adverse effects of topical antiglaucoma medication: i. The conjunctival cell profile. Arch Ophthalmol. 1994;112(11):1437–1445. doi:10.1001/archopht.1994.01090230051020.
- Baudouin C. Detrimental effect of preservatives in eyedrops: implications for the treatment of glaucoma. Acta Ophthalmol. 2008;86(7):716–726. doi:10.1111/j.1755-3768.2008.01250.x.
- Lavin MJ, Wormald RP, Migdal CS, Hitchings RA. The influence of prior therapy on the success of trabeculectomy. Arch Ophthalmol. 1990;108(11):1543–1548. doi:10.1001/archopht.1990.01070130045027.
- Pérez-Bartolomé F, Martínez-de-la-Casa JM, Arriola-Villalobos P, Fernández-Pérez C, Polo V, García-Feijoó J. Ocular surface disease in patients under topical treatment for glaucoma. Eur J Ophthalmol. 2017;27(6):694–704. doi:10.5301/ejo.5000977.
- Ramli N, Supramaniam G, Samsudin A, Juana A, Zahari M, Choo MM. Ocular surface disease in glaucoma: effect of polypharmacy and preservatives. Optometry Vision Sci. 2015;92(9):e222–e226. doi:10.1097/OPX.0000000000000542.
- Ammar DA, Noecker RJ, Kahook MY. Effects of benzalkonium chloride-preserved, polyquad-preserved, and sofZia-preserved topical glaucoma medications on human ocular epithelial cells. Adv Ther. 2010;27(11):837–845. doi:10.1007/s12325-010-0070-1.
- Rolando M, Crider JY, Kahook MY. Ophthalmic preservatives: focus on polyquaternium-1. Expert Opin Drug Deliv. 2011;8(11):1425–1438. doi:10.1517/17425247.2011.617736.
- Brignole-Baudouin F, Riancho L, Liang H, Baudouin C. Comparative in vitro toxicology study of travoprost polyquad-preserved, travoprost BAK-preserved, and latanoprost BAK-preserved ophthalmic solutions on human conjunctival epithelial cells. Curr Eye Res. 2011;36(11):979–988. doi:10.3109/02713683.2011.578781.
- Britt M, Burnstine MA. Iopidine allergy causing lower eyelid ectropion progressing to cicatricial entropion. British J Ophthalmol. 1999;83(8):987. doi:10.1136/bjo.83.8.987f.
- Armisen M, Vidal C, Quintans R, Suarez A, Castroviejo M. Allergic contact dermatitis from apraclonidine. Contact Dermatitis. 1998;39(4):193. doi:10.1111/j.1600-0536.1998.tb05893.x.
- Scruggs J, Whiteside-Michel J, Brodsky MC. The teardrop sign: a rare dermatological reaction to brimonidine. British J Ophthalmol. 2000;84(6):667. doi:10.1136/bjo.84.6.667e.
- Kalavala M, Statham B. Allergic contact dermatitis from timolol and dorzolamide eye drops. Contact Dermatitis. 2006;54(6):345. doi:10.1111/j.0105-1873.2006.0645b.x.
- Grassberger M, Baumruker T, Enz A, et al. A novel anti-inflammatory drug, SDZ ASM 981, for the treatment of skin diseases: in vitro pharmacology. Br J Dermatol. 1999;141(2):264–273. doi:10.1046/j.1365-2133.1999.02974.x.
- Lai C-H, Lai I-C, Chi -C-C. Allergic Contact Dermatitis Caused by Latanoprost Ophthalmic Solution. London, England: SAGE Publications Sage UK; 2006.
- Zimmerman TJ, Wheeler TM. Side effects and ways to avoid them. Ophthalmology. 1982;89(1):76–80. doi:10.1016/S0161-6420(82)34866-6.
- Cusano F, Luciano S, Capozzi M, Verrilli D. Contact dermatitis from pilocarpine. Contact Dermatitis. 1993;29(2):99. doi:10.1111/j.1600-0536.1993.tb03495.x.
- Reynolds A, Murray P, Colloby P. Darkening of eyelashes in a patient treated with latanoprost. Eye. 1998;12(4):741–743. doi:10.1038/eye.1998.181.
- Johnstone MA. Hypertrichosis and increased pigmentation of eyelashes and adjacent hair in the region of the ipsilateral eyelids of patients treated with unilateral topical latanoprost. Am J Ophthalmol. 1997;124(4):544–547. doi:10.1016/S0002-9394(14)70870-0.
- Butler P, Mannschreck M, Lin S, Hwang I, Alvarado J. Clinical experience with the long-term use of 1% apraclonidine: incidence of allergic reactions. Arch Ophthalmol. 1995;113(3):293–296. doi:10.1001/archopht.1995.01100030047020.
- Wilkerson M, Lewis RA, Shields MB. Follicular conjunctivitis associated with apraclonidine. Am J Ophthalmol. 1991;111(1):105–106. doi:10.1016/S0002-9394(14)76907-7.
- Adamsons IA, Polis A, Ostrov CS, Boyle JE. Two-year safety study of dorzolamide as monotherapy and with timolol and pilocarpine. Dorzolamide safety study group. J Glaucoma. 1998;7(6):395–401. doi:10.1097/00061198-199812000-00007.
- Gupta R, Vernon S. An unusual appearance of limbal conjunctival follicles in a patient on brimonidine and dorzolamide. Eye. 2005;19(3):356–358. doi:10.1038/sj.eye.6701498.
- Jackson WB. Differentiating conjunctivitis of diverse origins. Surv Ophthalmol. 1993;38:91–104. doi:10.1016/0039-6257(93)90034-5.
- Dog˘ an AL, Orhan M, Söylemezog˘ Lu F, İrkeç M, Bozkurt B. Effects of topical antiglaucoma drugs on apoptosis rates of conjunctival epithelial cells in glaucoma patients. Clin Exp Ophthalmol. 2004;32(1):62–66. doi:10.1046/j.1442-9071.2003.00760.x.
- Pérez Bartolomé F, Martínez de la Casa JM, Arriola Villalobos P, Fernández Pérez C, Polo V, Sánchez Jean R, and García Feijoó J. Ocular Redness Measured with the Keratograph 5M in Patients Using Anti-Glaucoma Eye Drops. Semin Ophthalmol. 2018;33(5):643–650. doi:10.1080/08820538.2017.1395891.
- Weissman S, Asbell P. Effects of topical timolol (0.5%) and betaxolol (0.5%) on corneal sensitivity. British J Ophthalmol. 1990;74(7):409–412. doi:10.1136/bjo.74.7.409.
- Van Buskirk EM. Corneal anesthesia after timolol maleate therapy. Am J Ophthalmol. 1979;88(4):739–743. doi:10.1016/0002-9394(79)90675-5.
- Wirtitsch MG, Findl O, Heinzl H, Drexler W. Effect of dorzolamide hydrochloride on central corneal thickness in humans with cornea guttata. Arch Ophthalmol. 2007;125(10):1345–1350. doi:10.1001/archopht.125.10.1345.
- Ammar DA, Kahook MY. The effects of combination glaucoma medications on ocular surface epithelial cells. Adv Ther. 2009;26(10):970–975. doi:10.1007/s12325-009-0076-8.
- Edwards R. A comparative study of Ocuserta® Pilo 40, intensive pilocarpine and low-dose pilocarpine in the initial treatment of primary acute angle-closure glaucoma. Curr Med Res Opin. 1997;13(9):501–509. doi:10.1185/03007999709113323.
- Johnson DH, Kenyon KR, Epstein DL, Van Buskirk EM. Corneal changes during pilocarpine gel therapy. Am J Ophthalmol. 1986;101(1):13–15. doi:10.1016/0002-9394(86)90459-9.
- Massry GG, Assil KK. Pilocarpine-associated allograft rejection in postkeratoplasty patients. Cornea. 1995;14(2):202–205. doi:10.1097/00003226-199503000-00015.
- Morales J, Shihab ZM, Brown SM, Hodges MR. Herpes simplex virus dermatitis in patients using latanoprost. Am J Ophthalmol. 2001;132(1):114–116. doi:10.1016/S0002-9394(01)01012-1.
- Wistrand PJ, Stjernschantz J, Olsson K. The incidence and time-course of latanoprost-induced iridial pigmentation as a function of eye color. Surv Ophthalmol. 1997;41:S129–S138. doi:10.1016/S0039-6257(97)80020-3.
- Narioka J, Ohashi Y. Effects of beta-adrenergic antagonist on width of nasolacrimal drainage system lumen. J Ocul Pharmacol Ther. 2007;23(5):467–475. doi:10.1089/jop.2007.0025.
- Gipson IK, Argüeso P, Beuerman R, et al. Research in dry eye: report of the research subcommittee of the international dry eye workShop (2007). Ocul Surf. 2007;5:179–193.
- Pinho Tavares F, Fernandes RS, Bernardes TF, and Bonfioli AA. Carneiro Soares EJ Dry eye disease. Seminars in Ophthalmology. Vol. 25. Taylor & Francis; 2010:84–93.
- Wong AB, Wang MT, Liu K, Prime ZJ, Danesh-Meyer HV, Craig JP. Exploring topical anti-glaucoma medication effects on the ocular surface in the context of the current understanding of dry eye. Ocul Surf. 2018;16(3):289–293. doi:10.1016/j.jtos.2018.03.002.
- Fiore PM, Jacobs IH, Goldberg DB. Drug-induced pemphigoid: a spectrum of diseases. Arch Ophthalmol. 1987;105(12):1660–1663. doi:10.1001/archopht.1987.01060120058023.
- Bernauer W, Broadway DC, Wright P. Chronic progressive conjunctival cicatrisation. Eye. 1993;7(3):371–378. doi:10.1038/eye.1993.75.
- Nelson JD. Impression cytology. Cornea. 1988;7(1):71–81. doi:10.1097/00003226-198801000-00012.
- Hong S, Lee CS, Seo KY, Seong GJ, Hong YJ. Effects of topical antiglaucoma application on conjunctival impression cytology specimens. Am J Ophthalmol. 2006;142(1):185–186. doi:10.1016/j.ajo.2006.02.056.
- Brandt JD, Wittpenn JR, Katz LJ, Steinmann WN, Spaeth GL. Conjunctival impression cytology in patients with glaucoma using long-term topical medication. Am J Ophthalmol. 1991;112(3):297–301. doi:10.1016/S0002-9394(14)76730-3.
- Nuzzi R, Finazzo C, Vercelli A, Cracco C. Conjunctiva and subconjunctival tissue in primary open-angle glaucoma after long-term topical treatment: an immunohistochemical and ultrastructural study. Graefes Arch Clin Exp Ophthalmol. 1995;233(3):154–162. doi:10.1007/BF00166608.
- Sherwood MB, Grierson I, Milgar L, Hitchings RA. Long-term morphologic effects of antiglaucoma drugs on the conjunctiva and Tenon’s capsule in glaucomatous patients. Ophthalmology. 1989;96(3):327–335. doi:10.1016/S0161-6420(89)32888-0.
- Cvenkel B, Ihan A. Ocular surface changes induced by topical antiglaucoma monotherapy. Ophthalmologica. 2002;216(3):175–179. doi:10.1159/000059624.
- Baudouin C, Garcher C, Haouat N, Bron A, Gastaud P. Expression of inflammatory membrane markers by conjunctival cells in chronically treated patients with glaucoma. Ophthalmology. 1994;101(3):454–460. doi:10.1016/S0161-6420(94)31322-4.
- Guglielminetti E, Barabino S, Monaco M, Mantero S, Rolando M. HLA-DR expression in conjunctival cells after latanoprost. J Ocul Pharmacol Ther. 2002;18(1):1–9. doi:10.1089/108076802317233162.
- Huang LC, Wong JR, Alonso-Llamazares J, et al. Pseudopemphigoid as caused by topical drugs and pemphigus disease. World J Ophthalmol. 2015;5(1):1–15. doi:10.5318/wjo.v5.i1.1.