ABSTRACT
Purpose
Epiphora in childhood is a frequent symptom that is typically associated with Congenital nasolacrimal duct obstruction (CNLDO). Nevertheless, inflammatory pathologies of the ocular surface as well as inside the eye, or even congenital glaucoma, must be considered in the differential diagnosis.
Methods
A comprehensive literature review concerning CNLDO was conducted. Different therapeutic steps are categorized and summarized in order to reflect the existing staged therapeutic concept.
Results
For CNLDO, a staged therapeutic concept is applicable, resulting in a cure rate of approximately 95% with only conservative or minimally invasive intervention. This concept includes five steps that encompass therapeutic interventions with increasing complexity. It includes conservative techniques, followed by probing and syringing, transcanalicular approaches without or with lacrimal intubation, and dacryocystorhinostomy which is the ultima ratio.
Conclusion
To preserve the topographic anatomy as much as possible, therapeutic recommendations enable stepwise and individualized management of children with CNLDO
INTRODUCTION
Watery eyes (epiphora) are one of the most common symptoms that lead to young patients being examined by ophthalmologists. Typically, this is caused by a condition called Congenital nasolacrimal duct obstruction (CNLDO). Although approximately 70% of newborns have a persistent Hasner’s membrane, only about 6–20% of children develop chronic epiphora,Citation2–4 illustrating that the majority of patients (over 95%) show spontaneous healing within the first few weeks of life.Citation4
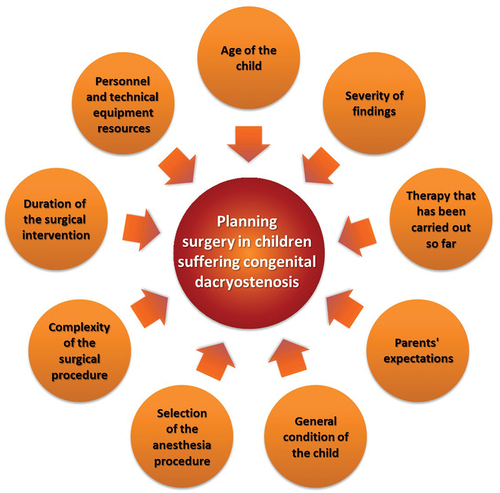
However, the clinical diagnosis of CNLDO is not uniform. Owing to the high spontaneous healing rate, different initial clinical situations, and young age of the patients, therapeutic interventions must be critically reconsidered.Citation5 Therefore, physicians find themselves in an area of conflict among various influencing factors ().
Figure 1. Factors affecting the therapeutic decision in children with congenital nasolacrimal duct obstruction.
The complex anatomy of the lacrimal drainage system must be considered when planning the treatment. A typical cause of CNLDO is membranous atresia of the nasolacrimal duct due to persistent Hasner’s membrane.Citation1,Citation6 Nevertheless, depending on the localization and extent of the stenosis, as well as the underlying general diseases, different therapeutic approaches can be considered. These strategies can be integrated into a step-by-step therapeutic approach. It is intended to provide decision-making aid for the treatment of CNLDO without claiming to be exhaustive. Like any therapeutic regimen, it is subject to ongoing influences and developments and must be adapted to the current (scientific) state-of-the-art. There have been constant advancements in this field from the 19th century to the present day.
EMBRYOLOGY OF THE LACRIMAL DUCTS
The fusion of the maxillary processes with the neighboring lateral nasal processes results in the formation of the nasolacrimal sulcus. Cell formation can be detected at a gestational age of approximately six weeks. This ectodermal anlage separates from the surface and forms an epithelial strand that proliferates and expands in the craniocaudal direction, whereby apoptotic processes lead to lumen formation. These processes continue until the 7th month of development and the eyelid edges are attained through the formation of lacrimal puncta.Citation1,Citation7
Caudal sprouting in the direction of the lower nasal passage is often incomplete, and a bone or mucosal bridge (Hasner’s membrane) may persist. Additionally, premature or incomplete sprouting and a lack of involution can lead to malformations (e.g., lacrimal sac fistulas or even the complete absence of the lacrimal tube). The most common cause is membranous atresia of the nasolacrimal duct. In contrast, aplasia (non-formation in the presence of organ anlage) is very rare.Citation1,Citation2,Citation7
CLINICAL PICTURE OF CONGENITAL NASOLACRIMAL DUCT OBSTRUCTION
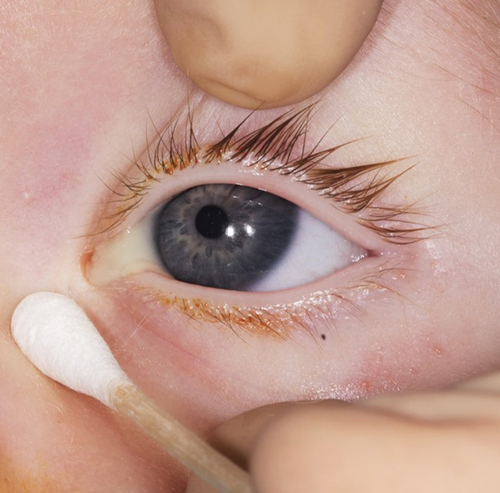
Epiphora is the primary symptom of CNLDO. It can often be ascertained from family history. Over 90% of children show this symptom within the first four weeks of life.Citation4 Clinically, a standing lacrimal lake and an enlarged lacrimal meniscus are present. In cases of unilateral involvement, a comparison can be made with the unaffected side. However, it should be noted that in up to 30% of cases, bilateral manifestations can be found.Citation8 Mucopurulent secretion is another classic clinical sign. Compression of the lacrimal sac can be performed to verify the same ().
The presence of these mucopurulent secretions indicates intra- or postsaccal localization of the stenosis (see below). Nevertheless, several important differential diagnoses must be considered ().Citation9 Despite mucopurulent secretions, clinically visible red eye is not observed. However, it may have resulted from a tear drainage disorder in the sense of an ascending infection with conjunctivitis.
Table 1. Selection of possible differential diagnoses of epiphora due to congenital dacryostenosis (modified from [9]).
LACRIMAL DIAGNOSTICS IN PEDIATRIC PATIENTS
Before the examiner examines the tear ducts, the following examinations are generally recommended when the child presents for the first time:
Orienting Visual Acuity Test
Testing red fundus reflex (Brückner’s test)
Testing pupillary reactions
Macroscopic inspection of the periocular region (facial skull, cleft formation, and scars)
Assessment of hypertelorism, lid position, palpebral fissure width, and lid edges
Macroscopic inspection of the lacrimal sac region for possible lacrimal sac fistulas, swelling, and change in skin coloration (DD: amniotocele with livid coloration)
Microscopic inspection of the anterior segment of the eye
Digital palpation of intraocular pressure
The use of a handheld slit lamp can be helpful in the differential diagnosis of epiphora in the context of CNLDO.
Fluorescein Dye Disappearance Test – FDDT
The spontaneous drainage of tears can be checked by instilling 1% fluorescein into the conjunctival sac. After 5 min, no fluorescence should be detectable under blue light illumination. This test is highly specific for the detection of CNLDO (sensitivity: 90%; specificity: 100%).Citation10
Compression of the Lacrimal Sac (Canaliculus Test According to West)
Only after FDDT has been performed should the lacrimal sac be examined for the presence of mucous secretion. By compressing the lacrimal sac from the outside against its medial border (fossa sacci lacrimalis), the contents of the lacrimal sac can be expressed towards the lacrimal puncta. In case of intra- or postsaccal stenosis localization, there was a more or less pronounced purulent discharge of secretions (). The previous administration of dye-containing eye drops (FDDT) results in a reflux of dye.Citation11
Sonography of the Lacrimal Sac Region
The filling status of the lacrimal sac, particularly for the differentiation of other masses (e.g., tumors, vascular malformations, meningoceles), can be delineated by sonography using a B-scan. In the case of an amniotocele, a cystically altered lacrimal sac with sonographically low internal reflectivity can be detected.Citation12
Diagnostic Probing and Irrigation of the Draining Tear Ducts
Diagnostic intended lacrimal irrigation to rule out CNLDO is obsolete, as there is a risk of iatrogenic damage (mucosal bleeding) with secondary stenosis (scarring).Citation13 However, if therapeutic lacrimal irrigation is to be considered, irrigation via the canaliculi should be performed at the start of probing. If irrigation is already possible, probing deeper sections of the lacrimal ducts can be dispensed.
Classification of Congenital Nasolacrimal Duct Obstruction
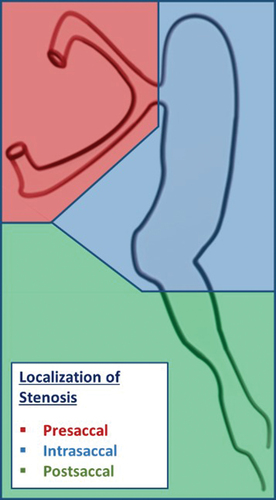
In general, lacrimal stenosis can be classified according to different criteria ().Citation11 Depending on the degree of the stenosis, a narrowing (relative stenosis) must be differentiated from an occlusion (absolute stenosis) of the lacrimal ducts. Due to the expected growth, relative stenoses rarely require treatment in children, as it can be assumed that they improve spontaneously as the child grows. Transient symptoms of epiphora and mucopurulent secretions occur, particularly in cases of mucosal swelling in the context of rhinitis (functional units from the nasal and lacrimal mucosa). These are regarded as functional stenoses, as they disappear spontaneously when the cold subsides.
Table 2. Classification of lacrimal stenosis.
This type of stenosis can be differentiated. If epiphora occurs but the lacrimal ducts can be flushed freely, functional stenosis can be assumed. If lacrimal irrigation shows complete blockage or if significantly increased irrigation pressure is required, mechanical stenosis can be assumed. Depending on the localization in relation to the lacrimal sac, a distinction is made between pre-, intra-, and postsaccal lacrimal stenosis ().Citation11
CLASSICAL CONGENITAL NASOLACRIMAL DUCT OBSTRUCTION
The “classic form” of CNLDO is the persistence of Hasner’s membrane. It can be assumed that many newborns (50–70%) have not yet completed canalization of the nasolacrimal duct. In over 90% of cases, the children have symptoms corresponding to CNLDO (epiphora, mucopurulent discharge) just a few days after birth. It is interesting to note that 90% of these children heal spontaneously within the first four weeks and sometimes do not become symptomatic at all. A study by MacEwen and Young showed that epiphora spontaneously stops at the end of the first year of life in approximately 96% of cases.Citation2,Citation4 However, this study did not differentiate between epiphora with and without clinical signs of dacryocystitis. Differentiating epiphora between chronic dacryocystitis and other eye diseases is important. The latter requires intra- or postsaccal drainage disorder and treatment.
Chronic dacryocystitis can be acutely exacerbated, leading to further inflammatory complications.Citation14 Additionally, prolonged inflammation of the lacrimal sac is associated with poor prognosis after surgical intervention.Citation15 Therefore, lacrimal sac inflammation must be treated.
COMPLEX CONGENITAL NASOLACRIMAL DUCT OBSTRUCTION
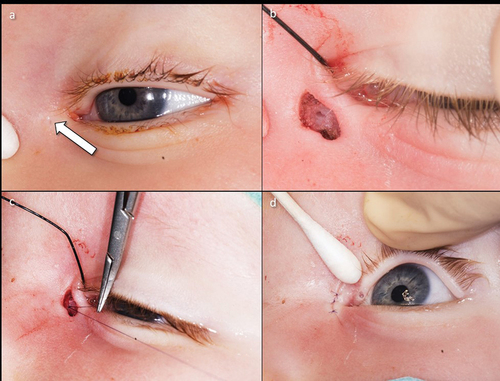
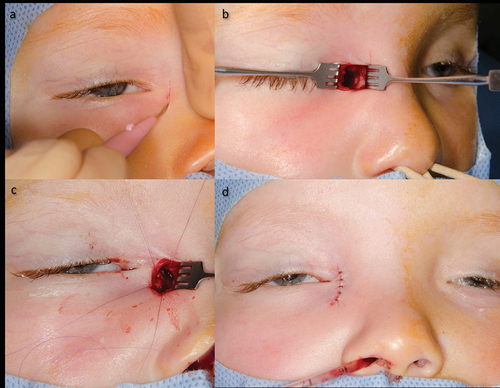
Complex forms of CNLDO include therapy-refractory courses and lacrimal malformations. True malformations are rare and occur in approx. 1:1.000 newborn.Citation16 Duplications or fistulas were frequently observed in the malformations (). Treatment is only necessary if these become symptomatic (delayed tear drainage, mucopurulent secretion, or secondary infection). Complex dacryostenoses include tear drainage disorders, as well as head and neck malformations. These include craniofacial dystrophy, cranial dysostosis, and rare facial cleft formation. Clinical features are found in isolation or as part of a syndrome. However, other genetic disorders also frequently show a deviating anatomy of the lacrimal ducts (trisomy 21, Rubinstein-Taybi syndrome, etc.).Citation2,Citation16,Citation17
Figure 4. Lacrimal fistula and its surgical treatment.
TREATMENT OPTIONS FOR CONGENITAL NASOLACRIMAL DUCT OBSTRUCTION
CNLDO treatment includes both conservative and invasive measures. Indications for conservative therapy should be generally considered. Invasive measures, on the other hand, must be carefully considered due to the high spontaneous healing rate. The reasons for invasive treatment are as follows:
Lack of success with conservative therapy
Recurrent secondary infections peri-ocularly (eyelid eczema) or on the ocular surface area (conjunctivitis)
Development of an acute pyomucocele of the lacrimal sac with phlegmonous spread
Chronic purulent dacryocystitis with a risk of fibrotic remodeling of Hasner’s membrane, and
Development of amblyopia with long-lasting (>18 months) symptoms.Citation14,Citation15,Citation18–20
Stage 1 - Conservative Measures
Conservative treatment includes massage of the lacrimal sac, astringent nose drops, and the use of topical and systemic antibiotics in cases of acute exacerbation. Even in the 19th century, the possibility of compressing the lacrimal sac to treat CNLDO was pointed out.Citation6 Crigler described a structured procedure for compressing the lacrimal sac by increasing the pressure in the direction of the Hasner’s membrane. The lacrimal sac is first compressed horizontally in the medial direction while compressing the canaliculi to increase the pressure in an inferior direction to the lacrimal sac and cause a perforation of Hasner’s membrane.Citation21 Stolovitch et al. reported a success rate for this method within the first year of life of 56% in children under 2 months, 46% in children aged 2 to 6 months, and 28% in children older than 6 months.Citation22
A more recent study by Bansal et al. was even able to show better treatment success. Depending on age, success rate was 87.3%, 78.9%, 77.9%, and 76.8% in children aged up to 3, 6, 9, and 12 months. Although the differences were not significant, these findings highlight the importance of early treatment initiation. The authors also emphasized that the correct performance of lacrimal sac massage is an important influencing factor. The success rate after the initial incorrect procedure was only 61.2%.Citation23
Therefore, the use of topical antibiotics should be minimized. Indications were ascending infections (severe conjunctivitis with red eyes) and acute exacerbations (dacryocystitis). The use of antibiotic eye drops carries the risk of developing bacterial resistance.Citation24 The extent to which a chronic bacterial infection of the lacrimal sac negatively influences the success of treatment is controversial.Citation3,Citation15,Citation24,Citation25 Nevertheless, a possible fibrotic remodeling of the Hasner’s membrane as a result of chronic inflammation is considered to be the cause of reduced treatment success in older children.Citation15 Therefore, tear sac massage is of therapeutic importance. However, parents should be shown how to perform this precisely, taking into account the anatomical conditions and the force to be applied. Finally, lacrimal sac massage should be administered generously in any case of suspected CNLDO as there is no evidence of a negative effect in the event of overtreatment.
Additionally, warm compress and regular cleaning of eyelids can be part of the conservative management to relieve eye irritation and reduce inflammation.
Stage 2 - Probing and Pressurized Lacrimal Irrigation Under Local Anesthesia
Indications for invasive therapy by probing and pressurized lacrimal irrigation include chronic dacryocystitis with ongoing symptoms since birth and amniotocele. Treatment under local anesthesia can be performed within the first year of life.Citation2,Citation3,Citation8,Citation9,Citation15,Citation25
After topical anesthesia and oral administration of 40% glucose solution, an atraumatic TNW cannula according to Bangerter is inserted into the lacrimal puncta vertically to the edge of the eyelid after bougienage of the lacrimal puncta. Further canalicular probing with the cannula is performed after approximately 1 mm parallel to the lid edge until the periosteal stop is reached in the lacrimal sac (“hard stop”). A slight over-inflation of the lacrimal sac by syringing helps protect the intraluminal mucosa of the lacrimal sac. The irrigation cannula was positioned vertically to probe caudally, in the direction of the nasolacrimal duct. If the persistent Hasner’s membrane cannot be opened by increasing the irrigation pressure alone, the stenosis is ruptured by the direct cannula pressure through further advancement (). Physician experience and care are fundamental prerequisites for successful application. The probing direction, force, and irrigation volume (maximum of 2 ml physiological saline solution) are the most important parameters that must be considered. In experienced hands, healing is possible in almost 90% of patients.Citation3,Citation8,Citation12,Citation25 The instruments required are straightforward and limited to a fine conical probe, a 0.6 mm Bangerter cannula, 2 ml physiological saline solution in a syringe, and eye drops for anesthesia ().
Figure 5. Lacrimal probing and syringing under local anesthesia (upper row) and general anesthesia (lower row).
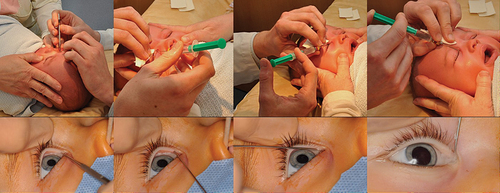
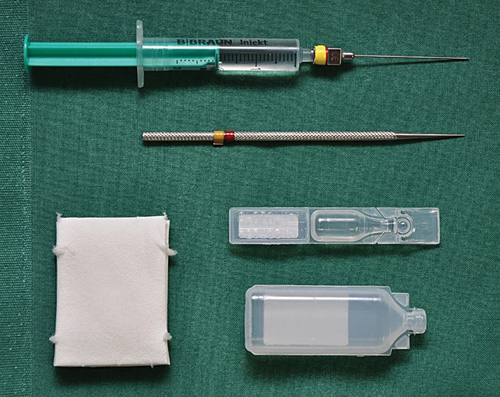
Figure 6. Instruments and materials for lacrimal syringing (glucose 40% solution, sponges, local anesthetic eye drops, probe for dilatation, syringe with Bangerter’s cannula and saline solution).
The treatment shows age dependency within the first year of life. The risk of an unsuccessful application of the irrigation treatment increases by a factor of 1.2 with each month.Citation8 Despite a higher, albeit short-term, acute stress load compared with treatment under general anesthesia, the procedure is well tolerated by children and parents. The acceptance of the therapy was very high. Parents whose children were treated under local anesthesia would choose the same procedure again or recommended it to other parents in 96% of cases.Citation26
Stage 3 - Probing and Positive Pressurized Lacrimal Irrigation Under General Anesthesia with or without Lacrimal Intubation
Irrigation treatment under local anesthesia is no longer an option if the patient is >12 months old. Children are sometimes too strong and show well-developed social interactions with regard to pain processing and pain memory.Citation26 Therefore, treatment under general anesthesia is preferred. The therapeutic procedure does not differ from that of local anesthesia.
This procedure should be selected for older children with complex anatomical conditions or those with multiple unsuccessful prior treatments. The demands on physicians’ care and experience are no less, but the practitioner’s environment is much more relaxed. The time and calmness gained from this helps one cope better with complex situations. Another decisive advantage is that the treatment can be supported by additional techniques, such as the insertion of a silicone intubation material into the lacrimal ducts. If the patient is older than 24 months, additional lacrimal intubation is recommended, as patients in this age group have a lower success rate than with lacrimal irrigation alone.Citation15,Citation25 Securing lacrimal ducts with silicone support materials after two unsuccessful irrigation treatments is also recommended.Citation8,Citation15,Citation25
Monocanalicular autostable intubation has been available for more than 20 years.Citation27 Recent studies have shown the superiority of this procedure over bicanaliculonasal intubation using loop-shaped knotting of a silicone indwelling tube (U-intubation). The advantages of autostable intubation include a lower risk of tube dislocation into the lacrimal sac and easier tube removal without the need for sedation ().Citation27–30
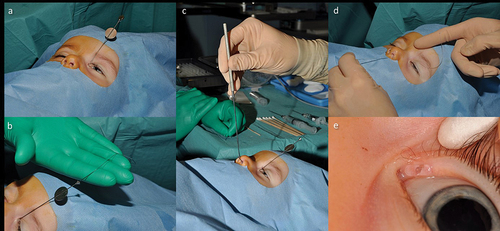
Figure 7. Monocanaliculonasal intubation in an 18-month-old child (left eye).
Stage 4 - Dacryoendoscopy or Balloon Dacryoplasty
Dacryoendoscopy () can be considered in the event of repeated unsuccessful irrigation treatments, including the use of temporary lacrimal intubation. Modern endoscopy systems have an outer diameter of ≤0.75 mm, which corresponds to the thickness of the available Bangerter cannulas. The advantage of this method is seen in visual control, with the possibility of considering individual anatomies. Furthermore, additional information regarding its pathogenesis is obtained. It is possible to describe the type and localization of the stenosis more precisely and to rule out intrasaccal pathologies (e.g., foreign bodies).Citation31,Citation32 More recent studies have shown good results using dacryoendoscopy as the primary treatment option, even in very young children.Citation33–35
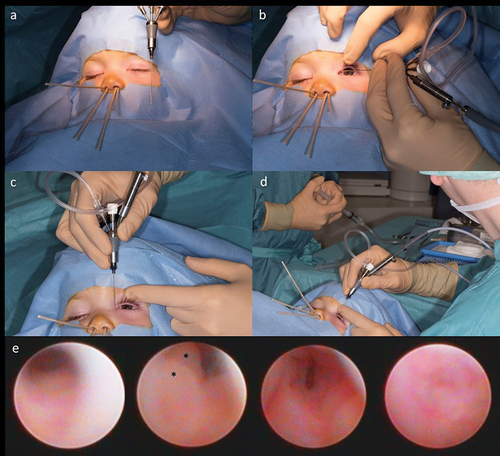
Figure 8. Dacryoendoscopy in a 14-month-old child (left eye).
Balloon catheter dacryoplasty can also be performed transcanalicularly and can increase the success rate after failed probing.Citation36 A balloon is inserted into the draining lacrimal duct via a catheter, which can then be inflated to 2 or 3 mm, depending on its size. Success rates of 77–90% were attributed to the treatment.Citation37,Citation38
Stage 5 - Dacryocystorhinostomy
Dacryocystorhinostomy (DCR) is considered the last possible treatment option for CNLDO after ensuring that none of the other treatment approaches are alternatives. This is particularly the case for postsaccal bony atresia. In principle, DCR can be performed transcutaneously (external; ) or via an endonasal (internal) approach. The aim was to open the medial saccus wall and connect it to the nasal cavity at the level of the middle nasal turbinate via an osteotomy. The goal is to create a targeted sacconasal mucosal anastomosis.Citation39–43 In terms of success rates, both access routes are comparable and are around 90%.Citation39–42 Nevertheless, the procedure should not be performed before the age of one year, as other surgical techniques, including dacryoendoscopy, are possible alternatives even for thin bony atresia.Citation32,Citation41,Citation44
Figure 9. Transcutaneous dacryocystorhinostomy.
Complete agenesis of the lacrimal ducts is an indication for conjunctivorhinostomy with permanent bypass tube. In order to avoid repeated revisions, surgery should not be performed before the age of 10–12 years, as this is when the growth of the facial bones is largely finished.Citation2
ACQUIRED DACRYOSTENOSES IN CHILDHOOD AS DIFFERENTIAL DIAGNOSIS TO CONGENITAL NASOLACRIMAL DUCT OBSTRUCTION
Acquired lacrimal duct stenosis in children is similar to that in adults but is rarer and less scientifically documented. Besides trauma, the most common causes in children are viral infections (herpes simplex, herpes zoster, and adenoviruses), which typically lead to scarring in the area of the lacrimal tubes. However, intra- to postsaccal stenosis localization is also possible.Citation45 As these are mostly older children, treatment is largely independent of the age of the patients. Recanalizing transcanalicular surgery, followed by temporary lacrimal intubation, is the treatment of choice. However, recurrences are frequent, especially if the length of the stenosis is greater than 3 mm or if there are multiple strictures.Citation46
Trauma to draining tear ducts is another common cause. Classically, these are the results of dog bites. However, rare lacrimal duct injuries, such as those during cesarean section deliveries, have also been reported. The canaliculi are affected in over 70% of cases. Primary microsurgical wound management should include reconstruction of the lacrimal ducts with intubation because a good prognosis is often assumed.Citation47 Iatrogenic injuries also play a role, which is why irrigation of the draining tear ducts is obsolete for purely diagnostic reasons in infants and children without sufficient cooperation.Citation13
Other causes may include encephaloceles or mucoceles, which may secondarily lead to compression of the draining tear ducts. Diseases of the nose and paranasal sinuses should be carefully ruled out. Therefore, the treatment of the underlying disease is essential. Depending on the extent of the damage to the draining tear ducts, they must be treated separately (functional lacrimal stenosis). Combined surgeries are often required in interdisciplinary settings.Citation48,Citation49
In this respect, indirect imaging techniques (e.g. CT-scan, MRI) are important instruments for the differential diagnosis of unclearly failed CNLDO and secondary acquired nasolacrimal stenosis in children.
DISCUSSION
There are several causes of lacrimal stenosis in children. Malformations and secondary acquired lacrimal stenosis must be distinguished from CNLDO. The therapeutic stage concept of CNLDO is versatile and dynamic. The concepts presented herein must be interpreted individually and implemented accordingly. Numerous influencing factors play a role in this process (see ). Therefore, it should not be used as a dogmatic sequence for therapeutic interventions. A summary of the therapeutic stage concepts for CNLDO is presented in .
Table 3. Staged therapeutic concept for Congenital Dacryostenosis [12].
The main aim of an early treatment is the prevention of complications. Besides inflammatory and infectious complications, prevention of amblyopia is mandatory.Citation18–20 A persistent lacrimal drainage disorder in childhood carries an increased risk of anisometropia or amblyopia.Citation18,Citation20 A significantly higher rate of anisometropia > 1.5 dpt (5.5%) is found in unilateral CNLDO. Complex CNLDO, which lead to a delay in healing due to increased treatment effort, also have a higher prevalence of anisometropia (9%).Citation20 Another study by Piotrowski et al. showed a prevalence of anisometropia (>1.0 dpt) of 9.8%.Citation18 The anisometropia rate in this patient group is significantly higher as the average prevalence of anisometropia greater than 1.0 dpt in early childhood is approximately 2%.Citation50
Physicians were already aware of the successful application of lacrimal sac massage at the end of the 19th century and the beginning of the 20th century.Citation6 The necessity of probing the draining tear ducts in the absence of successful massage treatment is well known.Citation51 The uncritical use of topical antibiotics should be avoided as they have no direct effect on CNLDO.Citation3,Citation24 To the best of our knowledge, this approach prevents complications.
With regard to transcanalicular (minimally invasive) surgical techniques, probing and pressurized lacrimal irrigation under local anesthesia in an outpatient setting should be mentioned in particular.Citation2,Citation3,Citation52 One disadvantage is that the procedure can generally only be performed within the first year of life and requires sufficient experience on the part of the surgeon. If a procedure is planned under general anesthesia, lacrimal intubation should be considered. Arguments for this include previous unsuccessful probing or patient age being greater than 24 months.Citation8,Citation25,Citation28,Citation52,Citation53,Citation54
Temporary lacrimal intubation has undergone significant developments. Flexible materials have been used in addition to rigid indwelling probes. Among other things, insertion into the lacrimal ducts was made possible by retrograde flexible probes.Citation55
For many years, these procedures have been replaced by bicanaliculonasal intubation using the “Münsteraner” technique (so-called U-intubation).Citation2,Citation56 Further autostable monocanaliculonasal intubation techniques are available and show various advantages in their application (easier placement, removal possible without sedation).Citation28,Citation29,Citation57 Dacryoendoscopy in children appears to offer a promising alternative but has not yet been established. The first available studies showed successful application without an increased complication rate compared to adults.Citation32–35 An additional benefit is the possibility of visually inspecting the individual anatomy. Further development and improvement of the dacryoendoscopic technique should be emphasized. The extent to which balloon dacryoplasty has retained its status as a minimally invasive transcanalicular surgical technique remains unknown.
DCR, as an anastomosis-forming surgical technique, is also showing increasing miniaturization and thus a decrease in invasiveness due to further development of the technical equipment. However, it remains reserved for selected cases.Citation39–43 The available monocanalicular intubation techniques can be combined with this therapeutic approach.
CONCLUSION
Knowledge of the various causes of CNLDO, as well as possible therapeutic approaches, allows for individualized care of young patients. This step-by-step therapeutic concept has been subject to constant development and numerous influences. Taking this into account, a successful treatment can be realized in most cases.
ACKNOWLEDGEMENTS
The author would like to thank Mrs. Nancy Horn (clinical photographer) for taking the photos.
DISCLOSURE STATEMENT
No potential conflict of interest was reported by the author(s).
Additional information
Funding
REFERENCES
- Cassady JV. Developmental Anatomy of Nasolacrimal Duct. AMA Arch Ophthalmol; 1952;47:141–158.
- Konnatale Dakryostenosen BH. Connatal dacryostenoses. Clinical picture and treatment. Ophthalmologe. 2004;101(9):945–954. doi:10.1007/s00347-004-1100-7.
- Takahashi Y, Kakizaki H, Chan WO, et al. Management of congenital nasolacrimal duct obstruction. Acta Ophthalmol. 2010;88(5):506–513. doi:10.1111/j.1755-3768.2009.01592.x.
- MacEwen CJ, Young JD. Epiphora during the first year of life. Eye (Lond). 1991;5(5):596–600. doi:10.1038/eye.1991.103.
- Heichel J, Struck HG. Current aspects on treatment of congenital dacryostenosis. Ophthalmologe. 2015;112(7):603–604. doi:10.1007/s00347-015-0094-7.
- Peters A. Ueber die sogenannte Thränensack-Blennorrhoe bei Neugeborenen. Klin Monatsbl Augenheilkd. 1891;29:376–388.
- Paulsen F, Garreis F, Schicht M, et al. Anatomy and physiology of the nasolacrimal ducts. HNO. 2016;64(6):354–366. doi:10.1007/s00106-016-0164-4.
- Heichel J, Bachner F, Schmidt-Pokrzywniak A, et al. Treatment of congenital lacrimal duct obstruction: A prospective clinical cohort study. Ophthalmologe. 2015;112(10):840–847. doi:10.1007/s00347-015-0067-x.
- Ballard EA. Excessive tearing in infancy and early childhood. The role and treatment of congenital nasolacrimal duct obstruction. Postgrad Med. 2000;107(6):149–154. doi:10.3810/pgm.2000.5.15.1100.
- MacEwen CJ, Young JD. The fluorescein disappearance test (FDT): an evaluation of its use in infants. J Pediatr Ophthalmol Strabismus. 1991;28(6):302–305. doi:10.3928/0191-3913-19911101-04.
- Struck HG. Das nasse Auge - Diagnostik und Therapie von Tränenabflussstörungen. Klin Monatsbl Augenheilkd. 2004;221(04):R29–R49. doi:10.1055/s-2004-821347.
- Heichel J. Connatal dacryostenosis due to persistence of Hasner’s Membrane. A frequent issue in pediatric ophthalmology. Monatsschr Kinderheilkd. 2017;165(8):697–706. doi:10.1007/s00112-016-0155-2.
- Lyon DB, Dortzbach RK, Lemke BN, et al. Canalicular stenosis following probing for congenital nasolacrimal duct obstruction. Ophthalmic Surg Lasers Imaging. 1991;22(4):228–232. doi:10.3928/1542-8877-19910401-14.
- Lawless M, Martin F. Orbital cellulitis and preseptal cellulitis in childhood. Aust N Z J Ophthalmol. 1986;14(3):211–219. doi:10.1111/j.1442-9071.1986.tb00038.x.
- Maheshwari R. Success rate and cause of failure for late probing for congenital nasolacrimal duct obstruction. J Pediatr Ophthalmol Strabismus. 2008;45(3):168–171. doi:10.3928/01913913-20080501-17.
- Heichel J, Heindl LM, Struck HG. Angeborene Fehlbildungen der ableitenden Tränenwege. Klin Monbl Augenheilkd. 2022;239(1):46–56. doi:10.1055/a-1717-2110.
- Heichel J, Bredehorn-Mayr T, Böhm K, et al. Bony lacrimal duct stenosis and hand abnormalities as signs of systemic disease. HNO. 2016;64(6):424–428. doi:10.1007/s00106-016-0132-z.
- Piotrowski JT, Diehl NN, Mohney BG. Neonatal dacryostenosis as a risk factor for anisometropia. Arch Ophthalmol. 2010;128(9):1166–1169. doi:10.1001/archophthalmol.2010.184.
- Kipp MA, Kipp MA Jr, Struthers W. Anisometropia and amblyopia in nasolacrimal duct obstruction. J Aapos. 2013;17(3):235–238. doi:10.1016/j.jaapos.2012.11.022.
- Eshraghi B, Akbari MR, Fard MA, et al. The prevalence of amblyogenic factors in children with persistent congenital nasolacrimal duct obstruction. Graefes Arch Clin Exp Ophthalmol. 2014;252(11):1847–1852. doi:10.1007/s00417-014-2643-1.
- Crigler LW. The treatment of congenital dacryocystitis. JAMA. 1923;81(1):23–24. doi:10.1001/jama.1923.02650010027009.
- Stolovitch C, Michaeli A. Hydrostatic pressure as an office procedure for congenital nasolacrimal duct obstruction. J Aapos. 2006;10(3):269–272. doi:10.1016/j.jaapos.2006.02.009.
- Bansal O, Bothra N, Sharma A, et al. Congenital nasolacrimal duct obstruction update study (CUP study): paper I—role and outcomes of Crigler’s lacrimal sac compression. Eye (Lond). 2021;35(6):1600–1604. doi:10.1038/s41433-020-01125-1.
- MacEwen CJ, Phillips MG, Young JD. Value of bacterial culturing in the course of congenital nasolacrimal duct (NLD) obstruction. J Pediatr Ophthalmol Strabismus. 1994;31(4):246–250. doi:10.3928/0191-3913-19940701-11.
- Katowitz JA, Welsh MG. Timing of initial probing and irrigation in congenital nasolacrimal duct obstruction. Ophthalmology. 1987;94(6):698–705. doi:10.1016/S0161-6420(87)33392-5.
- Heichel J, Bachner F, Hübner G, et al. Medical and psychological aspects of the treatment of connatal dacryostenosis: Parental evaluation of their own and their child’s stress. HNO. 2016;64(6):376–385. doi:10.1007/s00106-016-0167-1.
- Müllner K. Ritleng-Intubationsset zur Schienung verletzter Tränenwege. Klin Monbl Augenheilkd. 1998;213(10):238–240. doi:10.1055/s-2008-1034979.
- Yalaz M, Ozcan AA, Akcali C, et al. Lacrimal intubation with the Ritleng system in recurrent congenital nasolacrimal duct obstruction in children. ORL J Otorhinolaryngol Relat Spec. 2004;66(1):35–37. doi:10.1159/000077231.
- Lee H, Ahn J, Lee JM, et al. Clinical effectiveness of monocanalicular and bicanalicular silicone intubation for congenital nasolacrimal duct obstruction. J Craniofac Surg. 2012;23(4):1010–1014. doi:10.1097/SCS.0b013e31824dfc8a.
- Komínek P, Cervenka S, Pniak T, et al. Monocanalicular versus bicanalicular intubation in the treatment of congenital nasolacrimal duct obstruction. Graefes Arch Clin Exp Ophthalmol. 2013;249(11):1729–1733. doi:10.1007/s00417-011-1700-2.
- Heichel J, Bredehorn-Mayr T, Struck HG. Tränenwegsendoskopie im Kindesalter. Klin Monatsbl Augenheilkd. 2015;232(7):881–885. doi:10.1055/s-0034-1383407.
- Heichel J, Bredehorn-Mayr T, Stuhltraeger U, et al. Dacryoendoscopy for lacrimal duct obstruction manifesting in childhood. J Clin Exp Ophthalmol. 2015;6:394.
- Sasaki H, Takano T, Murakami A. Direct endoscopic probing for congenital lacrimal duct obstruction. Clin Exp Ophthalmol. 2013;41(8):729–734. doi:10.1111/ceo.12108.
- Fujimoto M, Ogino K, Matsuyama H, et al. Success rates of dacryoendoscopy-guided probing for recalcitrant congenital nasolacrimal duct obstruction. Jpn J Ophthalmol. 2016;60(4):274–279. doi:10.1007/s10384-016-0445-1.
- Heichel J, Struck HG, Fiorentzis M, et al. A case series of dacryoendoscopy in childhood: a diagnostic and therapeutic alternative for complex congenital nasolacrimal duct obstruction even in the first year of life. Adv Ther. 2017;34(5):1221–1232. doi:10.1007/s12325-017-0517-8.
- Goldstein SM, Goldstein JB, Katowitz JA. Comparison of monocanalicular stenting and balloon dacryoplasty in secondary treatment of congenital nasolacrimal duct obstruction after failed primary probing. Ophthal Plast Reconstr Surg. 2004;20(5):352–357. doi:10.1097/01.IOP.0000134271.25794.96.
- Chen PL, Hsiao CH. Balloon dacryocystoplasty as the primary treatment in older children with congenital nasolacrimal duct obstruction. J Aapos. 2005;9(6):546–549. doi:10.1016/j.jaapos.2005.08.002.
- Goldich Y, Barkana Y, Zadok D, et al. Balloon catheter dilatation versus probing as primary treatment for congenital dacryostenosis. Br J Ophthalmol. 2011;95(5):634–636. doi:10.1136/bjo.2010.183301.
- Vanderveen DK, Jones DT, Tan H, et al. Endoscopic dacryocystorhinostomy in children. J Aapos. 2001;5(3):143–147. doi:10.1067/mpa.2001.114910.
- Barnes EA, Abou-Rayyah Y, Rose GE. Pediatric dacryocystorhinostomy for nasolacrimal duct obstruction. Ophthalmology. 2001;108(9):1562–1564. doi:10.1016/S0161-6420(01)00699-6.
- Struck HG, Weidlich R. Indications and prognosis of dacryocystorhinostomy in childhood. A clinical study 1970-2000. Ophthalmologe. 2001;98(6):560–563. doi:10.1007/s003470170119.
- Leibovitch I, Selva D, Tsirbas A, et al. Paediatric endoscopic endonasal dacryocystorhinostomy in congenital nasolacrimal duct obstruction. Graefes Arch Clin Exp Ophthalmol. 2006;244(10):1250–1254. doi:10.1007/s00417-006-0273-y.
- Nemet AY, Fung A, Martin PA, et al. Lacrimal drainage obstruction and dacryocystorhinostomy in children. Eye (Lond). 2008;22(7):918–924. doi:10.1038/sj.eye.6702769.
- Avgitidou G, Koch KR, Cursiefen C, et al. Stufentherapie konnataler Tränenwegsstenosen. Ophthalmologe. 2015;112(7):605–606. doi:10.1007/s00347-015-0095-6.
- Kay KM, Woo KI, Kim JH, Chang HR. Acquired nasolacrimal duct obstruction in children. Jpn J Ophthalmol. 2007;51(6):437–441. doi:10.1007/s10384-007-0478-6.
- Heichel J. Ophthalmic surgical procedures for nasolacrimal duct stenosis in children. Z Prakt Augenheilkd. 2021;42:205–218.
- Struck HG. Lacrimal system lacerations and their surgical repair. Ophthalmologe. 2009;106(3):223–228. doi:10.1007/s00347-008-1906-9.
- Heichel J, Struck HG, Hammer T, et al. Pediatric acute dacryocystitis due to frontoethmoidal mucocele. HNO. 2019;67(6):458–462. doi:10.1007/s00106-019-0671-1.
- Weiner X, Kohnen T, von Jagow B. Einseitige, mediale Lidschwellung und Exotropie bei einem Neugeborenen. Ophthalmologe. 2016;113(3):248–249. doi:10.1007/s00347-015-0118-3.
- Donahue SP. Relationship between anisometropia, patient age, and the development of amblyopia. Am J Ophthalmol. 2006;142(1):132–140.e1. doi:10.1016/j.ajo.2006.02.040.
- Fuchs E. Lehrbuch der Augenheilkunde 12. Aufl. Leipzig-Wien: Deuticke; 1910.
- Heichel J. Graduated treatment concept for connatal Dacryostenosis. Klin Monbl Augenheilkd. 2017;234(10):1250–1258. doi:10.1055/s-0043-100655.
- Heichel J, Bredehorn-Mayr T, Struck HG. Congenital nasolacrimal duct obstruction from an ophthalmologist’s point of view: Causes, diagnosis and staged therapeutic concept. HNO. 2016;64(6):367–375. doi:10.1007/s00106-016-0124-z.
- Al-Faky YH, Mousa A, Kalantan H, et al. A prospective, randomised comparison of probing versus bicanalicular silastic intubation for congenital nasolacrimal duct obstruction. Br J Ophthalmol. 2015;99(2):246–250. doi:10.1136/bjophthalmol-2014-305376.
- Struck HG, Fikentscher R. Die Behandlung der therapieresistenten angeborenen postsakkalen Tränennasenwegsstenose mit der flexiblen Verweilsonde. Klin Monbl Augenheilkd. 1984;185(10):286–288. doi:10.1055/s-2008-1054617.
- Prokosch V, Busse H, Thanos S, et al. Impact of age on success rate of lacrimal duct irrigation with silicone tube intubation in connatal lacrimal duct stenosis. Klin Monbl Augenheilkd. 2013;230(10):1020–1024. doi:10.1055/s-0033-1350785.
- Andalib D, Mansoori H. A comparison between monocanalicular and pushed monocanalicular silicone intubation in the treatment of congenital nasolacrimal duct obstruction. Int J Ophthalmol. 2014;7(6):1039–1042. doi:10.3980/j.issn.2222-3959.2014.06.24.