ABSTRACT
Background
Traditionally, visual acuity gain and central retinal thickness have been used to measure outcomes when investigating the efficacy of vascular endothelial growth factor (VEGF) inhibitors for patients with neovascular age-related macular degeneration (nARMD). However, localization of retinal fluid may offer additional prognostic value for treatment. The primary aim of this retrospective clinical audit was to investigate whether the presence and location of subretinal fluid has an effect on the visual outcomes of treatment naïve patients with nARMD treated in the real-world setting with VEGF inhibitors. Secondary aims included investigation of change to visual and anatomical outcomes and investigation of the dosing schedule.
Methods
Retrospective observational study of patients attending one suburban and one regional ophthalmology clinic requiring treatment with VEGF inhibitors for nARMD using single-user non-identifiable data from the Fight Retinal Blindness! Registry from 2014 to 2020. Visual acuity (VA) and central subfield thickness (CST) were recorded at baseline, 3, 6, 12 and 24 months.
Results
Forty-nine eyes of 42 treatment naïve patients were included for analysis (aged 62–89 years). Almost half (49%) presented with a combination of intra- and subretinal fluid at baseline. Intraretinal fluid was present in 75% of eyes but decreased to 22.7% of eyes by 24 months. VA at baseline was 55 letters, and this improved by 6 letters. The change in VA from baseline to 3, 6 and 12 months was statistically significant (p < .05). The mean change in CST from baseline to 3 months was significant (−76 µm). This change was also observed at the other milestones (p < .001).
Conclusions
The findings of this study suggest that allowing some subretinal fluid to remain will not affect treatment outcomes.
INTRODUCTION
Age-related macular degeneration is the leading cause of severe, irreversible and progressive vision loss in the elderly population worldwide.Citation1 Neovascular age-related macular degeneration (nARMD) is responsible for most cases associated with severe vision loss due to growth of abnormal blood vessels under the retina which are weak and are more prone to leakage, thereby contributing to sight-threatening complications of this disease.Citation2,Citation3
Vascular endothelial growth factor (VEGF) inhibitors have been shown to be the most effective treatment option for nARMD as they decrease angiogenic stimulation and vessel leakage, reducing fluid accumulation in the retina. Real-world studiesCitation4–6 show that treatment outcomes are highly variable and are influenced by the treatment regimen.
Traditionally the use of visual acuity gains and central retinal thickness has been used to measure outcomes when investigating the efficacy of VEGF inhibitors. However, it has been suggested that localising retinal fluids in different compartments of the retina offers superior prognostic value for treatment. The presence of intraretinal fluid may have a negative impact on visual acuity;Citation7,Citation8 however, the impact of residual subretinal fluid in terms of treatment outcomes is not completely understood. Therefore, the primary aim of this retrospective clinical audit was to investigate whether the presence and location of subretinal fluid is associated with negative visual acuity outcomes among treatment naïve patients with nARMD following treatment with VEGF inhibitors over a two-year period in a real-world setting. The secondary aims were to investigate dosing pattern and central subfield thickness among this cohort.
MATERIALS AND METHODS
Setting and Patients
A retrospective observational study of treatment naïve patients with nARMD receiving treatment with VEGF inhibitors was conducted. Single user non-identifiable subset data pertaining to these patients was extracted from the ARMD module of the Fight Retinal Blindness! (FRB!) RegistryCitation9 from 13 November 2014 to 14 November 2020. This project received approval from the Human Ethics Committee, La Trobe University (Approval number: 21162).
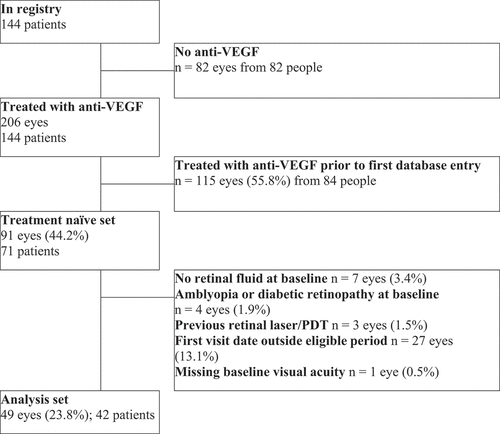
Eyes of patients 50 years or older at first injection were included with a clinical diagnosis of nARMD made by the presence of intraretinal or subretinal fluid on Heidelberg Spectralis HRA + OCT (Heidelberg Engineering, Heidelberg, Germany) Spectral Domain Optical Coherence Tomography (SD-OCT) imaging. All eyes underwent treatment with an intravitreal anti-VEGF agent, and the first entry in the database was at least 24 months before the end of the study period (November 2020). Only eyes with complete values at all milestone visits were included. Eyes were excluded if they were recorded as having no retinal fluid at baseline, had an additional disease or did not have data for all milestone visits. Also prior use of anti-VEGF intravitreal injection or retinal laser.
Clinical Assessment
Visual acuity (VA) was measured under usual clinical conditions using an electronic chart (Med Tech SRL LCD version 4.8 visual acuity chart, Torino Italy®), with spectacle correction. VA was recorded in the number of ETDRS letters for analysis. Visual impairment was classified according to the World Health Organization impairment definition. (No impairment: 70–95 letters; mild: 60–69 letters; moderate: 35–59 letters, Severe or blind: 0–34 letters.Citation10)
Central subfield thickness (CST) was calculated as the average distance from the vitreoretinal interface to the inner border of the retinal pigment epithelium in the central 1 mm ETDRS grid as processed by the default software.
Statistical Methods
Baseline date was allocated separately for each eye and defined as the date of first treatment and included VA and CST measurements using the methods previously described. Milestone visits included those at baseline, 3, 6, 12 and 24 months, and the visits closest to a pre-set target date were chosen.
Analysis of VA was conducted using Spearman rank order correlation. The change in VA was document in two ways: the observed mean VA (using number of ETDRS letters) at each milestone visit (baseline, 3, 6, 12 and 24 months) for all eyes where the number of eyes varied at each milestone, and the observed mean VA for eyes which had data for each milestone visit. A mixed-effects linear regression with random intercepts (patient and eye) and random slopes (eye) to account for intra-eye correlation was undertaken to analyse CST values. A logistic regression, adjusted for baseline CST, was also performed to determine whether there were any predictors of high CST at 24 months.
RESULTS
Patient Characteristics
Of the 144 patients entered into the ARMD module of the FRB registry, 71 patients (91 eyes, 44.2%) were treatment naïve. Forty-nine eyes of 42 treatment naïve patients were eligible for inclusion ().
Figure 1. Flow chart of study participants. IVI = intravitreal injection, PDT = photodynamic therapy, VEGF = vascular endothelial growth factor.
Age ranged from 61 to 89 years (mean 81.9 years, SD 8.7). Median baseline VA was 68 letters (6/12-2, IQR: 45–74), and median CST at baseline was 315 µm (IQR: 278–420) ().
Table 1. Characteristics at baseline (n = 49 eyes).
Treatment and Loss to Follow-Up
Within the 24-month follow-up period, the number of injections ranged from 3 to 15 in the first 12 months (median = 10), with a range of 3 to 25 injections over the 2 years (median = 17). The median number of injections within the first 6 months was 6, and this decreased to 4 per year. In the first 6 months, the median time between injections was 4 weeks, from 6 to 12 months the time was 5 weeks and then at each 6-month interval up to 24 months the median time between injections increased slightly to 6 weeks.
Sixteen eyes (32%) required a switch of anti-VEGF. Eight eyes switched from bevacizumab to aflibercept, 6 switched from aflibercept to ranibizumab, 1 switched from ranibizumab to aflibercept and 1 switched from aflibercept to bevacizumab. The median time interval until first switch was 4.7 months (IQR: 2.9–9.4).
Retinal Fluid
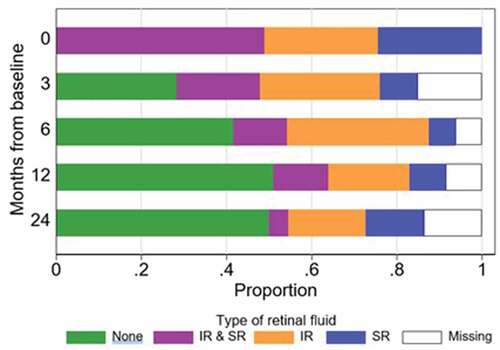
Twenty-four eyes (49.0%) presented with both intra- and subretinal fluid. Thirteen (26.5%) had only intraretinal fluid and 12 eyes (24.5%) had only subretinal fluid at baseline. The proportion of patients with any retinal fluid substantially decreased by 3 months’ proportion of fluid is shown in . Presence of subretinal fluid alone at baseline was associated with better VA compared to having both types of fluid or only intraretinal fluid at baseline. Having subretinal fluid alone at baseline was associated with lower odds of losing ≥5 letters from baseline compared to the presence of both types of fluid (p = .003) but not compared to eyes with intraretinal fluid only at baseline (p = .341).
Change in Visual Acuity
Analysis suggests that VA at 3 months is highly associated with the level of VA at 24 months (r = 0.76, n = 40, p < .05) and is predictive, and VA becomes relatively stable after the first 3 months of treatment. VA at baseline was 55 letters, and this improved by 6 letters. The change in VA from baseline to 3, 6 and 12 months was statistically significant. A Kruskal-Wallis test showed no association between retinal fluid type and VA at baseline (p = .112).
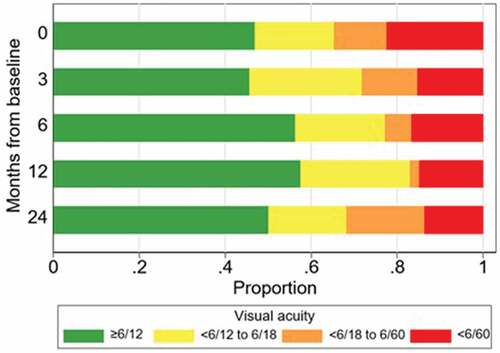
shows the proportion of eyes with visual impairment at the various milestone visits. The proportion of eyes with mild impairment (VA < 6/12-6/18) remained fairly stable, and those with moderate impairment (VA <6/18-6/60) decreased significantly at 6 and 12 months. The number of eyes with severe impairment or blindness (VA<6/60) decreased from 22.4% at baseline to 13.6% at 24 months. Non-adjusted univariate logistic regression showed that baseline VA is a continuous predictor. For any five-letter increase in VA at baseline the estimated odds of visual impairment at 24 months decreased by 11% (p = .015). Also, eyes with less than 6/60 VA at baseline had 14 times the odds of vision impairment at 24 months compared to eyes with vision of 6/12 or better at baseline (p = .023). Univariate logistic regression was used to investigate whether the number of injections had a predictive effect and showed that eyes with = /<13 injections were estimated to have 14 times the odds of visual impairment at 24 months compared to eyes treated with 19 to 24 injections (p = .031).
Figure 3. Proportion of eyes with visual impairment at milestone visits (n = 49, 46, 48, 47 and 44 at months 0, 3, 6, 12 and 24, respectively).
Eyes mostly gained ≥5 or ≥10 letters at 3, 6, 12 and 24 months and only a small proportion of eyes (8.7% to 18.2%) gained ≥ 15 letters ().
Table 2. ETDRS letters gained and lost from baseline at milestone visits.
Change in Central Subfield Thickness
The mean change from baseline to 3 months was significant (−76 µm). This change was also observed at the other milestones (p < .001). Logistic regression indicated that VA; CST; fluid type; visual impairment category; number of anti-VEGF injections by 24 months or switch in anti-VEGF medication were not found to be associated with high values of CST at 24 months (p > .05 for each).
DISCUSSION
The primary aim of this retrospective clinical audit was to investigate whether the presence and location of subretinal fluid has an effect on the visual outcomes of treatment naïve patients with nARMD treated in the real-world setting with VEGF inhibitors. Additional investigation of dosing schedule and visual and anatomical outcomes was also included.
The influence of fluid on the retina and the location of that fluid upon visual outcomes is still not settled.Citation11 Despite the limitations related to a small proportion of missing data by 24 months in our study, the findings suggest that presence of subretinal fluid either at baseline or over time does not affect visual outcomes. A very large proportion of eyes (75%) in this study presented with intraretinal fluid (alone or in combination with subretinal fluid), and this decreased to 22.7% by 24 months, whilst there was a gain in visual acuity. It appears that some remaining subretinal fluid does not affect visual outcomes after treatment treating the retina until all fluid is resolved is probably not necessary.
VA improved by ~5 letters from baseline and generally stablised after the first 3 months. Change in VA was less than that reported in clinical trials;Citation3,Citation11–14 however, it is known that VA outcomes in real-world studies vary and do not approach the outcomes reported in the trials. When compared with some real-world studies, VA gain in this study was similar or better.Citation4,Citation5,Citation15 When comparing the proportion letters gained and lost across the clinical trials our outcomes differ and are worse. It is difficult to make inferences between our outcomes in terms of letters lost or gained due to the differing nature of how outcomes are reported. For example, the proportion of eyes that gained ≥15 letters in our audit by 24 months was 18.2% and Gillies et al.Citation4 reported this proportion to be 16%, but the milestone was 7 years, not 24 months. The RAINBOW studyCitation15 reports that 25.2 to 28.9% of eyes gained ≥15 letters at 12 months compared to 12.8% in this audit at 12 months. The difference, however, is that eyes in the RAINBOW study only received aflibercept, compared to this audit where 79.6% of eyes were treated with aflibercept. The PERSEUS studyCitation6 does not report percentage gain and only reports proportion of eyes with worsening VA of > 5 letters at 12 months (13.6% for eyes with regular dosing and 29.6% for eyes with irregular dosing) compared with 19.1% in our audit.
To gain better insight into visual outcomes in our study, eyes were categorised by visual impairment classification at each milestone. The findings indicate that the proportion of eyes with severe impairment (<6/60) drops significantly within the first 3 months (from 22.4% to 15.2%). By the 24-month milestone a much smaller proportion of eyes are severely impaired (13%) when compared to baseline. The proportion of eyes classified as not impaired, with VA ≥ 6/12, was 46.9% at baseline and remained fairly stable at 50% by 24 months. Gillies et al.Citation4 reported that the proportion of eyes with VA > 6/12 at the 7-year milestone increased from 32% to 40%. The study found that eyes with better baseline VA tend to have better longer-term outcomes, and this is similar to the findings of this audit whereby VA at 3/12 is highly predictive of VA at 24 months. The ceiling and floor effects, that is “eyes with the poorest initial VA cannot get much worse, and eyes starting with the best VA cannot get any better” as described by Gillies et al.Citation4, also held true in this audit because analysis of the relationship between baseline VA and VA at 24 months showed the worse VA at baseline offered more room for improvement over time.
The median baseline CST for patients in our audit was 315 µm, with a large range (218 to 1095 µm). The CST significantly improved at all milestone visits, and the thickness was 85 µm less and 12 months and decreased by 91 µm by 24 months. The more recent clinical trials which report change in CST show a greater improvement after 1 year (with similar baseline values) compared with our study. HAWK and HARRIERCitation14 report a mean change between 143 and 172 µm, whilst VIEW I and VIEW II show CRT improved by 115 to 138 µm.Citation13 Much like VA, the improvement in CST is lower than reported in the clinical trials. Several real-world studies do not report change in CST;Citation4–6 however, it is reported in the RAINBOW studyCitation15 and the improvement ranges from 108.7 to 116.4 µm, from a starting baseline value of 395.6 µm. The limitation of our retrospective audit of treatment naïve patients is the number of cases included for analysis which is significantly smaller than the clinical trials and real-world studies, and this therefore may contribute to the outcomes.
At the 12-month milestone in our audit, patients received between 3 and 15 anti-VEGF injections, with a median of 10 and a median of 17 injections by 24 months (range = 3–25). This is slightly higher than the other real-world studies that report number of treatment injections over 12 months (RAINBOW = 6–6.8; LUMINOUS = 4.7; PERSEUS 7.4–8). The PERSEUS studyCitation6 suggests that patients on an irregular dosing schedule had more injections, compared to those on a regular regimen; however despite more frequent monitoring, their VA outcomes were worse, showing a 2.5-letter gain. Our audit showed that the number of treatment injections had a predictive effect. Eyes with 13 injections or less (i.e., a lower number) were 14 times more likely to have visual impairment at 24 months compared to eyes treated with between 19 and 24 injections. Eyes in out audit received a median of 4 injections for each 6-month period up to 24 months. This finding differs from that reported by WachtlinCitation6 in PERSEUS and suggests that in our cohort, a higher number of treatment occasions has a better effect on VA.
It is clear that visual and anatomical outcomes from a small single practice audit are inferior to the clinical trials. However, when compared to real-world studies, our outcomes compare well, particularly with regard to VA and the significant drop in proportion of eyes with vision impairment within 3 months and half of the eyes not having vision impairment by 24 months. This is despite the limitation of a small sample size of treatment naïve eyes and a small proportion of missing data at the 24-month milestone. However, better visual outcomes in this audit were expected. This could also be explained by ceiling and floor effects influencing this outcome given that median baseline VA was 6/12 (with a range from 6/10 to 6/38). The improvement in CST was also lower than expected, and this could explain VA outcomes. Another limitation relates to the relatively small number of eyes included in our study and treatment naivety. Whilst we are confident we have correctly classified these eyes as treatment naïve, it is possible that some of them may have had prior treatment which is unknown to us.
The results suggest that treatment from baseline to 3 months may be the “critical period” whereby visual gain and CST decrease are the greatest, vision at 3 months is predictive of vision by 24 months and the proportion of eyes with intraretinal fluid is significantly lower. Based on this a strict adherence to the monthly loading dose is warranted to maximise visual and anatomical outcomes.
Finally, this study does raise important issues with regard to treatment of retinal fluid. The findings suggest that allowing some subretinal fluid to remain will not affect treatment outcomes. Further research on the impact of fluid and the location of fluid in the retina is required.
Disclosure Statement
The authors do not have any competing or conflicting interests.
Additional information
Funding
References
- Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99(6):933–943. doi:10.1016/S0161-6420(92)31871-8.
- Mursch-Edlmayr AS, Luft N, Podkowinski D, Ring M, Schmetterer L, Bolz M. Effects of three intravitreal injections of aflibercept on the ocular circulation in eyes with age-related maculopathy. Br J Ophthalmol. 2020;104(1):53–57. doi:10.1136/bjophthalmol-2019-313919.
- Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi:10.1056/NEJMoa054481.
- Gillies MC, Campain A, Barthelmes D, et al. Long-term outcomes of treatment of neovascular age-related macular degeneration: data from an observational study. Ophthalmology. 2015;122(9):1837–1845. doi:10.1016/j.ophtha.2015.05.010.
- Holz F, Minnella A, Tuli R, et al. Ranibizumab treatment patterns in prior ranibizumab-treated neovascular age-related macular degeneration patients: real-world outcomes from the LUMINOUS study. PLoS ONE. 2020;15(12):1–17.doi:10.1371/journal.pone.0244183.
- Wachtlin J, Eter N, Hasanbasic Z, et al. Importance of continuous treatment with intravitreal aflibercept injections in patients with neovascular age-related macular degeneration-12-month post hoc analysis of the PERSEUS real-world evidence study. Graefes Arch Clin Exp Ophthalmol. 2021;259(3):601–611. doi:10.1007/s00417-020-04803-8.
- Holekamp NM, Sadda S, Sarraf D, et al. Effect of residual retinal fluid on visual function in ranibizumab-treated neovascular age-related macular degeneration. Am J Ophthalmol. 2022;233:8–17. doi:10.1016/j.ajo.2021.06.029.
- Schmidt-Erfurth U, Waldstein SM. A paradigm shift in imaging biomarkers in neovascular age-related macular degeneration. Prog Retin Eye Res. 2016;50:1–24. doi:10.1016/j.preteyeres.2015.07.007.
- Gillies MC, Walton R, Liong J, et al. Efficient capture of high-quality data on outcomes of treatment for macular diseases: the fight retinal blindness! Project. Retina. 2014;34(1):188–195. doi:10.1097/IAE.0b013e318296b271.
- WHO. International Classification of Diseases for Mortality and Morbidity Statistics [11th Revision]. World Health Organisation; 2022. https://www.who.int/standards/classifications/classification-of-diseases
- Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–1908.
- Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology. 2009;116(1):57–65.e5. doi:10.1016/j.ophtha.2008.10.018.
- Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–2548. doi:10.1016/j.ophtha.2012.09.006.
- Dugel P, Koh A, Ogura Y, et al. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127(1):72–84. doi:10.1016/j.ophtha.2019.04.017.
- Weber M, Velasque L, Coscas F, Faure C, Aubry I, Cohen SY. Effectiveness and safety of intravitreal aflibercept in patients with wet age-related macular degeneration treated in routine clinical practices across France: 12-month outcomes of the RAINBOW study. BMJ Open Ophthalmol. 2019;4(1):e000109. doi:10.1136/bmjophth-2017-000109.