ABSTRACT
Background
Retinal neovascularization is the major cause of vision loss that affects both adults and young children including premature babies. It has been a major pathology in several retinal diseases like age-related macular degeneration (AMD), diabetic retinopathy (DR) and retinopathy of prematurity (ROP). Current treatment modalities such as anti-VEGF therapy, laser are not suitable for every patient and response to these therapies is highly variable. Thus, there is a need to investigate newer therapeutic targets for DR, ROP and AMD, based on a clear understanding of disease pathology and regulatory mechanisms involved.
Method
Appropriate articles published till February 2021 were extracted from PUBMED using keywords like ocular angiogenesis, DR, ROP, AMD, miRNA, mRNA, and cirMiRNA and containvaluable information regarding the involvement of miRNA in causing neovascularization. After compiling the list of miRNA regulating mRNA expression in angiogenesis and neovascularaization, their interactions were studied using online available tool MIENTURNET (http://userver.bio.uniroma1.it/apps/mienturnet/). The pathways involved in these processes were also predicted using the same tool.
Results
Most of the studies have explored potential targets like HIF1-α, PDGF, TGFβ, FGF, etc., for their involvement in pathological angiogenesis in different retinal diseases. The regulatory role of microRNA (miRNA) has also been explored in various retinal ocular pathologies. This review highlights regulatory mechanism of cellular and circulatory miRNAs and their interactions with the genes involved in retinal neovascularization. The role of long noncoding RNA (ncRNA) in the regulation of genes involved in different pathways is also noteworthy and discussed in this review.
Conclusion
This review highlights the potential regulatory mechanism/pathways involved in retinal neovascularization and its implications in retinal diseases and for identifying new drug targets.
INTRODUCTION
Neovascularization is defined as the formation of new blood vessels from existing retinal vasculature onto the retina. It differs from vasculogenesis which is involved in the generation of primitive embryonic blood vessels from mesodermal cells called angioblasts. Infact, these newly formed blood vessels are a kind of abnormal vascular growth from existing retinal venules which occurs in specific diseased conditions. Neovascularization serves as major contributor of visual impairment and vision loss in several ocular diseases like proliferative diabetic retinopathy (PDR), retinopathy of prematurity (ROP), and age-related macular degeneration (AMD).
Often, the retinal neovascularization happens at the disc and near the large thicker retinal veins, but they may arise from any position of the retinal vascular bed. It can grow either superficially at the vitreous, or beneath the retina. Early neovascularization can appear as clump of abnormal fine and fragile vessels on the retinal surface. It can be clinically visualized via Fluorescein Fluorescence Angiography (FFA).
METHODS
Appropriate articles were searched out from PUBMED (https://pubmed.ncbi.nlm.nih.gov/) having valuable information for neovascularization caused by dysregulated miRNA-RNA expression. Search was made using specific keywords like ocular angiogenesis, miRNA, mRNA, ocular diseases, circulatory MiRNA, and exosomal miRNA. List of genes and its regulatory miRNA were compiled. MiRNA-mRNA interactions were drawn by using online available tools named as Mienturnet (http://userver.bio.uniroma1.it/apps/mienturnet/). This software also allowed us to predict miRNAs involved in the specific pathways causing neovascularization.
Molecular Mechanism Involved in Healthy and Pathological Neovascularization
Blood vessels are the first developing organ in the embryo that spreads as the largest network in our body. Organs, tissues and cells are mainly nourished by these supporting blood vessels by providing oxygen, nutrients and maintain their normal health. Normal physiological angiogenesis during adulthood involves wound healing and in female reproductive cycle.
Wound healing includes four step process namely, severe inflammation, re-epithelialization, granulated tissue formation, and finally tissue remodeling. During this process, activated platelet secretes cytokines/chemokines involves IL1-β, TNF-α, and VEGF-A to attract other inflammatory cells like monocytes, neutrophils, etc., to the injury site. In endothelial cells, plasminogen activators and PAI-1 production are regulated by VEGF-A. However, the mechanism of uPA and tPA by VEGF-A is not clear yet. VEGF-A induces production of several matrix metalloproteinase, collagenases that degrade the basal lamina and thus increase in permeability via disorganizing the junction proteins like VE-cadherin and occludins etc. in endothelial cells. This results in formation of granule like structures. And finally, endothelial cell proliferation occurs via an increase in VEGF receptors; VEGFR-1, and VEGFR-2. Eventually, granulated tissues remodeled and end results into scar largely composed of fibrocytes, blood vessels and collagen fibrils.Citation1
At the time of ovulation, corpus luteum is formed from the follicles. These corpus luteum get interconnected with outer capillary plexus. VEGF-A plays an important role in angiogenesis in luteum. Luteinizing hormone (LH) regulates angiogenesis in ovary. Studies have found an association of LH with increase VEGF-A level. However, further detailed research is needed to explore the mechanism of LH in vascularization. Thus, under these normal physiological processes there seems to be a tight regulation of molecules engaged in angiogenesis while under pathological condition, the balance between these is disturbed leading to an abnormal angiogenesis.Citation2
Ocular angiogenesis is a prime sign of several corneal diseases (any kind of trauma, infections etc. cause new blood vessels formation to the cornea) and retinal diseases like ROP, DR (mainly proliferative diabetic retinopathy; PDR) and AMD (wet-AMD). This further leads to irreversible visual loss caused by opacification of cornea or deleterious pathologic changes to neural retina. Dysregulated blood vessels formation may contribute to several other biological mechanisms involving hypoxia, inflammation, and infections. This phenomenon is basically associated with an increase in endothelial cells number caused by the imbalance in cell proliferation and apoptosis. The abnormal angiogenesis occurs due to an imbalance of angiogenic and anti-angiogenic factors that play a major role in its regulation. It also involves extracellular matrix proteins, cytokines, cell signaling molecules and other factors.Citation3
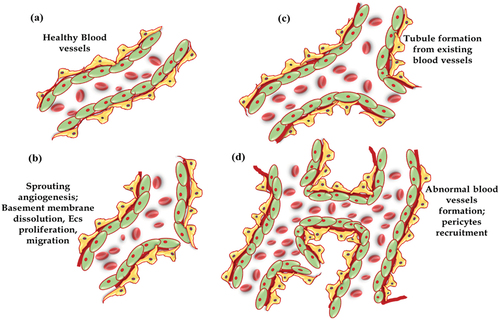
Neovascularization is the resultant pathological mechanism especially of ischemia. Differentiations of endothelial progenitor cells (EPCs) are the main contributor in new blood vessel formation under pathological condition.Citation4,Citation5 Generally, it can be described by four process; A) matrix degradation; where activated angiogenic factors like (VEGF, TGF-β, C3, ETS-1, eNOS3 etc.) bind to receptors on endothelial cells and pericytes; and facilitates the matrix degradation. Several family members of proteases like MMPs upon activation, digest matrix components (collagen, laminin, fibrin, and fibronectin etc.).Citation6,Citation7 B) migration; in response to angiogenic factor, endothelial cells migrate to surrounding region via chemokinesis or chemotaxis movement, C) proliferation; secretion of platelet derived growth factor, endothelial cells proliferation supplies the cells to make new blood vessels walls. D) survival; pericytes cells loss, secretion of anti-angiogenic molecules like Ang1, and THBS1 etc. which binds to Tie2 on endothelial cells and promotes endothelial cell survival, vessel maturation and stabilization. Finally, the endothelial cells assemble and facilitate the tube formation shown in .
The earlier histological feature of pathological angiogenesis involves neuronal damage, basement membrane thickening, pericytes loss and endothelial cell proliferation. However, at severe stage, persistent proliferation of abnormal fibrovascular cells cause subsequent bleeding, form scar over there and thus leads to retinal detachment. Identification of proangiogenic molecules (such as VEGF, FGFs, and Ang etc.) and inhibitors (such as platelet factor-4, endostatin, vasostatin, and angiostatin etc) provided therapeutic options for developing drug targets for clinical applications of different ocular pathologies. VEGF, one of the specific and critical inducer of angiogenesis moderate endothelial cell proliferation, motility, and survival.Citation8 Many research evidence implicated VEGF as an angiogenic inducer/mediator in intraocular neovascular syndromes, and still several clinical studies are trying to inhibit VEGF for therapeutic purposes.
Molecular Pathways Involved in Angiogenesis
Cdc42 engaged in filapodia formation acts as molecular sensor for guiding migratory mechanism of ECs. Regulation of endothelial cell migration in response to VEGFR-2 receptor activation involves activation of small GTPases of Rho family in early postnatal angiogenesis in retina. Sprouting of endothelial cells forming multiple filapodia occurs in response to level of VEGF expression. Higher level of VEGF results in vascular sprouting under pathological condition. Activation of Cdc42 establishes stress fibers via activation of p38 pathway. RhoA phosphorylates VEGFR-2 and in turn activates phosphatidylinositol 3-kinase (PI3K) pathway. This PI3K activation causes endothelial cell migration and also activates 3-phosphoinositide-dependent protein kinase-1 (PDK1), further activating Akt/PKB along with other kinases of AGC family. It further activates eNOS pathway by forming nitric oxide (NO). NO is vasoconstrictor and plays a crucial role in ECs migration and angiogenesis.Citation9
On the other hand, activation of VEGFR-2 causes its auto phosphorylation on residue Tyr1214 followed by recruitment of Nck. It sub sequentially causes activation of Fyn and Cdc42 (upstream of the p38 MAP kinase module). Nck elicits actin polymerization via activation of WASP-Arp2/3 pathway. Along with this, binding of VEGF to its receptor VEGFR-2 recruits HSP90 and thus initiates the activation of RhoA-ROCK pathway in turn causing the changes in the focal adhesion kinases (FAK) configuration. Taking all together, actin polymerization via p38 pathway and focal adhesions via FAK-mediated pathway contributes to cell migration by inducing contraction of ECs.Citation9
Notch proteins expression increased in injured vascular endothelial cells under regulation of VEGFR1. Notch receptors and its ligands (like Delta/Serrate) are known to play several roles throughout development as well as pathogenic conditions affecting cell cycle regulation and apoptosis processes. It contributes to angiogenesis by inducing cell proliferation and stabilizing the blood vessels.Citation10
Overall, VEGF induces endothelial cell migration, and proliferation, resulting from activation of several signaling pathways downstream to its receptors.
MicroRNA as Regulatory Manager and Its Biogenesis
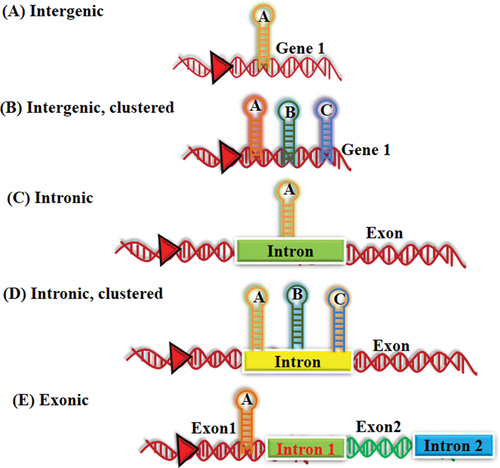
MicroRNAs are the small non-coding RNA sequences. Victor Ambros (Lee et al., 1993) and Gary Ruvkun (Wightman et al., 1993) were the first ones to discover the small non-coding RNA lin-4 molecules in C. Elegans. Lin-4 represses lin-14 translation, a gene which plays major role in developmental timing of C. Elegans.Citation11,Citation12 It plays pivotal role in regulating the mRNAs expression in multitude of pathways and processes as developmental, carcinogenesis, apoptosis and metabolism etc. MiRNA binds to a specific mRNA at 3´-untranslated region (3´ UTR), that may lead to either translational repression or degradation of mRNA. However, some studies have shown that miRNA can cause increase in translation.Citation13 Basically these are found in clustered form within the genome. These small RNAs are mostly present in both intergenic and within intronic region of the mRNAs. These regions are known initially as “junk DNA” as their functions were still unexplored (). However, the origin of precursor miRNA is very well explored with the usefulness of protein coding genes and very few miRNA precursors have also been found within exons of mRNA and in antisense RNA sequences.Citation14,Citation15
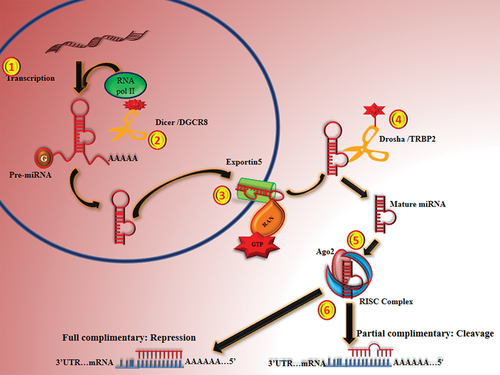
MiRNA biogenesis occurs in two cellular compartments with several cleavage events: nucleus and cytoplasm. In nucleus, RNA polymerase II which belongs to RNAs III family transcribes miRNAs into pri-miRNAs (primary miRNAs transcript- hairpin loop structure).Citation16 Most of the miRNAs are transcribed by polymerase II which is a component of a complex protein for transcribing DNA to RNA. However Borchert et al., (2006) found that Alu elements upstream of C19MC miRNAs require polymerase III to transcribe. Regulatory miRNA involved in cell cycle regulation and growth are found to be transcribed by pol III.Citation17–20
First processing of long miRNA transcript is done by Drosha/DGCR8 into 60–70nts length in the nucleus.Citation21 DGCR8 (RNA binding protein) plays a major role as a molecular ruler that measures the length from the bases of the stem loop and assist the Drosha (RNase III family member) for a precise cleavage.Citation22 Pri-miRNAs thus generated (and possibly mirtrons; miRNA generated from intronic region of gene) are exported to cytoplasm for further processing. This transportation involves nucleo-cytoplasmic transporter factor Exportin-5 (Expo-5, a member of the karyopherin family) and RanGTP. Pri-miRNA assembles with Exoprtin-5/RanGTPs, which prevents pri-miRNAs from nuclear degradation and facilitates its translocation.Citation23
In the cytoplasm, hydrolysis of RanGTP allows the release of pri-miRNA to cytoplasm; where second processing is done by Dicer (second RNase III family member), Tandem Repeat Binding Protein (TRBP) and Ago2 protein complex. Firstly, a single-strand of the pri-miRNA of 11–12 nucleotides cleaved by Ago2 from 3’ end and create a nicked hairpin structure, called as Ago2-cleaved precursor miRNA (Ac-pre-miRNA). Further Dicer cleaves ac-pre-miRNA and generates a double stranded miRNA duplex of length approx. 17–22nts.Citation24,Citation25
This generated pre-miRNAs are generally imperfect duplex (commonly denoted as miRNA:miRNA* duplex), of a passenger strand (miRNA*) and a mature miRNA strand (guide strand; miRNA). This duplex gets lump together with RNA induced silencing complex (RISC), where ATP-independent unwinding of miRNA:miRNA* duplex happens and mature miRNA generated.Citation26,Citation27
The unwinding of duplex is not clear, however several hypotheses were put forward to explain the involvement of Ago2, Dicer, Helicase A (RHA; also known as DHX9 or NDHll) in unwinding of miRNA:miRNA* duplex in RNA-induced silencing complex (RISC) complex.Citation28 Within the RISC complex the guide RNA binds to 3ʹUTR of target mRNA through Watson-Crick base-pairing.Citation11 Recognition of target and mature miRNA mainly depends on base pairing between seed sequences (2–8nts at 5’ end) of guide miRNA.Citation29
Finally, gene silencing mechanism is determined by grade and nature of complementarity between guide miRNA sequence and target mRNACitation30 explored as negative regulator of mRNA expression. A single miRNA has the ability to regulate several of target mRNA and a mRNA can be potentially targeted by many of the miRNAs ().Citation16
Here, in this review, we explored the miRNA regulatory role targeting genes as an important and common pathological change; neovascularization that happen almost in all retinal diseases.
Key Genes Involved in Angiogenesis
Retina has high demand for metabolic energy fulfilled by a complex network of vasculature formed in choroid and retina and by hyaloid vasculature during initial developmental phase.Citation31 The pathological condition like hypoxia/ischemia leads to initiation of angiogenesis from center to periphery of retina. While VEGF is a main inducer of blood vessel growth, it is important for neuronal survival as well. Retinal neovascularization is a complex pathophysiological event induced by an imbalance of angiogenic (like VEGF, HIF-α, PIGF, TGF-beta, FGF and EDN1) and anti-angiogenic (like THBS1, and TIMPs etc) key genes.Citation32 In the next section, we will discuss few key genes involved in neovascularization and their regulations by small RNAs.
Hypoxia Inducible Factor1-alpha (HIF1-α)
HIF1-alpha is a hetero-dimeric DNA-binding complex, acts as a master regulator of cellular and homeostatic response to hypoxia or ischemia. Under such conditions, it activates multiple genes involved in angiogenesis, oxidative stress, and apoptosis resulting into production of proteins that cause escalation of oxygen delivery or assisting for metabolic adaptation to hypoxia/ischemia.Citation33 Basically, under hypoxia, hydroxylation of HIF1-α is reduced. Hence, accumulated HIF1-α, enters into the nucleus and drives the transcription of several genes like VEGF, VHL etc.Citation34,Citation35 This accumulation causes an increased expression of miR-206-3p, miR-20b-5p, miR-20a-5p, miR-27b-3p, miR-27a-3p, and miR-381-3p in human retinal endothelial cells under hypoxia induced by CoCl2. These miRNAs cause upregulation of pro-angiogenic genes such as VEGFA, PIGF, and TGF-β signaling genes like TGF-β1, its receptors TGF-βR1, TGF-βR2 and SMAD2.Citation36 TGF-βR1 has a detrimental role of in retinal angiogenesis in DR pathology.Citation37 A study by Zhang Y. et al., (2019) showed hypoxia induced TGFβ-1 angiogenesis pathway activation by miR-27 in a model of corneal neovascularization.Citation38 MiR-18a-5p, a member of miR17-94 family (mostly studied in tumor genesis) was downregulated in retina of OIR model compared to healthy control retina indicating its pathophysiological role in diseased condition. The reduced expression of miR-18a-5p cause accumulation of HIF1-α stabilization along with increased expression of FGF. However, overexpression of miR-18a-5p cause significantly reduction in their expression both at mRNA and protein level indicating a direct regulation of both the genes via binding to their 3’ UTR and its potential use as a therapy for targeting angiogenesis in retinal diseases like ROP, DR etc.Citation39 An overexpression of cluster miR-183-96-192 under hypoxia upregulates HIF1-α and thereby induces the expression of VEGF/FGF-2/Ang2 in ECs that eventually stimulates multiple processes like angiogenesis, and autophagy as seen in several diseases like cancer, retinopathy etc.Citation40
Vascular Endothelial Growth Factor (VEGF)
VEGF, a homo-dimeric glycoprotein (mol. Wt. approx. 45 kDa) is a key player of neovascularization in several ocular disease. It has two receptors VEGF receptor-1 and VEGF receptor-2, mainly expressed on vascular endothelial cells. It is required during embryonic development and also for wound healing in adults. In human, let-7 family genes includes let-7a, let-7b, let-7d, let-7e, let-c, let-7 f, let-7 g, let-7i, miR-98 and miR-202. Over expression of let-7 has been found to repress migration, proliferation of endothelial cells. Let-7 anti-miR results in neovascularization in healthy retina and choroid both. However, overexpression of let-7 leads to retinal tortuosity and pericytes defect by regulating EC’s angiogenic properties via targeting HMGA2 gene which gives similar changes happen in non-proliferative diabetic retinopathy (NPDR) in vivo. However, the retinal changes seen in mice model did not progress to severe as in proliferative retinopathy stages due to some regulatory mechanism. Which is not explored yet or this could also showed its role specifically in NPDR.Citation41 Shi et al. have shown downregulation of miR-34 upon induction with VEGF in human microvascular retinal endothelial cells lead to higher expression of Notch1 that further promotes angiogenesis. Similar phenomenon was seen in OIR model confirming that miR-34a may attenuate pathological angiogenesis via targeting the Notch1. The anti-angiogenic effects of miR-34a are found to be clearly associated with the negatively regulation of cell proliferation and migration.Citation42
Another miRNA-126, mostly studied in different types of cancers, cystic fibrosis, asthma, and diabetes, was found to be expressed by endothelial cells in blood vessels. MiR-126 targets SPRED-1 (Sprouty Related EVH1 Domain Containing 1) which further enhanced VEGF expression in induced pluripotent stem cell model.Citation43 Other than MiR-126, several miRNAs like miR-212, miR-132, and miR-206 are also known to target SPRED-1.Citation44,Citation45 A study by Yang et.al. 2020 showed downregulation of miR-15b targets 3ʹUTR region of VEGF transcript and cause significant higher production of VEGF in the serum of PDR patients. Likewise a decreased expression of miR-96 was seen in hyperoxic/oxidative stress conditions, where, it binds to VEGF and Ang2 and further promote vaso-obliteration as seen in initial phase of retinopathy of prematurity. Thus, these two genes demonstrate a synergistic role in encouraging angiogenic process. Importantly, miR-96 also downregulates PTPN9 gene (anti-angiogenic phosphatase), causing inactivation of STAT3 that further suppress VEGF expression.Citation46 In addition, PTPN9 gene is found to be down regulated by miR-126 leading to activation of two major signaling pathways i.e. AKT and ERK signaling pathway. Induction of these pathways result in stimulating cell survival, proliferation, and migration.Citation47
Other Angiogenic Genes
PDGFB is another potent regulator of endothelial cells proliferation. A signaling mechanism activated by PDGFB where it binds with PDGFRβ. This signaling is well known for BRB formation and its maturation. It involves vigorous enrollment of pericytes onto new growing blood vessels in the retina.Citation48
Tang et.al (2020) showed downregulation of miR-29b-3p caused statistically significant increase in the expression of PDGFB at both mRNA and protein in retinal microvascular endothelial cells (RMECs). MiR-29b is a member of miR-29 family having other members as miR-29c, and miR-29a. However, miR-29b has been extensively explored in many pathological conditions like cancer, metabolic disorders, tissue fibrosis, and muscle atrophy etc. This study for the first time explained its role in ocular angiogenesis by regulating ECs proliferation. However, this finding needs further validation in-vivo using appropriate model.Citation49 Wang et.al. 2017 found miRNA-370 displays a pro-apoptotic role and causes transcriptional arrest of KDR gene by binding to its 3’ UTR, though in comparison to its other receptor VEGFR1, VEGF has higher affinity to KDR. MiR-370 induce up regulation of cell cycle progression related genes like CycD1, P21, and p27 along with apoptotic genes like Bim and FasL eventually causing suppression of ECs via promoting apoptosis.Citation50
Dll4/Notch signaling is well known for controlling sprouting and branching of blood vessels. Moreover, CXCR4 and FZD are receptor for SDF-1 and Wnt signaling respectively. All the above mentioned genes are studied in inducing angiogenesis in both laser-induced CNV and OIR models. MiR-150 is known to maintain the vascular latency in normal healthy retina by suppressing these genes. However under pathological condition, the abundant expression of miR-150 results in higher expression of these angiogenic genes, thereby, abnormal angiogenesis in ocular neovascular diseases.Citation51 Haque et.al. 2015 showed another angiogenic genes Pro-renin receptor (PRR) targeted by miR-152 and miR-21 via in-silico and in-vitro analysis respectively under hyperglycemic condition that promotes TGFβ-1 signaling by targeting SMAD7, PTEN and SPRY1 genes involved in angiogenesis pathway. Thus, miRNA could be used a therapeutic target for diabetes induced retinal microvascular alteration.Citation52,Citation53 On the other hand, Jiang et. al. 2015 showed TGFβ-1 is also regulated by miR-200b.Citation54 In an oxygen-induced retinopathy model, miR-145-5p was found to be upregulated during the neovascularization phase. However, a synthetic miR-145-5p inhibitor repress the neovascularization in the retina of OIR model. Tropomodulin 3 (Tmod3), which is an actin-capping protein confirmed as a direct target of miR-145-5p. Tmod3 plays role in maintenance of cytoskeletal structure in ECs.Citation55
Circulating miRNA
There are cell-free circulating miRNAs that are secreted into biofluids. These are extremely stable and can be considered as non-invasive biomarkers for many ocular diseases where obtaining samples proximal to the retina is not possible. In contrast to intracellular miRNAs, these extracellular/circulating miRNAs are found in diverse biofluids such as tears, serum, plasma, milk, and CSF etc and are highly stable. These can withstand room temperature, other condition like reverse high/low pH, multiple-freeze thaw cycles and boiling temperaturesCitation56 due to being encapsulated within micro-particles like exosomes, micro/macro vesicles and apoptotic bodies etc.Citation57
In context to this, a study showed eight circulatory miRNAs (miR-20 family (miR-20a-5p, miR-20a-3p, miR-20b), miR-106a-5p, miR-27b-3p, miR-27a-5p, miR-381-3p and miR-206-3p) in serum of DR patients.Citation58 Another study by Elshelmani et. al. 2020 found significantly increased expression of miR-19a, miR-126, and miR-410 that target apoptosis and angiogenesis pathways by binding to 3’ UTR of VEGF-A in serum of AMD patient. MiR-410 target C1q showing complement activation in AMD. AMD being a neurodegenerative disease, showed upregulation of two circulatory miRNAs; miR-626 and miR-486-5p and downregulation of miR-885-5p isolated from serum exosome that are known to contribute to apoptosis and neovascularization in AMD patients.Citation59
Vitreous, a jelly like fluid present very much nearer to retina, maintains the ocular shape. Six miRNAs (upregulated; hsa-miR-362-5p, hsa-miR-185-5p, hsa-miR-326, hsa-miR-23b-3p, and hsa-miR-142-3p, downregulated; hsa-miR-20a-5p) were detected upon comparison of in vitreous of anti-VEGF treated with those of untreated PDR patients.Citation60 While such studies have helped in understanding the genetic regulations in disease pathogenesis, cohesive efforts are needed to identify circulatory miRNA for noninvasive biomarker identification. Further many challenges are needed to overcome for their use for clinical applications.
Non-coding RNAs
Human genome contains approx. ~98% of noncoding RNAs that do not translate into functional proteins. Among ncRNA, long non-coding RNAs consists on 200nts and capping at 5ʹend makes it stable. However, lncRNAs are distinct from mRNA in terms of conserve sequences. Its mechanism of action depends upon their localization. LncRNAs regulate transcription in nucleus while being involved in the modulation of proteins functions. These lncRNAs bind to miRNA at specific site and regulate its functions. Recently, lncRNAs are being explored for their diverse roles. Streptozotocin induced diabetic rat model showed increased expression of lncRNA MALAT1 in retina that targets p38 mitogen-activated protein kinase signaling mechanism. Increased expression of p38 mitogen-activated protein kinase causes ECs proliferation and thus promote angiogenesis. However, upon inhibition of MALAT1 via shRNA, the proliferation of ECs is suppressed causing the alleviation of DR condition.Citation61 In addition to this, another study showed lnc MALAT1 also regulate inflammation in retina of mice via regulating inflammatory responses related genes. MALAT1 has also been seen in vitreous of DR patients indicating its major role in DR pathogenesis.Citation62
Diverse role of lncRNA have been described and associated with various essential function as well as pathological role in retinal neovascularization. They are now identified as key regulator with their unique mechanism of action. This would be helpful in understanding the disease mechanism and developing novel therapeutic targets.
MicroRNA and mRNA Interaction
MiRNA has property to target many different mRNAs. A mRNA can be regulated by several miRNAs either simultaneously or in milieu-dependent manner.Citation63 Notably, there is an additive or cooperative effect in regulatory function of miRNA as they are found in cluster.Citation64,Citation65 Thus it is very challenging to map miRNA-mRNA interactions. Nowadays, several tools available for prediction of miRNA-mRNA interaction following different algorithm.Citation66,Citation67 Here, in this review we have tried to build interactions between known reviewed genes (mRNA) and its regulatory molecule miRNA using Mienturnet as shown in . Regulation of genes by several miRNAs is listed in .
Figure 4. mRNA-miRNA interaction: mRNA shown in yellow circle are regulated by multiple miRNAs indicated in blue circle.
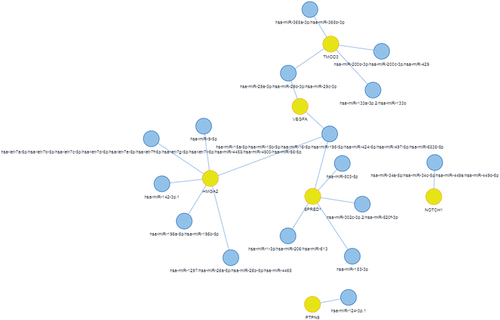
Table 1. Direct negative regulation of targeted genes by miRNAs.
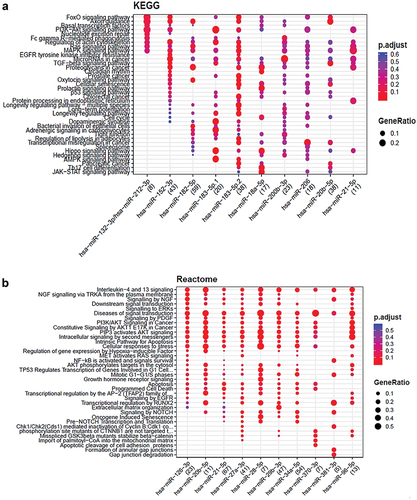
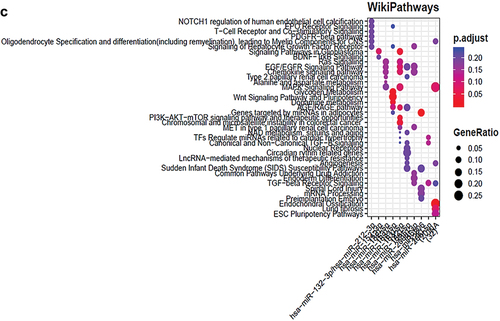
Upon functional enrichment analysis, it showed multiple pathways (from KEGG, REACTOME and WIKIPATHWAYS) interactions indicating involvement of multiple genes and miRNAs (Figure 6, and 8) in different signaling mechanisms. The major pathways indicated here are hypoxia inducible gene pathways, TGF-β signaling, Notch 1 signaling, JAK-STAT signaling, apoptosis, PI3K pathways and MAPK kinase pathways etc. These pathways plays a major role in angiogenesis of retinal disease. Several others pathways are also shown to be involved and their associated genes modulated by miRNAs that needs to be explored further ( (A–C)).
Concluding Remark
The pivotal roles of miRNAs are being studied in various ocular diseases, particularly in context with development of better diagnostic tool or for therapeutic purposes. The dynamic behavior of miRNA and complexity in regulation of mRNA have been explored widely that further underscores its utility. There are several factors contributing to their function like localization, abundance of mRNA, affinity for binding, cellular state and its type, and other components like RISC complex etc. Among these factors, localization, abundance and affinity between miRNA and mRNA are very crucial for regulating gene expression. Moreover, the exact biological mechanism involved in miRNA secretion and its uptake by receptor cells are still not well explored and requires further exploration.
The key genes involved in angiogenesis and their regulations by miRNAs could facilitate a better understanding of neovascularization and its regulated by miRNAs in different diseases. Many studies have explored the regulatory mechanism of miRNA in an in-vitro model using co-culture of several different cell types, thereby providing a valuable clues for understanding the role of dysregulated miRNAs in diseases like DR, ROP, and other retinopathies. These need further confirmation in human ocular tissues to better understand their role under normal physiological and pathological condition. The miRNAs identified from such studies can be explored for development of new therapeutic interventions for providing a better quality of life to the patients with retinal diseases. Lastly, the genetic regulation by miRNA could clearly explain the biological mechanisms underlying DR, ROP and AMD pathogenesis and their emerging potential as biomarkers and personalized therapeutics.
DISCLOSURE STATEMENT
No potential conflict of interest was reported by the author(s).
Additional information
Funding
REFERENCES
- Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004 Dec;56(4):549–580.
- Hazzard TM, Stouffer RL. Angiogenesis in ovarian follicular and luteal development. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000 Dec;14(6):883–900.
- Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011 May 19;473(7347):298–307.
- Takahashi T, Kalka C, Masuda H, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999 Apr;5(4):434–438.
- Tepper OM, Capla JM, Galiano RD, et al. Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow-derived cells. Blood. 2005 Feb 1;105(3):1068–1077.
- Rundhaug JE. Matrix metalloproteinases and angiogenesis. J Cell Mol Med. 2005 Apr-Jun;9(2):267–285.
- Van Hinsbergh VW, Engelse MA, Quax PH. Pericellular proteases in angiogenesis and vasculogenesis. Arterioscler Thromb Vasc Biol. 2006 Apr;26(4):716–728.
- Shibuya M. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J Biochem. 2013 Jan;153(1):13–19.
- Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007 Mar 30;100(6):782–794.
- Akil A, Gutiérrez-García AK, Guenter R, et al. Notch signaling in vascular endothelial cells, angiogenesis, and tumor progression: an update and prospective. Front Cell Dev Biol. 2021 Feb 16;9:642352. doi:10.3389/fcell.2021.642352.
- Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993 Dec 3;75(5):843–854.
- Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993 Dec 3;75(5):855–862.
- Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007 Dec 21;318(5858):1931–1934.
- Rodriguez A, Griffiths-Jones S, Ashurst JL, Bradley A. Identification of mammalian microRNA host genes and transcription units. Genome Res. 2004 Oct;14(10A):1902–1910.
- Lagos-Quintana M, Rauhut R, Meyer J, Borkhardt A, Tuschl T. New microRNAs from mouse and human. RNA. 2003 Feb;9(2):175–179.
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004 Jan 23;116(2):281–297.
- Borchert GM, Lanier W, Davidson BL. RNA polymerase III transcribes human microRNAs. Nat Struct Mol Biol. 2006;13(12):1097–1101.
- Goodfellow SJ, White RJ. Regulation of RNA polymerase III transcription during mammalian cell growth. Cell Cycle. 2007 Oct 1;6(19):2323–2326.
- Scott PH, Cairns CA, Sutcliffe JE, et al. Regulation of RNA polymerase III transcription during cell cycle entry. J Biol Chem. 2001 Jan 12;276(2):1005–14. 4.
- Costanzo G, Camier S, Carlucci P, Burderi L, Negri R. RNA polymerase III transcription complexes on chromosomal 5S rRNA genes in vivo: TFIIIB occupancy and promoter opening. Mol Cell Biol. 2001 May;21(9):3166–3178.
- Pantaleo V, Szittya G, Moxon S, et al. Identification of grapevine microRNAs and their targets using high-throughput sequencing and degradome analysis. Plant J. 2010 Jun 1;62(6):960–976.
- Han J, Lee Y, Yeom KH, et al. Molecular basis for the recognition of primary microRNAs by the Drosha-DGCR8 complex. Cell. 2006 Jun 2;125(5):887–901.
- Lund E, Güttinger S, Calado A, Dahlberg JE, Kutay U. Nuclear export of microRNA precursors. Science. 2004 Jan 2;303(5654):95–98.
- Diederichs S, Haber DA. Dual role for argonautes in microRNA processing and posttranscriptional regulation of microRNA expression. Cell. 2007 Dec 14;131(6):1097–1108.
- Haase AD, Jaskiewicz L, Zhang H, et al. TRBP, a regulator of cellular PKR and HIV-1 virus expression, interacts with Dicer and functions in RNA silencing. EMBO Rep. 2005 Oct;6(10):961–967.
- Maniataki E, Mourelatos Z. A human, ATP-independent, RISC assembly machine fueled by pre-miRNA. Genes Dev. 2005 Dec 15;19(24):2979–2990.
- Gregory RI, Chendrimada TP, Cooch N, Shiekhattar R. Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell. 2005 Nov 18;123(4):631–640.
- Robb GB, Rana TM. RNA helicase A interacts with RISC in human cells and functions in RISC loading. Mol Cell. 2007 May 25;26(4):523–537.
- Brennecke J, Stark A, Russell RB, Cohen SM. Principles of microRNA-target recognition. PLoS Biol. 2005 Mar;3(3):e85.
- Yekta S, Shih IH, Bartel DP. MicroRNA-directed cleavage of HOXB8 mRNA. Science. 2004 Apr 23;304(5670):594–596.
- Lutty GA, McLeod DS. Development of the hyaloid, choroidal and retinal vasculatures in the fetal human eye. Prog Retin Eye Res. Jan 2018;62:58–76. doi:10.1016/j.preteyeres.2017.10.001.
- Selvam S, Kumar T, Fruttiger M. Retinal vasculature development in health and disease. Prog Retin Eye Res. Mar 2018;63:1–19. doi:10.1016/j.preteyeres.2017.11.001.
- Weidemann A, Johnson RS. Biology of HIF-1alpha. Cell Death Differ. 2008 Apr;15(4):621–627.
- Jaakkola P, Mole DR, Tian YM, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001 Apr 20;292(5516):468–472.
- Semenza GL. Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology (Bethesda). Aug 2004;19:176–182. doi:10.1152/physiol.00001.2004.
- Lazzara F, Trotta MC, Platania CBM, et al. Stabilization of HIF-1α in human retinal endothelial cells modulates expression of miRNAs and proangiogenic growth factors. Front Pharmacol. 2020 Jul 17;11:1063. doi:10.3389/fphar.2020.01063.
- Gerhardinger C, Dagher Z, Sebastiani P, Park YS, Lorenzi M. The transforming growth factor-beta pathway is a common target of drugs that prevent experimental diabetic retinopathy. Diabetes. 2009 Jul;58(7):1659–1667.
- Zhang Y, Yuan F, Liu L, et al. The role of the miR-21/SPRY2 axis in modulating proangiogenic factors, epithelial phenotypes, and wound healing in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2019 Sep 3;60(12):3854–3862.
- Guan JT, Li PDW XX, Zhang WM, et al. MicroRNA-18a-5p administration suppresses retinal neovascularization by targeting FGF1 and HIF1A. Front Pharmacol. 2020 Mar 10;11:276. doi:10.3389/fphar.2020.00276.
- Li Y, Zhang D, Wang X, et al. Hypoxia-inducible miR-182 enhances HIF1α signaling via targeting PHD2 and FIH1 in prostate cancer. Sci Rep. 2015 Jul 24;5(1):12495.
- Zhou Q, Frost RJA, Anderson C, et al. let-7 contributes to diabetic retinopathy but represses pathological ocular angiogenesis. Mol Cell Biol. 2017 Jul 28;37(16):e00001–17.
- Shi S, Jin Y, Song H, Chen X. MicroRNA-34a attenuates VEGF-mediated retinal angiogenesis via targeting Notch1. Biochem Cell Biol. 2019 Aug;97(4):423–430.
- Ye L, Peng Y, Mo J, Yao Y. MiR-126 enhances VEGF expression in induced pluripotent stem cell-derived retinal neural stem cells by targeting spred-1. Int J Clin Exp Pathol. Feb 1, 2018;11(2):1023–1030.
- Sharma SB, Lin CC, Farrugia MK, et al. MicroRNAs 206 and 21 cooperate to promote RAS-extracellular signal-regulated kinase signaling by suppressing the translation of RASA1 and SPRED1. Mol Cell Biol. 2014 Nov 15;34(22):4143–4164.
- Desjarlais M, Wirth M, Rivera JC, et al. MicroRNA-96 promotes vascular repair in oxygen-induced retinopathy-a novel uncovered vasoprotective function. Front Pharmacol. 2020 Feb 3;11:13. doi:10.3389/fphar.2020.00013.
- Hong Y, Liang H, Wang Y, et al. miR-96 promotes cell proliferation, migration and invasion by targeting PTPN9 in breast cancer. Sci Rep. 2016 Nov 18;6:37421. doi:10.1038/srep37421.
- Qu M, Pan J, Wang L, et al. MicroRNA-126 regulates angiogenesis and neurogenesis in a mouse model of focal cerebral ischemia. Mol Ther Nucleic Acids. 2019 Jun 7;16:15–25. doi:10.1016/j.omtn.2019.02.002.
- Park DY, Lee J, Kim J, et al. Plastic roles of pericytes in the blood-retinal barrier. Nat Commun. 2017 May 16;8(1):15296.
- Tang W, Guo J, Gu R, et al. MicroRNA-29b-3p inhibits cell proliferation and angiogenesis by targeting VEGFA and PDGFB in retinal microvascular endothelial cells. Mol Vis. Feb 24, 2020;26:64–75.
- Wang XH, Chen L. MicroRNA-370 suppresses the retinal capillary endothelial cell growth by targeting KDR gene. Bratisl Lek Listy. 2017;118(4):202–207.
- Liu CH, Sun Y, Li J, et al. Endothelial microRNA-150 is an intrinsic suppressor of pathologic ocular neovascularization. Proc Natl Acad Sci U S A. 2015 Sep 29;112(39):12163–12168.
- Haque R, Hur EH, Farrell AN, Iuvone PM, Howell JC. MicroRNA-152 represses VEGF and TGFβ1 expressions through post-transcriptional inhibition of (Pro)renin receptor in human retinal endothelial cells. Mol Vis. Mar 7, 2015;21:224–235.
- Jiang Q, Zhao F, Liu X, Li R, Liu J. Effect of miR-200b on retinal endothelial cell function under high glucose environment. Int J Clin Exp Pathol. Sep 1, 2015;8(9):10482–10487.
- Haque R, Iuvone PM, He L, et al. The MicroRNA-21 signaling pathway is involved in prorenin receptor (PRR) -induced VEGF expression in ARPE-19 cells under a hyperglycemic condition. Mol Vis. Apr 14, 2017;23:251–262.
- Liu CH, Wang Z, Huang S, Sun Y, Chen J. MicroRNA-145 regulates pathological retinal angiogenesis by suppression of TMOD3. Mol Ther Nucleic Acids. 2019 Jun 7;16:335–347. doi:10.1016/j.omtn.2019.03.001.
- O’Brien J, Hayder H, Zayed Y, Peng C. Overview of MicroRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol (Lausanne). 2018 Aug 3;9:402. doi:10.3389/fendo.2018.00402.
- Tkach M, Théry C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016 Mar 10;164(6):1226–1232.
- Platania CBM, Maisto R, Trotta MC, et al. Retinal and circulating miRNA expression patterns in diabetic retinopathy: an in silico and in vivo approach. Br J Pharmacol. 2019 Jul;176(13):2179–2194.
- ElShelmani H, Wride MA, Saad T, Rani S, Kelly DJ, Keegan D. Identification of novel serum micrornas in age-related macular degeneration. Transl Vis Sci Technol. 2020 Mar 30;9(4):28.
- Friedrich J, Steel DHW, Schlingemann RO, et al. microRNA expression profile in the vitreous of proliferative diabetic retinopathy patients and differences from patients treated with anti-VEGF therapy. Transl Vis Sci Technol. 2020 May 19;9(6):16.
- Liu JY, Yao J, Li XM, et al. Pathogenic role of lncRNA-MALAT1 in endothelial cell dysfunction in diabetes mellitus. Cell Death Dis. 2014 Oct 30;5(10):e1506.
- Biswas S, Thomas AA, Chen S, et al. MALAT1: an epigenetic regulator of inflammation in diabetic retinopathy. Sci Rep. 2018 Apr 25;8(1):6526.
- Uhlmann S, Mannsperger H, Zhang JD, et al. Global microRNA level regulation of EGFR-driven cell-cycle protein network in breast cancer. Mol Syst Biol. 2012 Feb 14;8(1):570.
- Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell. 2007 Jul 6;27(1):91–105.
- Saetrom P, Heale BS, O S Jr, Aagaard L, Alluin J, Rossi JJ. Distance constraints between microRNA target sites dictate efficacy and cooperativity. Nucleic Acids Res. 2007;35(7):2333–2342.
- Lu Y, Leslie CS. Learning to predict miRNA-mRNA interactions from AGO CLIP sequencing and CLASH data. PLoS Comput Biol. 2016 Jul 20;12(7):e1005026.
- Riffo-Campos ÁL, Riquelme I, Brebi-Mieville P. Tools for sequence-based miRNA target prediction: what to choose? Int J Mol Sci. 2016 Dec 9;17(12):1987.