ABSTRACT
Purpose
To understand the impact of dry eye disease (DED) on the components of ocular biometry and ways to optimize the visual outcomes of cataract surgery in eyes with DED.
Methodology
A thorough literature review of the components pertaining to this review was undertaken using the databases, PubMed (from the year 2000), MEDLINE, CENTRAL (including Cochrane Eyes and Vision Trials Register; Cochrane Library: Issue 12 of 12 December 2019), metaRegister of Controlled Trials (mRCT) (www.controlled-trials. com), ClinicalTrials.gov (www.clinicaltrial.gov) and WHO International Clinical Trials Registry Platform (www.who.int/ictrp/search/en). The keywords used for the search included “cataract surgery” or “phacoemulsification” combined with “dry eye”, “dry eye disease”,”biometry”, “keratometry”.
Results
Publications considered for this review included meta-analysis, systematic reviews, case–control and cohort studies, case series, and laboratory-based studies. Published articles reporting tear film alteration in DED, its impact on the quality of vision and optical aberrations, the effect of topical medications on keratometry variations, and reports on optimizing the ocular surface before cataract surgery were included.
Conclusions
DED is a common entity seen in patients presenting to routine cataract clinics and is known to impact the accuracy, reliability, and repeatability of ocular biometry and IOL power calculations in them. This review intends to emphasize the preoperative screening for the presence of DED, initiation of appropriate medical management for optimization of the ocular surface before cataract surgery, and recommendations for performing biometry. The algorithmic approach proposed will help the general ophthalmologists in routine practice to provide quality care and acceptable visual outcomes in patients with pre-existing DED.
INTRODUCTION
The last couple of decades has seen a significant upsurge in the prevalence of dry eye disease (DED), which is estimated to be about 5–50% around the world.Citation1–5 As defined by TFOS DEW II, DED is a “multifactorial disease of the ocular surface characterized by loss of homeostasis of the tear film with associated tear film instability, hyperosmolarity, ocular surface inflammation and neurosensory abnormalities playing etiological roles”.Citation6 DED is broadly classified as evaporative, aqueous deficient, and mixed forms. While the evaporative type is associated with tear film instability leading to a rapid breakdown of the precorneal tear film, aqueous deficiency is associated with subnormal production of the tear film volume from the lacrimal glands. A mixed form of DED includes a combination of both the aforementioned entities.Citation6–8 All these variants are associated with a significant impact on the vision, ocular surface milieu, mental health, and quality of life (QOL) of the individuals suffering from it.Citation9,Citation10 The tear film plays an essential role in lubrication and sustaining the integrity of the ocular surface, ensuring the maintenance of a smooth refractive surface that aids in optimal visual performance.Citation11 Pre-corneal tear film being the first refracting medium of the ocular surface needs to be healthy to obtain precise topography, tonometry, and other biometric measurements of the eye. In DED, the disturbed tear film homeostasis results in ocular surface damage due to the induced tear film hyperosmolarity and chronic ocular surface inflammation. The resultant altered tear film dynamics and ocular surface damage lead to inaccuracies in optical measurements, thereby impacting the outcomes of interventions like cataract surgery that largely depend on these assessments.Citation12–14
Modern-day cataract surgery delivers excellent surgical outcomes along with rapid postoperative recovery and a lower rate of complications.Citation15 While the technological advancements in cataract surgery over the past five decades have had a positive impact on the QOL of millions of individuals globally, they have also created a rise in surgeon and patient expectations from these interventions.Citation16,Citation17 Despite evolving as a successful refractive procedure in the modern era, a commonly performed surgical intervention like cataract surgery becomes quite challenging when performed in the background of other associated ocular co-morbidities like DED.Citation15,Citation18 The sub-optimal outcomes achieved can be attributed to the associated ocular surface alterations, chronic inflammation, inaccuracies in IOL power calculations, epithelial healing issues, and postoperative worsening of DED itself.Citation1,Citation19,Citation20
DED has been reported to increase after cataract surgery even in eyes without pre-existing disease, thereby affecting the surgical outcomes and increasing dissatisfaction among patients. Dasgupta and Gupta reported that at 12 weeks post-cataract surgery, all the patients showed abnormalities in tear film break-up time (TBUT), Schirmer I test values, and DED symptomatology questionnaire scores.Citation21 Choi et al. reported that even at 3 months following cataract surgery, 27% of patients experienced persistent DED symptoms with clinical evidence of reduced TBUT, increased ocular surface staining with fluorescein, and meibomian gland dropouts.Citation22 In addition to aggravated symptoms, patients with DED are more prone to refractive surprises post-surgery due to the inaccuracies in pre-operative biometry assessments.Citation23 In this modern era of premium intraocular lenses (IOL) and heightened patient expectations, having a better understanding of the factors influencing biometry and surgical outcomes in patients with DED becomes extremely vital.
Thus, in this narrative review, we aim to provide an update on existing literature to improve understanding of the preoperative keratometric fluctuation and methods to optimize biometry in patients with DED presenting for cataract surgery. Additionally, we aim to identify the existing lacunae in literature which will act as a base for future research.
METHODOLOGY
A search was undertaken using the databases, PubMed (from the year 2000), MEDLINE, CENTRAL (including Cochrane Eyes and Vision Trials Register; Cochrane Library: Issue 12 of 12 December 2019), metaRegister of Controlled Trials (mRCT) (www.controlled-trials. com), ClinicalTrials.gov (www.clinicaltrial.gov) and WHO International Clinical Trials Registry Platform (www.who.int/ictrp/search/en). The search terms included “cataract surgery or phacoemulsification” combined with “dry eye”, “dry eye disease”, “biometry”, “keratometry”. Published articles in English were preferentially selected. Published data focusing on the potential burden of pre-existing and postoperative DED following cataract surgery, the effect of dry eye on the ocular surface, variations in biometry due to dry eye, and current evidence on the management options to ameliorate refractive surprises after cataract surgery in eyes with DED were reviewed.
TEAR FILM ALTERATIONS IN DED
DED is characterized by subnormal production of tears, rapid breakup of the tear film layer, and accelerated evaporation of tears from the ocular surface.Citation6 The increased osmolarity of the tear film along with tear film instability is the core pathophysiologic processes involved in DED. Tear film hyperosmolarity due to any etiology of DED is responsible for increased tear evaporation and low TBUT.Citation7 TFOS DEWS II report suggests that dry eye irrespective of the subtype enters a final common pathway in which hyperosmolarity of the tear film and ocular surface inflammation creates a vicious cycle perpetuating DED.Citation11 The inflammatory cascade along with tear hyperosmolarity causes reduced expression of glycocalyx mucins and goblet cell damage.Citation11 Low levels of MUC5AC in the tear film result from the goblet cell loss seen in DED.Citation11 Reduced expression of glycocalyx mucins is the most likely cause for inadequate ocular surface wetting leading to early tear film breakup. All the above changes further perpetuate dry eye disease, affect the ocular surface integrity and thereby impact any optical measurements that need to be performed on these eyes.
OPTICAL ABERRATIONS IN DED AND ASSOCIATED IMPACT ON QUALITY OF VISION
The tear film serves as the first refracting interface of the optical system of the eye, thereby exerting a significant impact on the quality of vision. Conventional measurements like visual acuity testing do not detect all aspects of compromised visual functions.Citation12 Quantitative objective assessments for detecting the quality of vision like corneal topography and wavefront sensors have made remarkable advances supplementing visual acuity testing in this aspect.
Corneal topographers like the Placido disc-based systems measure the anterior corneal curvature contours by analyzing reflected concentric mires on the air–tear interface.Citation24 The changes in the tear film can thus be detected by this system. The TMS-2 N corneal topography instrument (Tomey Technology, Nagoya, Japan) developed and inculcated a novel software called tear film stability analysis system (TSAS). This software enables the topographer to capture serial topography scans once every second for 10 s. The reverse-engineered Placido ring-based corneal topographic map calculations utilized in this system enable it to detect subtle tear film changes from distortion of mires.Citation25 Various studies in the past have also highlighted that irregular astigmatism is seen more markedly among patients with DED and is caused by the increased fluctuations and irregularities of the tear film.Citation26–28
Studies have taken into account surface regularity index (SRI) and surface asymmetry index (SAI) to better understand the changes occurring at the level of the cornea in DED.Citation29–31 As per these studies, both SRI and SAI were found to be higher in DED as compared to normal eyes. In a study by Kojima et al., TSAS software was used to analyze tear film stability, regularity, and asymmetry indices named TSRI and TSAI, which emanated from SRI and SAI respectively.Citation32 This study found out that TSRI and TSAI were significantly more in dry eyes than in normal eyes. Additionally, the insertion of punctal plugs as a therapeutic tool in treating DED significantly reduced both SRI and SAI.Citation32
The utility of wavefront analysis for aberrometry has been growing worldwide. Wavefront sensing has been utilized in the detection and quantification of irregular astigmatism as higher-order aberrations. Subjective visual outcomes in patients with DED were correlated with progression index for corneal higher-order aberrations (HOA).Citation33 One study highlighted that optical quality degraded in patients with DED and was associated with visual impairment during driving.Citation34 Total ocular HOAs are usually found to be stable between blinks in normal eyes, but one study reported that HOAs can fluctuate dynamically after blinking even in clinically normal subjects.Citation35 Short TBUT DED is a terminology that has been gaining importance in recent years, which is reserved for subjects with symptoms of DED with a reduced tear film break-up time (TBUT) without ocular surface damage or aqueous tear deficiency.Citation36 In eyes with short TBUT, HOAs have been found to increase with time after blinking and in the 10 seconds inter-blink interval as noted on TSAS systems.Citation36 Subjects with aqueous deficiency DED have higher total ocular HOAs and these were even more marked in subjects having superficial punctate keratopathy (SPK) due to DED.Citation37 Kaido et al. confirmed these findings and reported that optical degradation in the central zone of corneas with DED may affect the visual quality.Citation38 This also applies to short TBUT dry eyes where patients often complain of severe dry eye symptoms and visual disturbances despite having a good visual acuity on regular assessment.Citation39 The impact of dry eye on HOA and visual quality has been demonstrated in . By utilizing functional visual acuity and sequential wavefront analysis, clinicians can now understand the importance of treating short TBUT dry eye in a better way.Citation40,Citation41 In a study by Koh et al., it was found that ocular forward light scattering and corneal backward light scattering were significantly greater in DED subjects as compared to normal eyes. SPKs as commonly found in DED might be responsible for causing backward light scattering from the anterior surface of the cornea.Citation42 The above-cited studies on quantitative and qualitative analysis with corneal topography and wavefront sensing highlight that instability of tear film and ocular surface damage significantly degrade the quality of vision in patients with DED, despite their distant visual acuity being recorded as reasonable .
Figure 1. Impact of optical aberrations on the image quality in DED. The image shows the image quality in normal subjects with a stable pre-corneal tear film resulting in a sharp image(a). Image b shows the scatter of light in eyes with dry eye disease (DED) resulting in higher-order aberrations (HOAs) and blurred images.
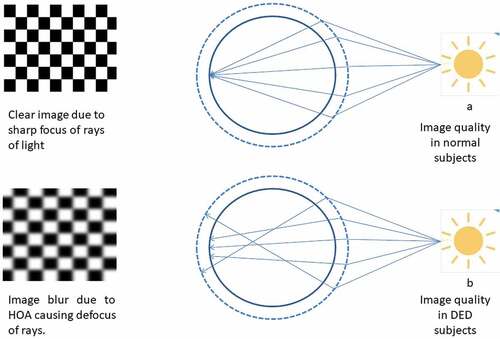
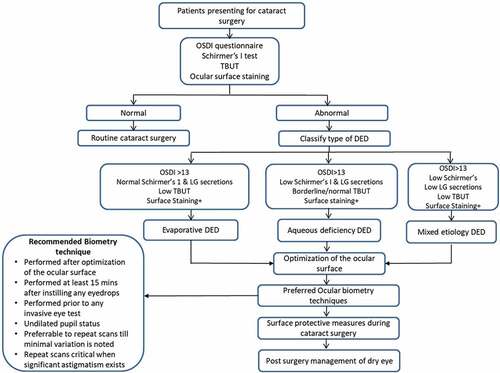
Figure 2. Algorithm for appropriate management of DED patients presenting in cataract clinics. The algorithm highlights a systematic approach in screening, evaluating, and optimizing dry eye disease (DED) in patients presenting for cataract surgery. It also discusses the recommendations for obtaining reliable ocular biometry in eyes with DED to improve the postoperative outcomes after cataract surgery.
VARIABILITY OF KERATOMETRY IN DED
According to the Royal College of Ophthalmologists cataract surgery guidelines, the outcomes of biometry can be considered excellent when at least 85% of the eyes attain target refraction within 0.5–1.0 diopter.Citation43 Accurate IOL power calculation is vital in providing satisfactory visual outcomes in patients undergoing cataract surgery and keratometry serves as a key component in this endeavor. The measurement and magnitude of anterior corneal astigmatism is an important factor in determining the need for toric IOL. A Manual keratometer, IOLMaster (Carl Zeiss Meditec AG), and Lenstar (Haag-Streit USA) are a few of the many instruments used widely for the calculation of keratometry preoperatively.
Keratometers rely on a good reflection of mires from the precorneal tear film, and the tear film instability can affect the reliability and sensitivity of keratometry measurements. This is due to the hampered quality of corneal reflections caused by the unstable tear film in eyes with DED.Citation44 The tear film instability is responsible for the dynamic changes of the anterior refractive surface of the cornea and affects the methods of ocular biometry that rely on the reflected light from the anterior corneal surface. Furthermore, the prevalence of dry eye is known to increase with age, and hence tear film instability is a common concern in the patients presenting with cataracts who often belong to the geriatric age group. In clinical practice, patients with DED have high short-term and long-term keratometry fluctuations and the ocular biometry needs to be repeated multiple times.Citation13 A study by Epitropoulos et al. showed that subjects with tear film hyperosmolarity had poor repeatability of keratometry values and IOL power calculation as compared to subjects with normal tear film osmolarity.Citation13 Thus, preoperative measurement of tear film osmolarity can identify patients who have higher chances of refractive surprises after cataract surgery.Citation13 illustrates the impact of dry eyes on ocular biometry parameters.
Table 1. Inaccuracies of biometry in DED and impact of treatment.
A study by Roggla et al. focused on the influence of instillation of artificial tears on keratometry measurements before cataract surgery.Citation45 This study observed significant changes in keratometry values after instilling both low- and high-viscosity lubricating eye drops lasting for a duration of 5 min. These fluctuations were noted to be higher among patients with dry eyes with lower reproducibility and reliability. Therefore, they concluded that biometry measurements should be taken either before or 5 min after instillation of lubricating eye drops in patients with DED with the former being more preferred.Citation45 Numerous eyedrops have been reported to impact optical biometry and illustrates the impact of various such eyedrops on keratometry measurements.
Table 2. Effect of different topical medications on keratometry and optical aberrations in DED.
A study by Aysun Sanal Dogan et al. studied the repeatability of Scheimpflug-based Sirius corneal topography system in normal versus mild-to-moderate dry eyes. It was concluded that the anterior segment topographers (dual Schiempflug imaging plus Placido disc-based system) like Sirius had excellent repeatability of anterior segment parameters including Sim K values in both healthy as well as dry eye candidates.Citation49 This might give us some hope for a more reliable calculation of IOL power in mild-to-moderate dry eye patients.
NEED FOR PREOPERATIVE OPTIMIZATION OF THE OCULAR SURFACE IN DED
The ocular surface disease is highly prevalent in patients presenting for cataract surgery as measured by objective tests for DED.Citation1 Cataract surgery has been implicated in causing or worsening DED.Citation8,Citation50–52 According to a few studies, 50% of patients presenting to cataract clinics with meibomian gland dysfunction were asymptomatic and 77% of patients with DED had positive corneal staining.Citation19,Citation20 A compromised ocular surface affects the preoperative planning including keratometry and topography measurements, IOL power calculations, and toric IOL axis and magnitude. Cataract surgeons should be cognizant of the fact that intraoperative factors like antiseptic procedures, dilating drops and solutions, anesthesia type and duration, surgical technique (small incision cataract surgery/phacoemulsification), length of the incision, phacoemulsification technique, surgical time and duration of intraoperative exposure to light can cause or worsen dry eye postoperatively, especially among those vulnerable or with subclinical disease.Citation53 Also, DED might affect visual recovery after surgery, leading to refractive surprises and affecting the postoperative outcomes. Hence, ophthalmic practitioners must recognize, diagnose and manage DED optimally before planning cataract surgery. As visual outcomes would be better if ocular surface dysfunction is treated before surgery, it is worth considering preoperative screening for DED and tear film instability, especially in those patients where premium IOLs are being planned.Citation1
OPTIMIZATION OF THE OCULAR SURFACE BEFORE CATARACT SURGERY IN EYES WITH DED
The secondary level of prevention in DED aims at early and effective management of DED for reducing ocular morbidity.Citation54 Proper optimization of the ocular surface before performing surgery usually has good visual outcomes with a low rate of complications intraoperatively.Citation55 Artificial tears remain the first-line therapy in the medical management of DED, but it may have to be supplemented with other forms of treatment, based on the underlying mechanisms of dry eye. In the case of MGD, eyelid hygiene, warm compresses, and lid massage is the mainstay of therapy. Additionally, an option of using a short course of topical or systemic antibiotics, fatty acid intake, androgen use, intense pulsed light therapy, or thermal pulsation therapy may be tried. In cases where ocular surface inflammation is evident, a short-term regimen of non-preserved low potency steroids, cyclosporine A and lifitegrast have consistently produced encouraging outcomesCitation56,Citation57 and may be proposed as a supplementary treatment in moderate-to-severe cases.Citation58,Citation59 However, it should be borne in mind that usage of artificial tears or any topical ophthalmic medication just before keratometry should be discouraged due to its impact on astigmatism measurements in dry eyes.Citation45 The use of Rebamipide for mucin deficient DED and lipid-based formulations for EDE have been found to have a promising effect on the improvement of the ocular surface and the tear film.Citation18,Citation21 Rebamipide is effective in improving the higher-order aberrations and discrepancies in astigmatism power and axis as compared to artificial tears thereby enhancing the accuracy of intraocular lens power calculations in patients with dry eyes.Citation48
Certain preoperative considerations like avoiding epitheliotoxic non-steroidal anti-inflammatory agents (NSAIDs), avoiding any benzalkonium chloride (BAK/BKC) containing eye drops, treatment with artificial tears and treatment of pre-existing meibomian gland dysfunction before surgery will help in improving postoperative outcomes.Citation60
Preoperative ocular surface irregularity leads to variability in keratometry and improper selection of intraocular lens leading to refractive surprises. Therefore, improving the ocular surface in dry eye patients will lead to greater accuracy in lens power selection.Citation47,Citation61,Citation62 A study conducted by Hovesian et al. assessed the effect of 0.09% cyclosporine on regularisation of the ocular surface and the predictive accuracy of preoperative keratometry measurements in patients with dry eye presenting for cataract surgery. The study concluded that patients who were prescribed 0.09% cyclosporine preoperatively showed significantly improved accuracy in prediction error of spherical equivalent outcome following surgery.Citation46 Dissatisfaction among patients implanted with multifocal IOL has commonly been linked to HOA. The study by Hovesian et al.,Citation46 also found out that pretreatment with 0.09% cyclosporine results in significant improvement of root mean square HOA. A similar patient population was recruited by the same group and DED was treated with lifitegrast.Citation47 In both the studies it was evident that there was a significant improvement in predictive spherical equivalent power for IOL implants, and pretreatment with pharmacological agents approved for DED significantly improved clinical signs of severity in DED. Both the studies help us to reiterate the fact that adequate treatment of DED plays a vital role before performing ocular biometry to achieve optimal visual outcomes.
SUMMARY
DED is associated with variability in keratometry and poor repeatability of IOL power calculations, often resulting in refractive surprise and dissatisfaction among patients after cataract surgery. DED is implicated in the deterioration of optical quality in the form of dynamic fluctuations over the anterior corneal surface, variable keratometry magnitude and axis of astigmatism, induction of HOA due to tear film instability, and ocular surface inflammation.
Objective as well as subjective testing of dry eye in the form of dry eye questionnaires, Schirmer’s testing, TBUT analysis and tear film osmolarity before cataract surgery in a vulnerable subgroup of patients is recommended. This will help us in diagnosing and treating pre-existing dry eyes adequately before cataract surgery that can help attain more optimal and satisfactory visual outcomes. Adequate control of DED before cataract surgery with medications like artificial tears, topical cyclosporine, lifitegrast, warm compresses and lid massage would certainly help in reducing preoperative biometric fluctuations and postoperative aggravation of dry eye symptomatology. Instillation of topical medications just before biometry should be discouraged and it is recommended to repeat the biometry multiple times till minimal fluctuations are seen to have more reliable results. Future studies aimed to understand the various factors that impact the ocular biometry and modifications that can help us arrive at more reliable IOL power calculations in eyes with DED can help achieve optimal results.
Abbreviations
TBUT: tear film break-up time, LG: lacrimal gland, OSDI: Ocular Surface Disease Index
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Gupta P, Drinkwater O, VanDusen K, Brissette A, Starr C. Prevalence of ocular surface dysfunction in patients presenting for cataract surgery evaluation. J Cataract Refract Surg. 2018;44(9):1090–1096. doi:10.1016/j.jcrs.2018.06.026.
- Paulsen AJ, Cruickshanks KJ, Fischer ME, et al. Dry eye in the Beaver Dam offspring study: prevalence, risk factors, and health-related quality of life. Am J Ophthal. 2014;157:799–806. doi:10.1016/j.ajo.2013.12.023.
- Stapleton F, Alves M, Bunya VY, et al. TFOSDEWS II epidemiology report. Ocul Surf. 2017;15:334–365. doi:10.1016/j.jtos.2017.05.003.
- Titiyal JS, Falera RC, Kaur M, Sharma V, Sharma N. Prevalence and risk factors of dry eye disease in North India: ocular surface disease index based cross-sectional hospital study. Indian J Ophthal. 2018;66:207–211. doi:10.4103/ijo.IJO_698_17.
- Donthineni PR, Kammari P, Shanbhag SS, Singh V, Das AV, Basu S. Incidence, demographics, types and risk factors of dry eye disease in India: electronic medical records driven big data analytics I. Ocul Surf. 2019 Apr;17(2):250–256. doi:10.1016/j.jtos.2019.02.007.
- Craig J, Nichols K, Akpek E, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–283. doi:10.1016/j.jtos.2017.05.008.
- Gayton JL. Etiology, prevalence, and treatment of dry eye disease. Clin Ophthalmol. 2009;3:405–412. 28. doi:10.2147/OPTH.S5555.
- The epidemiology of dry eye disease: report of the epidemiology subcommittee of the international dry eye workshop. Ocul Surf. 2007;5(2):93–107. doi:10.1016/S1542-0124(12)70082-4.
- Miljanovic B, Dana R, Sullivan DA, et al. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007;143:409–415. doi:10.1016/j.ajo.2006.11.060.
- Li M, Gong L, Chapin WJ, et al. Assessment of vision related quality of life in dry eye patient. Invest Ophthalmol Vis Sci. 2012;53:5722–5727. doi:10.1167/iovs.11-9094.
- Willcox M, Argueso P, Georgiev G, et al. TFOS DEWS II tear film report. Ocul Surf. 2017;15(3):366–403. doi:10.1016/j.jtos.2017.03.006.
- Koh S. Irregular astigmatism and higher-order aberrations in eyes with dry eye disease. Invest Ophthalmol Vis Sci. 2018;59(14):DES36–DES40. doi:10.1167/iovs.17-23500.
- Epitropoulos AT, Matossian C, Berdy GJ, et al. Effect of tear osmolarity on repeatability of keratometry for cataract surgery planning. J Cataract Refract Surg. 2015;41(8):1672–1677. doi:10.1016/j.jcrs.2015.01.016.
- Koh S, Tung C, Inoue Y, et al. Effects of tear film dynamics on quality of vision. Br J Ophthalmol. 2018;102(12):1615–1620. doi:10.1136/bjophthalmol-2018-312333.
- Day AC, Donachie PHJ, Sparrow JM, et al. The royal college of ophthalmologists’ national ophthalmology database study of cataract surgery: report 1, visual outcomes and complications. Eye. 2015;29:552–560. doi:10.1038/eye.2015.3.
- Addisu Z, Solomon B. Patients’ preoperative expectation and outcome of cataract surgery at Jimma University specialized hospital – department of ophthalmology. Ethiop J Health Sci. 2011;21:47–55. doi:10.4314/ejhs.v21i1.69044.
- Pager CK. Expectations and outcomes in cataract surgery: a prospective test of 2 models of satisfaction. Arch Ophthalmol. 2004;122:1788–1792. doi:10.1001/archopht.122.12.1788.
- Craig J, Muntz A, Wang M, et al. Developing evidence-based guidance for the treatment of drye eye disease with artificial tear supplements: a six-month multicentre, double masked randomised controlled trial. Ocul Surf. 2021;20:62–69. doi:10.1016/j.jtos.2020.12.006.
- Trattler W, Majmudar P, Donnenfeld E, McDonald M, Stonecipher K, Goldberg D. The prospective health assessment of cataract patients’ ocular surface (PHACO) study: the effect of dry eye. Clin Ophthalmol. 2017;11:1423–1430. doi:10.2147/OPTH.S120159.
- Cochener B, Cassan A, Omiel L. Prevalence of meibomian gland dysfunction at the time of cataract surgery. J Cataract Refract Surg. 2018;44:144–148.
- Dasgupta S, Gupta R. The course of dry eye following phacoemulsification and manual-SICS: a prospective study based on Indian scenario. Int Eye Sci. 2016;16:17891794.
- Choi YJ, Park SY, Jun I, et al. Perioperative ocular parameters associated with persistent dry eye symptoms after cataract surgery. Cornea. 2018;37(6):734–739. doi:10.1097/ICO.0000000000001572.
- Kim J, Kim M, Ha Y, Paik H, Kim D. Improved accuracy of intraocular lens power calculation by preoperative management of dry eye disease. BMC Ophthalmol. 2021; 21(1): doi: 10.1186/s12886-021-02129-5.
- Klyce SD. Computer-assisted corneal topography. High-resolution graphic presentation and analysis of keratoscopy. Invest Ophthalmol Vis Sci. 1984;25:1426–1435.
- Goto T, Zheng X, Klyce SD, et al. A new method for tear film stability analysis using videokeratography. Am J Ophthalmol. 2003;135:607–612. doi:10.1016/S0002-9394(02)02221-3.
- Cronje-Dunn S, Harris WF. Keratometric variation: the influence of a fluid layer. Ophthalmic Physiol Opt. 1996;16:234–236. doi:10.1046/j.1475-1313.1996.95001174.x.
- Chen S, Wang IJ. Effect of tear film stability on fluctuation of vision after photorefractive keratectomy. J Refract Surg. 1999;15:668–672. doi:10.3928/1081-597X-19991101-11.
- Buehren T, Collins MJ, Iskander DR, et al. The stability of corneal topography in the post-blink interval. Cornea. 2001;20:826–833. doi:10.1097/00003226-200111000-00010.
- Liu Z, Pflugfelder S. Corneal surface regularity and the effect of artificial tears in aqueous tear deficiency. Ophthalmology. 1999;106:939–943. doi:10.1016/S0161-6420(99)00513-8.
- Huang FC, Tseng SH, Shih MH, Chen FK. Effect of artificial tears on corneal surface regularity, contrast sensitivity, and glare disability in dry eyes. Ophthalmology. 2002;109:1934–1940. doi:10.1016/S0161-6420(02)01136-3.
- de Paiva Cs, Lindsey JL, Pflugfelder SC. Assessing the severity of keratitis sicca with videokeratoscopic indices. Ophthalmology. 2003;110:1102–1109. doi:10.1016/S0161-6420(03)00245-8.
- Kojima T, Ishida R, Dogru M, et al. A new noninvasive tear stability analysis system for the assessment of dry eyes. Invest Ophthalmol Vis Sci. 2004;45:1369–1374. doi:10.1167/iovs.03-0712.
- Denoyer A, Rabut G, Baudouin C. Tear film aberration dynamics and vision-related quality of life in patients with dry eye disease. Ophthalmology. 2012;119:1811–1818. doi:10.1016/j.ophtha.2012.03.004.
- Deschamps N, Ricaud X, Rabut G, et al. The impact of dry eye disease on visual performance while driving. Am J Ophthalmol. 2013;156:18. doi:10.1016/j.ajo.2013.02.019.
- Koh S, Maeda N, Hirohara Y, et al. Serial measurements of higher-order aberrations after blinking in normal subjects. Invest Ophthalmol Vis Sci. 2006;47:3318–3324. doi:10.1167/iovs.06-0018.
- Koh S, Maeda N, Hori Y, et al. Effects of suppression of blinking on quality of vision in borderline cases of evaporative dry eye. Cornea. 2008;27:275–278. doi:10.1097/ICO.0b013e31815be9c8.
- Koh S, Maeda N, Hirohara Y, et al. Serial measurements of higher-order aberrations after blinking in patients with dry eye. Invest Ophthalmol Vis Sci. 2008;49:133–138. doi:10.1167/iovs.07-0762.
- Kaido M, Matsumoto Y, Shigeno Y, Ishida R, Dogru M, Tsubota K. Corneal fluorescein staining correlates with visual function in dry eye patients. Invest Ophthalmol Vis Sci. 2011;52:9516–9522. doi:10.1167/iovs.11-8412.
- Toda I, Shimazaki J, Tsubota K. Dry eye with only decreased tear breakup time is sometimes associated with allergic conjunctivitis. Ophthalmology. 1995;102:302–309. doi:10.1016/S0161-6420(95)31024-X.
- Kaido M, Ishida R, Dogru M, Tsubota K. The relation of functional visual acuity measurement methodology to tear functions and ocular surface status. Jpn J Ophthalmol. 2011;55:451–459.
- Shimazaki-Den S, Dogru M, Higa K, Shimazaki J. Symptoms, visual function, and mucin expression of eyes with tear film instability. Cornea. 2013;32:1211–1218. doi:10.1097/ICO.0b013e318295a2a5.
- Koh S, Maeda N, Ikeda C, et al. Ocular forward light scattering and corneal backward light scattering in patients with dry eye. Invest Ophthalmol Vis Sci. 2014;55:6601–6606. doi:10.1167/iovs.14-15125.
- The Royal College of Ophthalmologists Cataract Surgery Guidelines. [ Online] 2010. Available from http://www.rcophth.ac.uk/core/core-picker/download.asp?id=544&filetitle=Cataract+Surgery+Guidelines+2010. (Accessed 21 September 2013).
- Kim P, Plugfelder S, Slomovic AR. Top 5 pearls to consider when implanting advanced-technology IOLs in patients with ocular surface disease. Int Ophthalmol Clin. 2012;52(2):51–58. doi:10.1097/IIO.0b013e31824b4504.
- Roggla V, Leydolt C, Schartmuller D, et al. Influence of artificial teras on keratometric measurements in cataract patients. Am J Ophthalmol. 2021;221:1–8. doi:10.1016/j.ajo.2020.08.024.
- Hovanesian J, Berdy G, Epitropoulos A, Holladay J. Effect of cyclosporine 0.09% treatment on accuracy of preoperative biometry and higher order aberrations in dry eye patients undergoing cataract surgery. Clin Ophthalmol. 2021;15:3679–3686. doi:10.2147/OPTH.S325659.
- Hovanesian J, Epitropoulos A, Donnenfeld ED, Holladay JT. The effect of lifitegrast on refractive accuracy and symptoms in dry eye patients undergoing cataract surgery. Clin Ophthalmol. 2020;14:2709–2716. doi:10.2147/OPTH.S264520.
- Teshigawara T, Meguro A, Mizuki N. Effects of rebamipide on difference in power and axis of corneal astigmatism between two intra-patient keratometric measurements in dry eyes. Ophthalmol Ther. 2021;10:891–904. Online ahead of print. doi:10.1007/s40123-021-00368-9.
- Dogan A, Gurdal C, Kovlu M. Does dry eye affect repeatability of corneal topography measurements? Turk Oftalmoloji Dergisi. 2018;57–60. doi:10.4274/tjo.10179.
- Khanal S, Tomlinson A, Esakowitz L, et al. Changes in corneal sensitivity and tear physiology after phacoemulsification. Ophthalmic Physiol Opt. 2008;28:127–134 4. doi:10.1111/j.1475-1313.2008.00539.x.
- Kasetsuwan N, Satitpitakul V, Changul T, Jariyakosol S. Incidence and pattern of dry eye after cataract surgery. PLoS One. 2013;8(11):e78657. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3827040/pdf/pone.0078657.pdf
- X-M L, Hu L, Hu J, Wang W. Investigation of dry eye disease and analysis of the pathogenic factors in patients after cataract surgery. Cornea. 2007;26(suppl 1):S16–S20. doi:10.1097/ICO.0b013e31812f67ca.
- Cho YK, Kim MS. Dry eye after cataract surgery associated and intraoperative risk factors. Korean J Ophthalmol. 2009;23:65–73. doi:10.3341/kjo.2009.23.2.65.
- Donthineni PR, Shanbhag SS, Basu S. An evidence-based strategic approach to prevention and treatment of dry eye disease, a modern global epidemic. Healthcare (Basel). 2021 Jan 17;9(1):89.
- Donthineni PR, Das AV, Shanbhag SS, Basu S. Cataract surgery in dry eye disease: visual outcomes and complications. Front Med (Lausanne). 2020 Oct 7;7:575834. doi:10.3389/fmed.2020.575834.
- Labetoulle M, Leonardi A, Amrane M, et al. Persistence of efficacy of 0.1% cyclosporin A cationic emulsion in subjects with severe keratitis due to dry eye disease: a nonrandomized open-label extension of the SANSIKA study. Clin Ther. 2018;40:1894–1906. doi:10.1016/j.clinthera.2018.09.012.
- Holland EJ, Luchs J, Karpecki PM, et al. Lifitegrast for the treatment of dry eye disease: results of a phase III, randomized, double-masked. Placebo-controlled trial (OPUS-3). Ophthalmology. 2017;124:53–60. doi:10.1016/j.ophtha.2016.09.025.
- Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15:575–628. doi:10.1016/j.jtos.2017.05.006.
- Labetoulle M, Baudouin C. From pathogenic considerations to a simplified decision-making schema in dry eye disease. J Fr Ophtalmol. 2013;36:543–547. doi:10.1016/j.jfo.2013.03.005.
- Labetoulle M, Rousseau A, Baudouin C. Management of dry eye disease to optimize cataract surgery outcomes: two tables for a daily clinical practice. J Fr Ophtalmol. 2019;42(8):907–912. doi:10.1016/j.jfo.2019.03.032.
- Sheard R. Optimising biometry for best outcomes in cataract surgery. Eye. 2014;28(2):118–125. doi:10.1038/eye.2013.248.
- Sahin A, Hamrah P. Clinically relevant biometry. Curr Opin Ophthalmol. 2012;23(1):47–53. doi:10.1097/ICU.0b013e32834cd63e.
