ABSTRACT
Background
Imaging plays a pivotal role in eye assessment. With the introduction of advanced machine learning and artificial intelligence (AI), the focus has shifted to imaging datasets in ophthalmology. While disparities and health inequalities hidden within data are well-documented, the ophthalmology field faces specific challenges to the creation and maintenance of datasets. Optical Coherence Tomography (OCT) is useful for the diagnosis and monitoring of retinal pathologies, making it valuable for AI applications. This review aims to identify and compare the landscape of publicly available optical coherence tomography databases for AI applications.
Methods
We conducted a literature review on OCT and AI articles with publicly accessible datasets, using PubMed, Scopus, and Web of Science databases. The review retrieved 183 articles, and after full-text analysis, 50 articles were included. From the included articles were identified 8 publicly available OCT datasets, focusing on patient demographics and clinical details for thorough assessment and comparison.
Results
The resulting datasets encompass 154,313 images collected from Spectralis, Cirrus HD, Topcon 3D, and Bioptigen devices. These datasets included normal exams, age-related macular degeneration, and diabetic maculopathy, among others. Comprehensive demographic information is available in one dataset and the USA is the most represented population.
Discussion
Current publicly available OCT databases for AI applications exhibit limitations, stemming from their non-representative nature and the lack of comprehensive demographic information. Limited datasets hamper research and equitable AI development. To promote equitable AI algorithmic development in ophthalmology, there is a need for the creation and dissemination of more representative datasets.
BACKGROUND
Imaging techniques play a crucial role in the field of ophthalmology, as they enable healthcare professionals to visualize and assess the structures of the eye.Citation1 Traditional imaging methods, such as fundus photography and fundus autofluorescence, have been widely used to diagnose and monitor ocular conditions. However, with the advent of new technologies, such as Optical Coherence Tomography (OCT), novel opportunities have emerged in the field of ophthalmology. OCT enables healthcare professionals to visualize and assess ocular structures with high-resolution, non-invasive, and in vivo imaging, facilitating fast diagnosis and real-time monitoring of ocular conditions.Citation2
Optical Coherence Tomography is used to visualize ocular structures in high resolution, through low-coherence interferometry.Citation2,Citation3 It provides cross-sectional slabs of the retina, using light waves to create three-dimensional images of the eye’s internal tissues.Citation2 OCT scans are particularly useful in diagnosing and monitoring various eye conditions, including diabetic macular edema, glaucoma, and age-related macular degeneration, providing information about the thickness and integrity of the ocular structures.Citation4 Additionally, OCT can be used to track changes in ocular diseases over time, assess the effectiveness of treatments, and guide surgical interventions.
The integration of artificial intelligence (AI) into ophthalmic imaging has opened up a landscape of exciting applications.Citation1 AI algorithms can analyze the vast amount of data generated by imaging modalities and assist ophthalmologists in patient care. One such area where AI shows great potential is in classifying and screening of ocular conditions.Citation5,Citation6 Furthermore, AI algorithms can assist in screening programs where large populations need to be assessed for eye diseases. This allows for efficient triaging and ensures that at-risk patients receive timely intervention.Citation7 In addition to classification and screening, AI can better our understanding of ocular conditions. By analyzing large datasets of imaging data, AI algorithms can identify subtle patterns and correlations that may not be apparent to the human eye.Citation8,Citation9 This can lead to new insights into disease progression, treatment response, and personalized medicine approaches.
OCT has emerged as a valuable source of data for AI algorithms as numerous publications have explored its use as a data input. Researchers have subsequently developed AI models that can automatically segment different retinal layers, detect and quantify pathological changes, and predict disease progression.Citation10–12 These models employ deep learning techniques, such as convolutional neural networks, to extract features and make predictions based on OCT images with the goal of improving diagnostics, treatment planning, and patient outcomes in ophthalmology.
Datasets are pivotal for training and developing AI algorithms, aiding in pattern learning, prediction, and insight generation. However, often overlooked is the diversity and quality of said datasets as these are crucial for generalizable and fair algorithms. It is well-documented that much of research is performed in high-income countries, and it is minorities who bear the brunt of this burden.Citation13,Citation14 Careful curation and balance in datasets are essential to avoid biased samples, and reduce AI biases that may perpetuate inequalities.
The field of ophthalmology faces specific challenges, including technical limitations, accessibility disparities, regulatory complexities, ethical considerations, and patient privacy issues. The need for standardized protocols and practices in ophthalmic imaging limits the interoperability and availability of data. In order to overcome these limitations, the FAIR (Findable, Accessible, Interoperable, and Reusable) principles should be applied in Optical Coherence Tomography (OCT) research.Citation15 FAIR principles involve processes like standardizing metadata, improving data accessibility, fostering interoperability, and enhancing data reusability. The use of FAIR principles enables data sharing and the development of equitable and accurate algorithms for ophthalmic imaging.
This review aims to identify and compare the landscape of publicly available optical coherence tomography databases.
MATERIALS AND METHODS
This review focused on publicly available Optical Coherence Tomography datasets. The literature review search included PubMed, Scopus, and Web of Science databases, before 10 July 2023. The search query utilized variations of the keywords “optical coherence tomography”, “dataset,” and “publicly available”. Document types were limited to journal papers, conference papers, and data papers with no language, year, or citation exclusion. The exact search queries can be found in Supplemental File 1.
Papers were considered eligible if they met the following criteria: i) Papers were published in academic journals, conference proceedings, and reputable sources; ii) Studies that focus on open OCT datasets; iii) Studies that provide information on the availability, accessibility, and use of open OCT datasets.
Papers were excluded if they did not meet all of the following: i) Non-academic sources such as blog posts, opinion pieces, and news articles; ii) No report of an Open OCT dataset. Analysis of documents and the assessment process for eligibility was performed by three authors (DR, JQ, LFN).
After screening and review of the full text, the applied datasets used in each of the articles were retrieved from the final list of documents. Of these datasets, only the datasets classified by the authors as open were included.
For the purpose of this paper, an open dataset refers to a comprehensive and publicly accessible repository of data that is made available without any restrictions on access, financial barriers, or usage limitations. Such datasets are typically provided in a format that allows researchers to freely download, analyze, modify, and share the data for academic, scientific, and educational purposes. Thus, in order to be considered open, a dataset must meet the following requirements:
The dataset must have a working link to the data. If the link is no longer working at the time of analysis, the dataset will also not be included in the review.
There should be no monetary cost for the use of the dataset.
The use of the dataset does not require extra permissions or requests via email to the authors, that is, it must be in an open repository.
For this review, the variables analyzed of the OCT datasets include the dataset name, device used for imaging, OCT technology employed, the number of images or eyes included in the dataset, demographic information of the subjects, proportions of different diseases present in the dataset, and the ratio of healthy and sick individuals, the count of abnormal slabs detected in the OCT images, the country of origin of the patients, and the labeling methodology employed in the dataset.
RESULTS
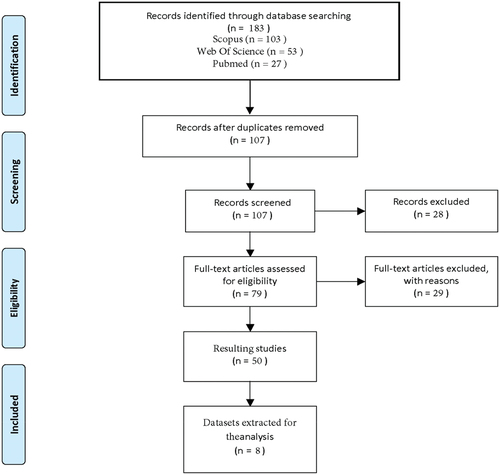
The search strategy yielded 183 articles, from which 76 duplicates were removed. From the remaining 107 articles, a primary screening based on the title and abstract assessment excluded 28 papers, and the subsequent full-text analysis excluded 29 papers. A total of 50 articles were used in the final analysis for dataset assessment. ()
From the 50 included articles, a total of 15 datasets were retrieved, 8 publicly available included in this review. The final list of datasets resulting in this review can be seen in .
Table 1. Open OCT datasets details.
OCT DEVICES
OCT exams offer detailed cross-sectional images of ocular tissue by reconstructing measurements of light reflection within ocular structures. These scans come in various modes, with the spectral domain and swept source technologies offering enhanced speed and resolution compared to the older time domain OCT systems.
While the capturing and underlying technology are similar, variation across different devices results in distinct images, which can compromise the generalizability of developed algorithms.
The datasets explored in this study encompass a range of OCT equipment and exams. Four datasets utilized exams conducted with the Spectralis, a high-resolution spectral domain OCT system manufactured by Heidelberg Engineering. Another dataset consisted of exams from the Cirrus HD-OCT (non-specified version), a spectral domain OCT. Additionally, two datasets featured exams from the Topcon 3D OCT-2000 and the Topcon 3D OCT-1000, both of which are spectral domain OCT devices. () Lastly, one dataset included exams conducted using the Bioptigen OCT (non-specified version), which is another spectral domain OCT system employed for ophthalmic imaging.
Figure 2. Comparative Samples of OCT Imaging Technologies: A. Cirrus HD OCT from OCTID, B. Topcon 3D OCT-1000 from data on OCT and fundus images, C. Spectralis OCT by Heidelberg Engineering from labeled OCT and chest X-Ray images.
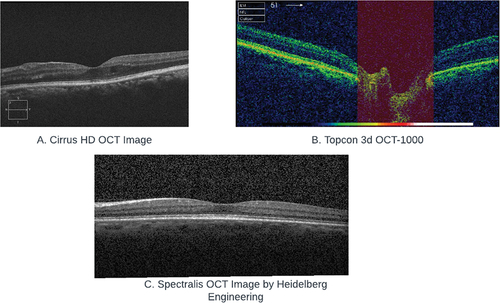
Notably, all datasets included spectral domain OCTs, without any time-domain or swept-source OCT examinations. Additionally, the Topcon 3D–1000 and 2000 were the pioneering models of Topcon’s SD OCTs, released in 2006 and 2009, respectively. However, these models have since been replaced by newer technologies.
DEMOGRAPHICS
There is an increased concern about the potential biases in AI systems when developed utilizing non-representative data.Citation16 To effectively address and mitigate such biases throughout the AI development process, it becomes essential to incorporate a comprehensive sociodemographic data.Citation16
Sociodemographic information was not widely available throughout all eight datasets. Patient age information is available in three datasets,Citation17–19 gender in two datasets,Citation17,Citation18 and race in one.Citation18 Only one dataset provided all three.Citation18
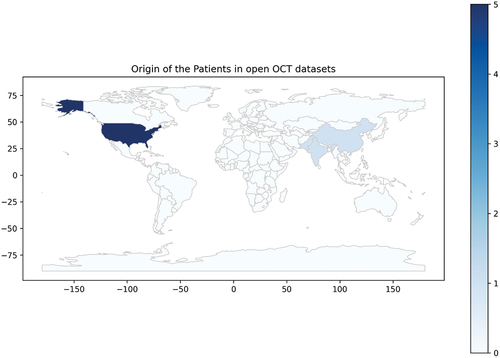
Artificial intelligence systems need to be trained and validated on-site and population-specific targets.Citation20 Of the reviewed datasets, five include patients from the USA, one from China, India, the UK, and Pakistan, showing a clear imbalance in geographic representation, as seen in .
Figure 3. Distribution of patients in the OCT datasets per country. Map created adapting naturalearth_lowres’s layer using (c) 2013–2022, GeoPandas developers, an open-source python package created under the liberal terms of the BSD-3-Clause license.Citation21.
EXAMS AND DISEASES
A total of 154,313 images were included, although specific counts for the number of eyes and patients remain undisclosed. The examination records encompass a variety of image compressed formats, including JPG, TIF, and MAT, with one dataset presenting OCT information in the form of OCT segmentation voxels, with no dataset in raw DICOM format available.Citation18
Seven of the datasets are dedicated to retinal diseases and feature macular slabs, while one dataset focuses on glaucoma and includes optic disc-centered slabs. These images include both healthy individuals and those afflicted with a diverse range of conditions such as choroidal neovascularization, diabetic macular edema, drusen, age-related macular degeneration, retinal vascular occlusion, macular holes, and glaucoma.
LABELING
Labeling and annotation are crucial for the exam interpretation and encompass manual annotation of structures within the OCT scan, image segmentation, and classification.
In three of the datasets labeling was done via classifications,Citation19,Citation22,Citation23 two datasets used layer segmentation,Citation17,Citation24 one used finding segmentation,Citation18 one utilized both classifications and layer segmentation,Citation25 and one was without labels and considered undefined.Citation26
When detailing the classification datasets, there are variations in the level of granularity. In two datasets, the eye macular slabs are available, yet the classification itself is performed at the eye-level.Citation19,Citation22 In contrast, the OCTID dataset offers image-level classification, but it comprises only one fovea-centered image per eye.Citation25 Lastly, the Data on OCT and Fundus Images provides image-level classifications along with the corresponding color fundus photographs.Citation23
All datasets with labels employed combinations of clinicians, ophthalmology residents, general ophthalmologists, and retina and glaucoma specialists for their labeling criteria. In one dataset, tiered labeling with adjudication is described in.Citation22
DISCUSSION
The performed review shines light on the current landscape of publicly-available OCT datasets. Of 183 papers yielded from the search strategy, only eight OCT datasets were found that met the requirements to be considered “open.” The OCT is widely implemented as an ancillary exam in ophthalmology to support retinal and glaucoma diagnosis and is considered valuable input for AI-automated systems. Nevertheless, the number of publicly available OCT datasets is limited. The lack of open data in such a widely applied test worldwide hinders and delays the advancement of research in this field.
These eight datasets have restricted geographical representation and limited demographic description. Latin America, Africa, and much of Europe and Southeast Asia are not represented in these datasets, highlighting massive gaps in research and knowledge generation that are multifactorial in nature. Many of these countries do not have the infrastructure to create datasets, educate personnel, and fund research, especially given the highly specialized nature of the equipment, and the countries with the necessary tools are not including the ones without. Additionally, variability in OCT scans is observed across different racial, gender, and age groups.Citation27,Citation28 For example, in pediatric ophthalmology, in vivo OCT studies provide evidence of ongoing retinal development in early childhood, suggesting that early and timely treatment of certain ophthalmologic conditions may optimize visual function.Citation29 This highlights how demographic information, such as age, in OCT scan data can contribute to an improved utility of the dataset. Ensuring that our data is both representative and diverse is imperative to detect and mitigate potential biases in algorithms relying on OCT exams. Unfortunately, the reviewed datasets often lack comprehensive demographic information. Moving forward, initiatives should be taken to promote the creation and sharing of more diverse and representative OCT datasets.
OCTs exams are not reproducible across devices and scan technology,Citation30,Citation31 and in clinical practice, it is not recommended for comparison between different devices. In our review, only five OCT devices are represented in OCT datasets, all using spectral domain technology. More diverse data from other devices and comparative studies are needed to assess the generalizability of AI algorithms.
Reliable labeling is a prerequisite for the development of AI algorithms. It is essential that datasets include clear documentation of the labeling protocol, criteria, and adjudication. Regrettably, the reviewed datasets do not provide adequate details regarding the labeling process.In addition, inter- and intra-observer variability should be taken into account to establish a robust dataset. Variations in interpretation and labeling between different clinicians or even the same clinician at different times can significantly impact the reliability of the dataset.
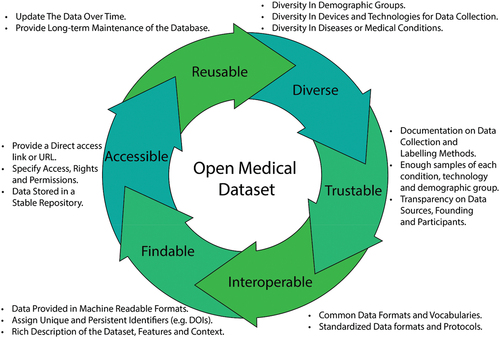
The ideal OCT dataset would encompass several critical criteria for its effectiveness. Firstly, it should be characterized by diversity, both in terms of demographics and devices. Demographic diversity ensures that the dataset represents a wide range of ages, genders, races, and ethnicities, making it applicable to a broader patient population. Similarly, including data from various OCT devices, with different specifications and technologies, enhances the dataset’s versatility and applicability across different clinical settings. Trustable labels are essential, ensuring that the dataset’s annotations and labels are accurate and reliable, often achieved through meticulous manual segmentation by qualified clinicians. A large number of images is crucial to support robust algorithm training and validation, allowing for more accurate and generalizable results. Moreover, the dataset should encompass multiple diseases, covering a spectrum of ocular conditions, to facilitate comprehensive research and diagnostic capabilities. Well-documented report characteristics, such as image formats and labeling methodologies, are vital for researchers to effectively utilize and interpret the dataset’s contents. Lastly, the datasets should also comply with the FAIR principles of findability, accessibility, interoperability, and reusability to allow reliability and usability. The structure of the ideal OCT dataset is suggested in .
Addressing the limitations in ophthalmic datasets requires collaborative efforts among researchers, healthcare institutions, and regulatory bodies. Initiatives that encourage data sharing, establish standardized protocols for data collection and annotation and ensure patient privacy can help alleviate some of these challenges. Furthermore, efforts should be made to actively collect data from diverse patient populations to ensure fairness and equity in the development of AI algorithms for ophthalmology.
Firstly, ophthalmic imaging data tends to be complex and highly specialized. The acquisition of high-quality ophthalmic images requires specialized equipment and expertise, making it challenging to collect large-scale datasets. The availability of diverse datasets can be further hindered by factors such as privacy regulations, limited access to patient records, and data-sharing restrictions. Secondly, ophthalmic conditions may exhibit significant variability within and between individuals. Factors like anatomical differences, disease severity, and treatment responses can contribute to variations in the data. This variability can make it challenging to develop algorithms that generalize well across different patients and ophthalmic conditions.
Furthermore, certain ocular conditions are relatively rare, which can limit the availability of large datasets specifically dedicated to those conditions. This scarcity of data can impede the development and training of AI models for these specific conditions, hindering progress in diagnosis, treatment, and research.
CONCLUSION
In conclusion, this review highlights the critical role of Optical Coherence Tomography in ophthalmology and its integration with artificial intelligence (AI) for enhanced diagnosis and patient care. OCT provides detailed insights into ocular structures, making it a valuable source of data for AI algorithms. AI shows great promise in classifying and screening ocular conditions, contributing to a better understanding of these conditions, and improving diagnostics and treatment planning.
However, the review also underscores several challenges in the field, particularly regarding the characteristics and availability of datasets. The limited diversity, geographical representation, and demographic description reveal a significant gap in research and knowledge generation, especially in regions like Latin America, Europe, and Africa. These challenges, along with the complex nature of ophthalmic imaging data, emphasize the need for collaborative efforts to promote data sharing, standardize data collection and annotation, and ensure patient privacy.
Addressing these limitations and actively collecting diverse datasets will be essential to advance the development of AI algorithms for ophthalmology. Despite these challenges, the integration of AI with OCT imaging holds great promise for enhancing the accuracy, efficiency, and accessibility of diagnosing and managing ocular conditions. As technology and research in this field continue to evolve, the synergy between OCT and AI will likely play a pivotal role in improving patient outcomes and expanding our understanding of eye diseases on a global scale.
While the use of OCT technology in AI is promising, the field is still lacking in terms of diversity and research in validation and identification of bias in OCT datasets. Further studies are needed to address the assets of OCT datasets in terms of enhancing diversity and mitigating bias, thereby ensuring more comprehensive and equitable advancements in the field of AI applied to ophthalmology imaging.
Supplemental Material
Download MS Word (14.6 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/08820538.2024.2308248.
Additional information
Funding
REFERENCES
- Benet D, Pellicer-Valero OJ. Artificial intelligence: the unstoppable revolution in ophthalmology Surv Ophthalmol. 2022;67(1):252–270. doi:10.1016/j.survophthal.2021.03.003.
- Aumann S, Donner S, Fischer J, Müller F. Optical coherence tomography (OCT): principle and technical realization. In: Bille J, ed. High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics. Cham (CH): Springer; 2019. doi:10.1007/978-3-030-16638-0.
- Gabriele ML, Wollstein G, Ishikawa H, Kagemann L, Xu J, Folio LS, Schuman JS. Optical coherence tomography: history, current status, and laboratory work. Invest Ophthalmol Vis Sci. 2011;52(5):2425–2436. doi:10.1167/iovs.10-6312.
- Wei X, Sui R. A review of machine learning algorithms for retinal cyst segmentation on optical coherence tomography Sensors. 2023;23(6):3144. doi:10.3390/s23063144.
- Ting DSW, Peng L, Varadarajan AV, Keane PA, Burlina PM, Chiang MF, et al. Deep learning in ophthalmology: the technical and clinical considerations. Prog Retin Eye Res. 2019;72:100759. doi:10.1016/j.preteyeres.2019.04.003.
- Esteva A, Robicquet A, Ramsundar B, Kuleshov V, DePristo M, Chou K, et al. A guide to deep learning in healthcare. Nat Med. 2019;25(1):24–29. doi:10.1038/s41591-018-0316-z.
- Ting DSW, Pasquale LR, Peng L, Campbell JP, Lee AY, Raman R, et al. Artificial intelligence and deep learning in ophthalmology. Br J Ophthalmol. 2019;103(2):167–175. doi:10.1136/bjophthalmol-2018-313173.
- Kim YD, Noh KJ, Byun SJ, Lee S, Kim T, Sunwoo L, et al. Effects of hypertension, diabetes, and smoking on age and sex prediction from retinal fundus images. Sci Rep. 2020;10(1):4623. doi:10.1038/s41598-020-61519-9.
- Gichoya JW, Banerjee I, Bhimireddy AR, Burns JL, Celi LA, Chen L-C, et al. AI recognition of patient race in medical imaging: a modelling study. Lancet Digit Health. 2022;4(6):e406–e414. doi:10.1016/s2589-7500(22)00063-2.
- Moraes G, Fu DJ, Wilson M, Khalid H, Wagner SK, Korot E, et al. Quantitative Analysis of OCT for Neovascular Age-Related Macular Degeneration Using Deep Learning. Ophthalmology. 2021;128(5):693–705. doi:10.1016/j.ophtha.2020.09.025.
- Anegondi N, Gao SS, Steffen V, Spaide RF, Sadda SR, Holz FG, et al. Deep learning to predict geographic atrophy area and growth rate from multimodal imaging. Ophthalmol Retina. 2023;7(3):243–252. doi:10.1016/j.oret.2022.08.018.
- Balaskas K, Glinton S, Keenan TDL, Faes L, Liefers B, Zhang G, et al. Prediction of visual function from automatically quantified optical coherence tomography biomarkers in patients with geographic atrophy using machine learning. Sci Rep. 2022;12(1):15565. doi:10.1038/s41598-022-19413-z.
- HealthLeaders. Root of some health disparities may be buried in technology. [cited 25 Aug 2023]. Available: https://www.healthleadersmedia.com/technology/root-some-health-disparities-may-be-buried-technology
- Gianfrancesco MA, Tamang S, Yazdany J, Schmajuk G. Potential biases in machine learning algorithms using electronic health record data JAMA Intern Med. 2018;178(11):1544–1547. doi:10.1001/jamainternmed.2018.3763.
- Wilkinson MD, Dumontier M, Aalbersberg IJ, Appleton G, Axton M, Baak A, et al. The FAIR guiding principles for scientific data management and stewardship. Sci Data. 2016;3(1):1–9. doi:10.1038/sdata.2016.18.
- Nakayama LF, Matos J, Quion J, Novaes F, Mitchell WG, Mwavu R, et al. Unmasking Biases and Navigating Pitfalls in the Ophthalmic Artificial Intelligence Lifecycle: A Review. 2023. doi:10.48550/ARXIV.2310.04997.
- IACL. https://iacl.ece.jhu.edu/Internet. [cited 1 Aug 2023]. Available: https://iacl.ece.jhu.edu/index.php?title=Main_Page
- Moraes G, Fu DJ, Wilson M, Khalid H, Wagner SK, Korot E, et al. Quantitative analysis of optical coherence tomography for neovascular age-related macular degeneration using deep learning. Dryad. 2020. doi:10.5061/dryad.2rbnzs7m4.
- Farsiu Ophthalmology. 2013. [cited 1 Aug 2023]. Available: https://people.duke.edu/~sf59/RPEDC_Ophth_2013_dataset.htm
- Youssef A, Pencina M, Thakur A, Zhu T, Clifton D, Shah NH. External validation of AI models in health should be replaced with recurring local validation Nat Med. 2023;29(11):2686–2687. doi:10.1038/s41591-023-02540-z.
- GeoPandas. [cited 2 Sep 2023]. Available: https://geopandas.org/en/stable/about/citing.html
- Kermany D. Large dataset of labeled optical coherence tomography (OCT) and chest X-Ray images. Mendeley. 2018. doi:10.17632/RSCBJBR9SJ.3.
- Raja H. Data on OCT and Fundus Images. Mendeley. 2020. doi:10.17632/2RNNZ5NZ74.2.
- Chiu dataset. https://people.duke.edu/Internet. [cited 18 Dec 2023]. Available: http://www.duke.edu/~sf59/Chiu_BOE_2014_dataset.htm
- Optical coherence tomography image retinal database. [cited 18 Dec 2023]. Available: https://borealisdata.ca/dataverse/OCTID
- Fang dataset. In: https://people.duke.edu/Internet]. [cited 18 Dec 2023]. Available: https://people.duke.edu/~sf59/Fang_TMI_2013.htm
- Girkin CA, McGwin G Jr, Sinai MJ, Sekhar GC, Fingeret M, Wollstein G, et al. Variation in optic nerve and macular structure with age and race with spectral-domain optical coherence tomography. Ophthalmology. 2011;118(12):2403–2408. doi:10.1016/j.ophtha.2011.06.013.
- Antar H, Tsikata E, Ratanawongphaibul K, Zhang J, Shieh E, Lee R, et al. Analysis of Neuroretinal Rim by age, race, and sex using high-density 3-dimensional spectral-domain optical coherence tomography. J Glaucoma. 2019;28(11):979–988. doi:10.1097/IJG.0000000000001381.
- Lee H, Proudlock FA, Gottlob I. Pediatric optical coherence tomography in clinical practice—recent progress Invest Ophthalmol Vis Sci. 2016;57(9):OCT69–OCT79. doi:10.1167/iovs.15-18825.
- Yılmaz H, Köylü MT, Yılmaz AC, Durukan AH, Uysal Y. Agreement between standard optical coherence tomography and optical coherence tomography-based angiography in estimating retinal nerve fiber Layer thickness Turk J Ophthalmol. 2020;50(5):264–270. doi:10.4274/tjo.galenos.2020.18488.
- Han IC, Jaffe GJ. Comparison of spectral- and time-domain optical coherence tomography for retinal thickness measurements in healthy and Diseased Eyes Am J Ophthalmol. 2009;147(5):847–858.e1. doi:10.1016/j.ajo.2008.11.019.