ABSTRACT
Background
Culture negative (CN) but presumed infectious endophthalmitis poses a huge diagnostic challenge in terms of clinical management. This article outlines the current state of knowledge of infectious endophthalmitis with negative cultures and summarizes the recommendations for the work up of this condition along with providing a simple algorithm, by putting into context the recent concerns about over-diagnosing endophthalmitis.
Methods
We searched the PubMed and Scopus databases for large hospital based studies on diagnosis of endophthalmitis, with emphasis on culture-negative infections in October 2021. Only clinical studies written in English were included. Basic science studies, letters to the editor and case reports on endophthalmitis were excluded.
Results
Twenty studies were included in this study. The prevalence of CN endophthalmitis ranged from 40% to 70%. Recent advances in PCR along with high throughput sequencing have helped identify the etiological agent in most cases but these technologies are not easily available, requires advanced bioinformatic analysis and are not cost effective. Role of other inflammatory and relatively low-cost biomarkers in diagnosing a presumed infection is yet to be validated clinically but hold promise in helping ophthalmologists identify the causative agent.
Conclusions
CN endophthalmitis is a relatively frequent finding and should not be labelled as sterile endophthalmitis. Recent advances provide a new perspective for ophthalmologist in diagnosis of presumed infectious endophthalmitis and further studies are needed to confirm their utility in clinical settings.
INTRODUCTION
Endophthalmitis is a vision-threatening complication of ocular surgeries or penetrating eye injuries and rarely by hematogenous spread from a distant site.Citation1 Studies on the overall incidence of endophthalmitis have differed widely. In western countries like USA, the incidence reported for post-surgical endophthalmitis was 0.05%,Citation2 whereas, post-traumatic endophthalmitis cases range between 3% and 17%. Similarly, in South-Asian countries, the incidence of post-operative endophthalmitis was 0.03–0.07%.Citation3 However, in India it was reported to be 0.02–0.09%.Citation4–7 Comparatively, post-traumatic endophthalmitis in Asian countries had a reported incidence of 2–5%.Citation8,Citation9 Likewise, among endogenous cases the incidence ranges from 2% to 15%.Citation10–12 The severity and clinical course of endophthalmitis depends on multiple factors like load and virulence of the inciting pathogen, time of presentation of infection and pre-operative measures taken. The etiology of endophthalmitis varies worldwide with Gram-positive bacteria being the predominant cause of endophthalmitis in the United States and Gram-negative bacteria and filamentous fungi in India.Citation13 Characteristic findings on examination include haze along with conjunctival congestion, and a hypopyon in majority of the cases.Citation14 The current management of suspected endophthalmitis involves “tap and inject”, which requires withdrawal of ocular fluid (aqueous or vitreous) and simultaneous injection of antibiotics. We and others have reported that only about ~30–50% of ocular fluids show culture positivity.Citation10,Citation15 A retrospective study of patients with endogenous endophthalmitis demonstrated that the positivity of intraocular fluid cultures was 28.6% overall, which decreased to 0% after systemic antibiotics was initiated.
Over the past few years, improved microbiological techniques, advanced surgical standards, and development of potent drugs, have led to a declined endophthalmitis incidence rate but culture-negative endophthalmitis continues to represent a serious threat in the field of ophthalmology.Citation13 As a result, many cases of suspected intraocular infections fail to respond due to incorrect and/or delayed antimicrobial therapy further leading to severe inflammation within the eye along with retinal injury and irreversible loss of vision. In addition, many non-infectious etiologies such as sterile endophthalmitis can mimic infectious endophthalmitis making diagnosis challenging. Due to overlapping features, it challenging to differentiate these conditions.Citation16 Thus, rapid accurate diagnostic technologies are needed for the identification of these non-cultivable pathogens from vitreous of patients with presumed infectious endophthalmitis. In the past decade, several techniques like metabolite biomarkers and advanced molecular sequencing have shown its utility in diagnostic microbiology by reducing the turnaround time and increasing positivity rate. Potential application of these techniques include distinguishing infectious from non-infectious inflammation as well as monitoring response therapy and outcome, all of which can aid the ophthalmologists in precise diagnosis and prognosis of patients with clinically presumed infectious endophthalmitis. The aim of the present study was to systematically review previous literature on culture-negative presumed infectious endophthalmitis, evaluate clinical outcomes, newer advances in diagnosis along with role of biomarkers, with an intention to understand the disease entities for successful treatment.
METHODS AND LITERATURE REVIEW
A systematic electronic search was performed using the MEDLINE via PubMed and the Scopus databases. The search was carried out in October 2021 using the following key search terms: “endophthalmitis”, “culture negative”, “sterile endophthalmitis”, “next generation sequencing”, and “biomarkers”. The search was restricted to English language publications regardless of the year of publication. Unpublished data (except our own), case reports, letters to editor, and instructional courses were not included. A total of 665 reports were revealed and included a huge number of case series, hence we further shortlisted to articles pertaining to diagnosis and biomarkers and finally 20 reports have been selected which are discussed in the subsequent paragraphs. The reference lists of the selected studies were further investigated for the identification of additional studies missed by the electronic search.
CULTURE-NEGATIVE (CN) PRESUMED INFECTIOUS ENDOPHTHALMITIS: A NEW ENTITY
A study conducted at our institute, which is a tertiary referral hospital in South India, showed that while culture proven bacterial and fungal cases accounted for 35.7% and 11.6% of the total cases seen, respectively, there was a steady increase in the proportion of culture-negative or presumed infectious cases from ~30% to over 70% in 25 years.Citation15 While the reasons for culture-negative endophthalmitis is not known completely, possible explanations include, infections with unculturable pathogens or immunological reaction to scant bacteria.Citation17 Some authors have also hypothesized that presence of fastidious or slow growing pathogens and prior systemic or topic antibiotic therapy that may inhibit microbial growth during culture.Citation17,Citation18 A study by Das et al. (2020), reported the role of small sample size, delay in processing the ocular samples, over diagnosis and sequestration of microorganisms in the capsular bag as other factors which might play an important role in the causation of culture negativity.Citation19 The other differential diagnosis includes a true “sterile” endophthalmitis, a variant of toxic anterior segment syndrome called Toxic Posterior Segment Syndrome (TPSS), or masquerade syndromes.Citation20
Clinical Outcomes of Culture-Negative Versus Culture-Positive Endophthalmitis
Traditional microbiology work up includes processing the undiluted vitreous sample for direct microscopy examination by Gram and Calcofluor white staining along with culture on an array of media including blood and chocolate agar, Potato/Sabouraud dextrose agar along with aerobic and anaerobic liquid media to allow growth of all types of fastidious and slow growing pathogens.Citation21 While bacterial and fungal isolates grown are identified based on microscopic features and colony characteristics followed by automated methods such as Vitek 2 compact system, or API for species identification. Antibiotic susceptibility testing for the culture grown isolates is then carried out and reported. A samples is declared culture negative when there is no growth of any organism after 7–14 days of incubation on any of the culture media. Comparing the initial clinical diagnosis of patients with culture-positive and -negative endophthalmitis, it has been reported that decreased vision, presence or absence of pain or hypopyon, vitritis and the interval between trauma or surgery and presentation does not help to distinguish culture-positive from culture-negative cases. While, achieving good visual recovery post treatment with pathogens like Staphylococcus aureus or Pseudomonas sp. infection is rare, Lobue. (2016) proposed that culture-negative fulminant endophthalmitis had a good visual outcome post treatment which may be attributed to a non-infectious toxic insultCitation22. Another study by Parver et al. (2003), reported that while the treatment success rate was 60% in culture-negative cases, comparatively no success was observed in culture-positive cases in their series.Citation23 Thus, as a rule of thumb, culture-positive endophthalmitis lead to change in clinical management and additional interventions compared to culture-negative infections.
Pathogenesis of CN Endophthalmitis: Role of Torque-Teno Virus (TTV)
Torque teno virus (TTV), and TTV-like mini virus (TLMV) are human anelloviruses, which are characterized by their prevalence worldwide, with literature having contradictory opinions on their pathogenic role.Citation24 Using RT-qPCR, the presence of TTV was detected in 63.4% of culture-negative vitreous samples in addition to culture-positive and non-infectious controls. Additionally, significantly higher repeat antibiotic injections were observed in patients who were TTV and /or TLMV positive, however significant correlation was not found in case of culture-negative cases. Lee and his colleagues (2014) evaluated 21 cases (11 culture positive and 10 culture negative) of endophthalmitis postoperatively using 16S metagenomics and BRiSK and found the presence of TTV in culture-negative specimens only.Citation25 They also suggested that the presence of TTV in the vitreous, indicates breakdown of the blood-retinal barrier due to endophthalmitis, thereby allowing TTV to enter the eye from the serum, or it could just be a marker of fulminant inflammation or bystander during leukocytic infiltration. In comparison, our results found this virus was found in non-infectious controls as well, indicating a possible stratification of TTV in the Indian population. Altogether, this study provides a novel insight into the prevalence and intra-host transmission of TTV in culture-negative endophthalmitis.Citation24
Comparative Assessment of Cytokines in CN Endophthalmitis
Culture-negativity being a challenging entity, specific cytokine biomarker might help in distinguishing culture-negative endophthalmitis from non-infectious endophthalmitis.Citation26 Our group had assessed the utility of 16 immune mediators as biomarkers for the diagnosis of infectious endophthalmitis. A comparative expression of various vitreal fluid cytokines with culture-positive and culture-negative endophthalmitis showed mixed clusters but they were distinctly separate from non-infectious samples. This result indicated that culture proven and culture-negative vitreous samples have similar cytokine and chemokine profiles. Additionally, immune mediators like IL-1RA alpha, IL-6, IL-8, and GM-CSF exhibited a significant difference between culture negative and control group which was postulated to serve as biomarkers for diagnosing culture negative but presumed infectious endophthalmitis. However, Otsuka et al. (2013) reported that in endophthalmitis associated with intravitreal triamcinolone acetonide (IVTA) group, which are potential sterile endophthalmitis cases, levels of IL-6 and IL-8 were elevated in eyes with inflammation compared to those without inflammation. Further studies on cytokine profiling are required from other cohorts to determine its utility as diagnostic biomarkers.Citation27
DIAGNOSTIC APPROACH TO CN ENDOPHTHALMITIS
Molecular Methods: PCR
Several studies have evaluated the utility of bacterial ribosomal 16S polymerase chain reaction (PCR) combined with sanger sequencing for detecting bacterial etiologies in endophthalmitis has been examined. In a study by Therese et al. (1998), of the 58 samples included, 53.5% were negative for any infective pathology by traditional microbiological methods, and 44.7% of the 38 bacteriologically negative specimens showed positivity for eubacterial genome by nested PCR. This method increased the diagnostic sensitivity from 46.5% to 75.8% for proving an infectious aetiology in vitreous fluids.Citation28 Another Study by Chiquet et al. (2008) showed that PCR detected bacteria in 70% of the cases including culture-negative cases in contrast to culture where it was only positive in 7% of the total cases included.Citation29 Pongsachareonnont P et al. (2017) reported that PCR analysis identified Haemophilus species in two vitreous specimens collected from patients with endophthalmitis, which was missed by both microbiological and blood culture methods.Citation30 This postulated that Haemophilus sp. are fastidious organisms, and that the sample probably had low bacterial load, leading to failure in detection by both methods of cultivation. They further reported that, Streptococcus species was also missed in two specimens and coagulase-negative staphylococci in one specimen, which could be due to the low volume of fluid collected, or the pathogen load was below the limit of detection. Several authors have additionally hypothesized that unsuitable DNA extraction methods could lead to inhibited in detection, or inhibitors which are present in the sample could also cause the amplification process to fail and all of which accentuates the drawback of the PCR technique. Thus while PCR and sequencing approach results in rapid identification of infectious etiology from a small amount of fluid, the reagents and equipment required is expensive and produces a high rate of false-positive results.Citation30
Newer Advances in Diagnostic Microbiology – Next-Generation Sequencing
To further understand the etiology of culture-negative endophthalmitis and to increase sensitivity in diagnosing culture-negative endophthalmitis, many recent studies have reported usage of advanced unbiased sequencing techniques (). Next-generation sequencing (NGS) is a high-throughput technique, which allows for an unbiased approach to detect pathogens and its application in microbiology settings are manifold.Citation31 Since then many other authors have explored the use of this technique and have been enlisted in . Since this technique is culture independent, a new perspective for detection of pathogens has emerged in a clinical setting. Our earlier pilot study showed that, 73.6% of the culture-negative cases showed presence of bacterial pathogens, and among them only 21% were monobacterial while the rest showed presence of polybacterial infections. Interestingly, the cases that were culture negative, revealed presence of routine bacteria that grows in culture like Pseudomonas sp., Streptococcus sp., Staphylococcus sp., Haemophilus sp., Acinetobacter sp. and Gemella sp. Similarly, the fungal DNA identified in the three samples which were culture negative also had predominantly culturable pathogens like Aspergillus sp., Malassezia sp., Scedosporium sp., and Candida sp.Citation17 Another study using high-throughput sequencing analysis, with increased number of clinical samples of endophthalmitis including culture-negative samples, found a higher rate of fungal DNA which were mostly Aspergillus sp.Citation32 While Streptococcus sp., Staphylococcus sp., Bacillus sp., and Klebsiella sp., were also found to be present in some of the culture-negative cases, a larger proportion belonged to the fungal category, signifying the tropical environment favouring growth and infection by these pathogens. Huang and group (2021) used Nanopore targeted sequencing (NTS) to study the feasibility of diagnosing infectious etiologies in culture-negative endophthalmitis. They found that NTS identified pathogens in 17 /18 cases (94.4%) with a high agreement between culture and NTS for culture-positive cases. They also reported that the average turn around times for reporting the results of cultures were 48–72 h, compared to NTS results, which was available in 12 h. Hence they proposed that NTS can be used as promising diagnostic platform for diagnosing culture-negative cases.Citation33 Mishra et al. (2021) examined vitreous fluids from 16 culture-negative patients by targeted metagenomics and found Operational taxonomic unit (OTUs) of multiple bacterial genera in 13 vitreous fluids from culture-negative patients and OTUs of a one bacterial genera in 2 culture negative and one culture-positive specimens; while one negative specimen had no bacterial DNA. All the organisms identified belonged to clinically relevant species.Citation34 Similarly, Lee et al. (2020), analysed 24 cases which were culture positive and 26 cases which were culture negative, using whole-genome sequencing, and detected infectious etiologies in 33% of culture-negative cases. Additionally, they also compared the visual outcome where they observed no variation in pathogen-positive cases versus pathogen-negative cases.Citation35
Table 1. Studies on the role of next-generation sequencing technology in the diagnosis of culture-negative endophthalmitis.
Thus nucleic acid extraction followed by next-generation sequencing may ultimately reveal diagnostic clues with regard to the causative pathogen in patient samples. NGS is proving to be a valuable tool especially in the identification of polymicrobial infections and provides better sensitivity and specificity.Citation17 The metagenomics sequencing of the non-cultivable microorganism may also provide information regarding virulence-associated genes and antibiotic resistance. Currently NGS is expensive and special bioinformatic skills are required for downstream processing and result interpretation. With decreasing cost and standardized bioinformatics pipeline NGS could be a feasible diagnostic tool in routine ocular microbiological laboratories.Citation34,Citation36
ROLE OF INFECTION BIOMARKERS
Biomarkers have become an important tool for decision-making process in clinical setting. Potential applications of biomarkers in infectious diseases include distinguishing infectious from sterile or non-infectious samples, monitoring therapeutic response, and predicting clinical outcomes. Biomarker should help establish a quick and reliable diagnosis in routine clinical practices. It provides an indication of disease prognosis because its levels remain elevated during prolonged and successive infections and it is always responsive to the infectious stimulus.Citation37
To be of greatest practical value, a candidate assay for a biomarker needs to be both reliable and reproducible, ideally rapid, easy and inexpensive to perform. Ilhan et al. (2021) recently investigated the changes in systemic inflammatory biomarkers, including neutrophil, lymphocyte, monocyte, and platelet counts, mean platelet volume (MPV), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) in post-operative endophthalmitis cases.Citation38 The authors found only NLR value of ≥2.10 as a significant predictor of endophthalmitis with a sensitivity and specificity of 72% and 63%, respectively. Serum inflammatory markers are commonly used clinically as an adjunct for early surveillance and diagnosis of invasive infections especially (1,3)-β-D glucan for predicting fungal etiology.Citation39 Other markers evaluated by our group include vitreous D-lactate and Procalcitonin for the diagnosis of bacterial endophthalmitisCitation40,Citation41 and Galactomannan and (1,3)-β-Dglucan for identification of fungal endophthalmitis (Unpublished data).
D-lactate and Procalcitonin (PCT)
D-lactate is one of the by-product of lower forms of life, its presence in sterile body fluid indicates bacterial infection. Our group tested the utility of D-lactate utility in 41 vitreous fluids from patients presumed clinically as infectious endophthalmitis and 20 samples from non-infectious retinal conditions by a simple colorimetric assay kit and calculated the receiver operating characteristic curves (ROC) of D-lactate. We reported that at a concentration greater than 47.07ng/μl, bacterial and culture-negative endophthalmitis were distinguishable with a 80% sensitivity and 100% specificity. We confirmed our findings of D-lactate production in patients with bacterial endophthalmitis in mouse models of endophthalmitis infected with bacterial pathogens. Therefore, measurement of D-lactate can be used as biomarker for identifying infectious culture-negative casesCitation40 and the measurement of D-lactate requires only a spectrophotometer which can be easily adapted in most basic clinical laboratories.
Several reports have reported the relation between elevated PCT levels and infection and is widely used as a marker of sepsis and systemic bacterial infections.Citation42–44 Our group evaluated the role of vitreous PCT as a diagnostic biomarker in 55 patients with infectious endophthalmitis and included 19 culture-negative samples using commercially available ELISA kits. We found that the average PCT in culture-negative cases (100.24 ± 12.9 pg mL-1) was higher than non-infectious controls (75.74 ± 26.8 pg mL-1) but was less than the levels found in culture-positive cases (126.41 ± 26.47 pg mL-1), which was not statistically significant. Therefore, the study concluded that unlike D-lactate, PCT is not a suitable biomarker of culture-negative casesCitation42 though it could help to differentiate between gram-positive and gram-negative infections in these cases. Larger studies from other populations are needed to test the true utility of this test.
Galactomannan (GM) and 1,3 β-D-Glucan (BDG)
Galactomannan (GM) is a polysaccharide and cell wall component in Aspergillus spp. and other closely related filamentous fungi. Dupont et al. (2020) reported that galactomannan testing of vitreous humour (index of 5.92) was useful for the diagnosis of fungal endophthalmitis in a case report wherein standard mycology was negative and PCR was not available, and an additional intravitreal injection of antifungal antibiotic along with systemic voriconazole therapy was reinitiated in a patient with endophthalmitis.Citation45 This process which allowed a rapid start of adapted antifungal treatment, helped prevent vision loss in that patient with good outcome. The authors also suggest that this antigen detection test can be run without delay, can be used by untrained staff and results can be available in less than an hour, while culture and PCR can take upto 48–72 hours.
Similarly, Beta-D-glucan is a cell wall component composed of polysaccharide which is found in several fungal species. During an invasive fungal infection, Beta-D-glucan is released into the bloodstream.Citation46 Serum BDG testing is used more routinely used in immune-compromised population as a means of early monitoring and diagnosis of invasive fungal infections. Ammar et al. (2021) evaluated serum beta-D-glucan (BDG) as a biomarker for endogenous fungal eye infection in four endophthalmitis patients. They reported that BDG levels ≥80 pg/mL could be considered test positive, with a sensitivity (95% confidence interval) of 100% (39.8–100%) and specificity of 74.4% (63.2–83.6%) for endophthalmitis. Thus with a cut-off value of ≥80 pg/mL for BDG and assuming a 1% endophthalmitis prevalence, the positive and negative predictive value was 3.8% (2.4%–5.2%) and 100% (99.1–100%), respectively.Citation47 Chen et al. (2020) reported a retrospective, observational case series of 5 fungal endophthalmitis cases, 4 of which were diagnosed as fungal endophthalmitis through microbiological analysis of intraocular fluid and all five had significantly elevated BDG levels, which was consistent with the pathological diagnosis. They quantified that BDG level below 100.5 pg/ml was negative and a BDG level above 151.5 pg/ml was positive, but these values depend on the various kits being used.Citation48 Kolomeyer et al. (2018) also suggested that ophthalmologists should consider using BDG testing as an adjunctive in cases of suspected fungal eye infections, especially when fever is also present or when the patient has a history of drug use.Citation39
Unpublished data by our group shows increased vitreous GM and BDG concentration in patients with culture-proven fungal endophthalmitis than culture-negative endophthalmitis group. The Area under the ROC curve (AUC) value for GM was 0.81 with a sensitivity and specificity of 0.88 and 0.73 for a cut-off value of 51.36 pg/ml, and the AUC value for BDG was 0.93 (95% CI: 0.84–0.1) with a sensitivity of 0.94 and a specificity of 0.82, respectively, with the cut-off value of 1.19 pg/ml. Similar to other studies, we report that these tests will be available is few hours and should be considered in combining with clinical and microbiological tests. Negative GM and BDG levels can help to potentially prevent resistance and toxicities arising due to usage of empiric antifungal therapy or additional diagnostic procedures in cases of low clinical suspicion. Larger clinical studies are needed to fully evaluate the utility of vitreous GM and BDG levels in diagnosing endophthalmitis in patients due to fungal etiologies.
SUMMARY
Endophthalmitis is an ocular emergency that requires immediate attention. However, prior antibiotic therapy and low load or fastidious nature of the organism leads to increased culture negativity, leading to a serious threat with regards to the prompt diagnosis and management and increase in anti-microbial resistance. While there are no current guidelines that exist to understand the utility and importance of various methods to diagnose culture-negative endophthalmitis, which are clinically presumed infectious. It is important to accurately rule out an infectious etiology by changing the current protocols that calls for only culture of vitreous or aqueous fluid. With the advent of polymerase chain reaction and next-generation sequencing, identifying the causative organism(s) has become easier but these techniques are not readily available, requires advanced bioinformatic analysis and are not cost effective. There are a number of more straightforward and relatively low-cost biomarkers to help ophthalmologists maximize the chances of diagnosing a presumed infection and identify the causative agent. When clinical suspicion of infectious endophthalmitis is high, especially for cases with negative cultures, we propose the following algorithm in , to be employed. The approach to detect these infections should include a complete microbiological work-up with a minimum of 4–6 cultures that are incubated for 14 days.
Figure 1. Schematic representation of diagnostic work-up for management of culture-negative endophthalmitis.
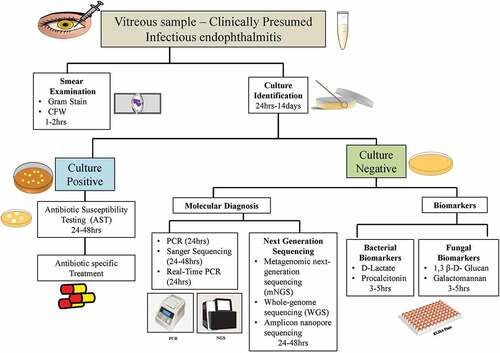
As shown, vitreous fluid is to be collected aseptically from all endophthalmitis cases in the operating room and immediately divided into two equal amounts and sent immediately for routine microbiological processing which includes smear examination and culture identification. Post culture identification, antibiotic susceptibility test is carried out, based on which the specific treatment is decided. The other half is further processed for PCR and biomarker based identification, especially if the fluid is negative after 7 days of incubation. This provides a plausible diagnostic approach when there is no prior knowledge of the bacterial species involved. For patients with both positive and completely negative results by PCR, it is further suggested for high-throughput sequencing for species identification. The microorganisms that are identified are classified as critical pathogens, or non-pathogenic microbes according to etiological trends and clinical guidelines in the literature. This particular identification technique takes around 5–6 days. Alternatively, biomarkers can be a useful surrogate for a fast and reliable diagnosis leading to timely management of patients with endophthalmitis. Biomarkers like D-lactate and procalcitonin are well-known useful markers in diagnosing bacterial and non-bacterial endophthalmitis. Likewise, in fungal suspected culture-negative cases B-glucan and galactomannan can be used as prompt diagnosis markers. Use of modern technologies for the identification of these microbes such as diagnostic biomarkers (D-lactate, procalcitonin, Galactomannan and 1,3, β- Glucan), assessment of cytokines level, and metagenomic sequencing approach should also be taken into consideration.
Though most clinical studies on culture-negative endophthalmitis, does not describe definite identification of infecting microorganisms or optimal treatment strategies, these techniques provide a new perspective for ophthalmologist in diagnosis of presumed infectious endophthalmitis in clinical settings. Further prospective studies need to be performed to establish the clinical utility of new technologies and biomarkers along with optimal treatment strategies for culture-negative infections.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Durand ML. Endophthalmitis. Clin Microbiol Infect. 2013;19(3):227–234. doi:10.1111/1469-0691.12118.
- Aaberg TM, Flynn HW, Schiffman J, Newton J. Nosocomial acute-onset postoperative endophthalmitis survey. A 10-year review of incidence and outcomes. Ophthalmology. 1998;105(6):1004–1010. doi:10.1016/S0161-6420(98)96000-6.
- Siak J, Win MZ, Chee SP. Epidemiology and Treatment Trend of Endophthalmitis in Asia. Singapore: Springer; 2018:29–44.
- Jambulingam M, Parameswaran SK, Lysa S, et al. A study on the incidence, microbiological analysis and investigations on the source of infection of postoperative infectious endophthalmitis in a tertiary care ophthalmic hospital: an 8-year study. Indian J Ophthalmol. 2010;58:297–302. doi:10.4103/0301-4738.64132.
- Das T, Hussain A, Naduvilath T, et al. Case control analyses of acute endophthalmitis after cataract surgery in South India associated with technique, patient care, and socioeconomic status. J Ophthalmol. 2012;2012:298459. doi:10.1155/2012/298459.
- Ravindran RD, Venkatesh R, Chang DF, et al. Incidence of post-cataract endophthalmitis at Aravind Eye Hospital: outcomes of more than 42,000 consecutive cases using standardized sterilization and prophylaxis protocols. J Cataract Refract Surg. 2009;35:629–636. doi:10.1016/j.jcrs.2009.01.002.
- Lalitha P, Rajagopalan J, Prakash K, et al. Postcataract endophthalmitis in South India incidence and outcome. Ophthalmology. 2005;112:1884–1889. doi:10.1016/j.ophtha.2005.05.020.
- Dehghani AR, Rezaei L, Salam H, et al. Post traumatic endophthalmitis: incidence and risk factors. Glob J Health Sci. 2014;6:68–72. doi:10.5539/gjhs.v6n6p68.
- Faghihi H, Hajizadeh F, Esfahani MR, et al. Posttraumatic endophthalmitis: report no. 2. Retina. 2012;32:146–151. doi:10.1097/IAE.0b013e3182180087.
- Dave VP, Pathengay A, Panchal B, et al. Clinical presentations, microbiology and management outcomes of culture-proven endogenous endophthalmitis in India. Indian J Ophthalmol. 2020;68(5):834–839. doi:10.4103/ijo.IJO_1091_19.
- Chee SP, Jap A. Endogenous endophthalmitis. Curr Opin Ophthalmol. 2001;12(6):464–470. doi:10.1097/00055735-200112000-00012.
- Connell PP, O’Neill EC, Fabinyi D, et al. Endogenous endophthalmitis: 10-year experience at a tertiary referral centre. Eye. 2011;25:66–72. doi:10.1038/eye.2010.145.
- Kernt M, Kampik A. Endophthalmitis: pathogenesis, clinical presentation, management, and perspectives. Clin Ophthalmol. 2010;24(4):121–135. doi:10.2147/OPTH.S6461.
- Lalwani GA, Flynn HW, Scott IU, et al. Acute-onset endophthalmitis after clear corneal cataract surgery (1996-2005). Clinical features, causative organisms, and visual acuity outcomes. Ophthalmology. 2008;115(3):473–476. doi:10.1016/j.ophtha.2007.06.006.
- Joseph J, Sontam B, Guda SJM, et al. Trends in microbiological spectrum of endophthalmitis at a single tertiary care ophthalmic hospital in India: a review of 25 years. Eye. 2019;33(7):1090–1095. doi:10.1038/s41433-019-0380-8.
- Park CY, Lee JK, Chuck RS. Toxic anterior segment syndrome-an updated review. BMC Ophthalmol. 2018;18(1):276. doi:10.1186/s12886-018-0939-3.
- Deshmukh D, Joseph J, Chakrabarti M, et al. New insights into culture negative endophthalmitis by unbiased next generation sequencing. Sci Rep. 2019;9:844. doi:10.1038/s41598-018-37502-w.
- Kolb M, Lazarevic V, Emonet S, et al. Next-generation sequencing for the diagnosis of challenging culture-negative endocarditis. Front Med. 2019;6:203. doi:10.3389/fmed.2019.00203.
- Das T. Endophthalmitis management: stain-culture, empirical treatment, and beyond. Asia Pacific J Ophthalmol. 2020;9:1–3. doi:10.1097/01.APO.0000617904.11979.ae.
- Safneck JR. Endophthalmitis: a review of recent trends. Saudi J Ophthalmol. 2012;26(2):181–189. doi:10.1016/j.sjopt.2012.02.011.
- Sharma S, Padhi TR, Basu S, et al. Endophthalmitis patients seen in a tertiary eye care centre in Odisha: a clinico-microbiological analysis. Indian J Med Res. 2014;139(1):91–98.
- Lobue S, Lobue TD. Fulminant culture negative endophthalmitis as a new entity, toxic posterior segment syndrome? Adv Ophthalmol Visual Syst. 2016;4:171–175.
- Parver D, El-Agha S, He Y. Treatment outcomes in different subgroups of endophthalmitis. Invest Ophthalmol Vis Sci. 2003;44:1855.
- Naik P, Dave VP, Joseph J. Detection of Torque Teno Virus (TTV) and TTV-like minivirus in patients with presumed infectious endophthalmitis in India. PLoS One. 2020;15(1):e0227121. doi:10.1371/journal.pone.0227121.
- Lee AY, Akileswaran L, Tibbetts MD, et al. Identification of torque teno virus in culture-negative endophthalmitis by representational deep DNA sequencing. Ophthalmology. 2015;122(3):524–530. doi:10.1016/j.ophtha.2014.09.001.
- Deshmukh D, Chakrabarti M, Jayasudha R, et al. Elevated cytokine levels in vitreous as biomarkers of disease severity in infectious endophthalmitis. PLoS One. 2018;13(10):e0205292. doi:10.1371/journal.pone.0205292.
- Otsuka H, Kawano H, Sonoda S, et al. Particle-induced endophthalmitis: possible mechanisms of sterile endophthalmitis after intravitreal triamcinolone. Invest Ophthalmol Vis Sci. 2013;54(3):1758–1766. doi:10.1167/iovs.12-11247.
- Therese KL, Anand AR, Madhavan HN. Polymerase chain reaction in the diagnosis of bacterial endophthalmitis. Br J Ophthalmol. 1998;82(9):1078–1082. doi:10.1136/bjo.82.9.1078.
- Chiquet C, Cornut PL, Benito Y, et al.; French Institutional Endophthalmitis Study Group. Eubacterial PCR for bacterial detection and identification in 100 acute postcataract surgery endophthalmitis. Invest Ophthalmol Vis Sci. 2008;49(5):1971–1978. doi:10.1167/iovs.07-1377.
- Pongsachareonnont P, Honglertnapakul W, Chatsuwan T. Comparison of methods for identifying causative bacterial microorganisms in presumed acute endophthalmitis: conventional culture, blood culture, and PCR. BMC Infect Dis. 2017;17(1):165. doi:10.1186/s12879-017-2264-5.
- Gu W, Talevich E, Hsu E, et al. Detection of cryptogenic malignancies from metagenomic whole genome sequencing of body fluids. Genome Med. 2021;13(1):98. doi:10.1186/s13073-021-00912-z.
- Gandhi J, Jayasudha R, Naik P, et al. Targeted high-throughput sequencing identifies predominantly fungal pathogens in patients with clinically infectious, culture-negative endophthalmitis in South India. Microorganisms. 2019;7(10):411.doi:10.3390/microorganisms7100411.
- Huang Q, Fu A, Wang Y, et al. Microbiological diagnosis of endophthalmitis using nanopore targeted sequencing. Clin Exp Ophthalmol. 2021;49:1060–1068. doi:10.1111/ceo.13992.
- Mishra D, Satpathy G, Chawla R, et al. Targeted metagenomics using next generation sequencing in laboratory diagnosis of culture negative endophthalmitis. Heliyon. 2021;7(4):e06780. doi:10.1016/j.heliyon.2021.e06780.
- Lee CS, Hong B, Kasi SK, et al.; Endophthalmitis Study Group. Prognostic utility of whole-genome sequencing and polymerase chain reaction tests of ocular fluids in postprocedural endophthalmitis. Am J Ophthalmol. 2020;217:325–334. doi:10.1016/j.ajo.2020.03.008.
- Zhong Y, Xu F, Wu J, Schubert J, Li MM. Application of next generation sequencing in laboratory medicine. Ann Lab Med. 2021;41:25–43. doi:10.3343/alm.2021.41.1.25.
- Póvoa P. Serum markers in community-acquired pneumonia and ventilator-associated pneumonia. Curr Opin Infect Dis. 2008;21(2):157–162. doi:10.1097/QCO.0b013e3282f47c32.
- Ilhan Ç, Citirik M, Uzel MM, et al. Investigation of systemic inflammatory biomarkers in acute post-cataract surgery endophthalmitis. Eur Eye Res, 2021;1(2):79–83.
- Kolomeyer AM, Murphy KM, Traband A, et al. Beta-d-glucan testing in patients with fungal endophthalmitis. Retina. 2018;38(4):650–659. doi:10.1097/IAE.0000000000002049.
- Naik P, Singh S, Dave VP, et al. Vitreous D-lactate levels as a biomarker in the diagnosis of presumed infectious culture negative endophthalmitis. Curr Eye Res. 2020;45(2):184–189. doi:10.1080/02713683.2019.1662057.
- Naik P, Satyashree G, Mohamed A, et al. Evaluation of vitreous Procalcitonin as a diagnostic biomarker in infectious endophthalmitis. Int Ophthalmol. 2021;41(10):3401–3409. doi:10.1007/s10792-021-01903-0.
- Gao Q, Li Z, Mo X, et al. Combined procalcitonin and hemogram parameters contribute to early differential diagnosis of Gram-negative/gram-positive bloodstream infections. J Clin Lab Anal. 2021;35(9):e23927.doi:10.1002/jcla.23927.
- Zhang Y, Zhang J, Zhao L, et al. Procalcitonin in infectious diseases: a bibliometric analysis. Ann Palliat Med. 2021;10(7):8003–8014. doi:10.21037/apm-21-1607.
- Tosoni A, Cossari A, Paratore M, et al.; On Behalf Of The Internal Medicine Sepsis Study Group. Delta-procalcitonin and vitamin D can predict mortality of internal medicine patients with microbiological identified sepsis. Medicina (B Aires). 2021;57(4):331. doi:10.3390/medicina57040331.
- Dupont D, Saison J, Miailhes P, et al. Aspergillus endophthalmitis: potential role for vitreous galactomannan testing? Int J Infect Dis. 2020;96:151–153. doi:10.1016/j.ijid.2020.04.037.
- Linder KA, Kauffman CA, Zhou S, et al. Performance of the (1,3)-beta-D-glucan assay on bronchoalveolar lavage fluid for the diagnosis of invasive pulmonary aspergillosis. Mycopathologia. 2020;185(5):925–929. doi:10.1007/s11046-020-00479-0.
- Ammar MJ, Carroll R, Kolomeyer A, et al. Clinical utility of beta-d-glucan testing for endogenous fungal chorioretinitis or endophthalmitis. Retina. 2021;41(2):431–437. doi:10.1097/IAE.0000000000002861.
- Chen L, Tao Y, Hu X. Utility of intraocular fluid β-D-glucan testing in fungal endophthalmitis: a series of 5 cases. Am J Case Rep. 2020;21:e921188. doi:10.12659/AJCR.921188.
