ABSTRACT
Declining ore grades, coupled with the increasing mineralogical complexity of ores, have forced the platinum group mineral (PGM) industry to search for collectors that outperform the conventional options. One such potential alternative is Nouryon’s Tecflote™ family of nitrile-based collectors for sulfide flotation; whilst notable success has been reported in the copper industry, the potential value of these in the PGM industry has not yet been evaluated. This study employed batch rougher flotation tests coupled with mineralogical analysis of tailings samples to investigate the application of Tecflote S11 as a partial or complete replacement of thiol collectors in the flotation of a PGM bearing Platreef ore. Tecflote S11 as sole collector and in combination with different thiol collectors were investigated. At low dosages of 80 g/t, the Tecflote and PAX as single collectors achieved similar 3E (Pt, Pd and Au), Ni, and Fe recoveries, with Tecflote proving to be the more powerful Cu collector. At higher dosages of 160 g/t, PAX outperformed Tecflote in terms of 3E, Cu, Ni and Fe recoveries. A 50:50 mass% combination of the Tecflote with either DTP, DTC or PAX showed improved recoveries without a loss in 3E grade. The Tecflote-PAX combination achieved the highest 3E, Cu and Ni recoveries. Under these conditions, fully liberated PGMs and those associated with liberated base metal sulfides (BMS) were recovered. Overall, the study demonstrated that the Tecflote requires the backing of a strong thiol-based collector to achieve an improved flotation response for the Platreef ore.
1. Introduction
For several years, a combination of xanthate and dithiophosphate (DTPs) has been the preferred, reagent suite in PGM flotation concentrators (Grobler, Sondashi and Chidley Citation2005). Processing the Platreef ore with this combination may, however, not represent the optimum solution, the ore posing particular challenges with regard to low concentrate PGM grade and high Fe and S content that impact on smelting operations (Sahu et al. Citation2020).
Declining ore grades and increasing mineralogical complexity of the South African platinum group mineral (PGM) ores have prompted the industry to investigate novel collectors that could outperform thiol collectors. One such example is TecfloteTM, a family of patented nitrile-based collectors for sulfide flotation by Nouryon, Sweden (Lewis and Lima Citation2018). The collector consists of a nitrile (-CN) functional group and a hydrocarbon chain length of between 12 and 36. The structures of two TecfloteTM collectors are shown in .
Table 1. Structures of Tecflote S10 and S11 collectors (Lewis et al. Citation2018)
Whilst thiol collectors bond to a mineral surface via the sulfur atom, Tecflote does so via the nitrogen atom (Lewis and Lima Citation2018; Lewis et al. Citation2018). Also, whilst thiol collectors hydrophobise the particles during the conditioning step, Tecflote collectors do so during particle-bubble collision (Lewis and Lima Citation2018; Lewis et al. Citation2018). Unlike thiol collectors which adsorb on the mineral–water interface, it is proposed that Tecflote adsorbs on the air–water interface (Lewis and Lima Citation2018; Lewis et al. Citation2018), enhancing film thinning resulting in improved particle–bubble interaction which leads to improved mineral recoveries.
Studies on the flotation of copper-bearing ore have demonstrated that Tecflote™ can be used as a primary collector or as a supplement to thiol-based collectors (Lewis et al. Citation2018) at both laboratory and plant scales. The aim of this study was to investigate the application of Tecflote S11 for partial or complete replacement of thiol collectors in the rougher flotation of PGM-bearing Platreef ores. Tecflote S11 as sole collector and in combination with different thiol collectors, namely, diethyl dithiophosphate (DTP), diethyl dithiocarbamate (DTC) and potassium amyl xanthate (PAX) were investigated, and compared to a xanthane-DTP combination (referred to as the ‘standard’).
2. Materials and methods
2.1. Ore chemistry and mineralogy
The ore was sourced from a mining operation on the Platreef, the northern limb of the Bushveld Complex in South Africa. The material was crushed to −1.7 mm in a jaw crusher and split into representative sub-samples, some of which were used for mineralogical and chemical analysis. The sub-sample was assayed for 3E (i.e. combined Pt, Pd and Au) using fire assay, ICP (Cu, Ni and Fe) and Total S analysis using a LECO sulfur analyzer. The head grades of the ore are shown in .
Table 2. Assay of the Platreef PGM ore
Mineralogical analysis showed that the ore had a low base metal sulfide (BMS) content; pyrrhotite (45%), pentlandite (35%) and chalcopyrite (17%) being the major constituents with minor traces of pyrite. The major gangue minerals are plagioclase, enstatite, hornblende and talc, a naturally floating alteration gangue which dilutes the concentrates.
2.2. Flotation tests and procedure
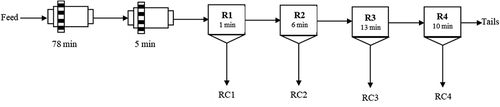
The standard flowsheet is shown in , with the corresponding reagent dosages and residence times depicted in . A 1.5 kg ore batch was initially milled in a laboratory rod mill for 78 min, after which the mill was opened and Flomin C7160 (a thionocarbamate collector) was added at 5 g/t. Milling was then continued for another 5 min. The milled slurry was conditioned in a 2.5 L Denver flotation cell at an impeller speed of 1500 rpm and a slurry density of 28% solids by mass. All the flotation reagents were prepared at 1% strength with exception of the Tecflote, which was added neat and dosed slowly and deep into the pulp by means of a micro syringe.
Table 3. Staged addition of reagents in the ‘standard’ test
All the flotation tests were conducted at the natural pH (~9) of the ore, only 35 g/t of Senfroth 522 frother being added to rougher 1. Sodium isopropyl xanthate (SIPX) and Aero 3477 (a sodium dialkyl dithiophosphate promoter) were then added to rougher stages 2 and 3, and floated for 6 and 13 minutes respectively, with stage 4 being conducted without further in-stage dosing.
Four concentrates were collected from each test. Tests were performed in triplicate and RC1 concentrates generated from each of the tests were dried, weighed separately and combined into a bulk RC1 which was assayed using methods highlighted in Section 2.1. The same procedure was used to produce bulk RC2, RC3, RC4 and tailings samples. To ensure reproducibility and procedural precision, mass pulls were compared, and any runs that deviated by more than 5% were rejected and repeated.
Since the focus of this study was on collector suites added to R2 and R3, all other chemistry parameters (e.g reagent type and dosages) and operational parameters (e.g. air flow rate (7 l/min), solids concentration and impeller speed) were kept constant during the tests.
3. Results and discussion
3.1. Flotation response
The effect of the various reagent conditions on the flotation performance of the PGMs and other minerals in the Platreef ore is shown in and respectively.
Table 4. Final mass pull, recoveries and grades under different reagent conditions
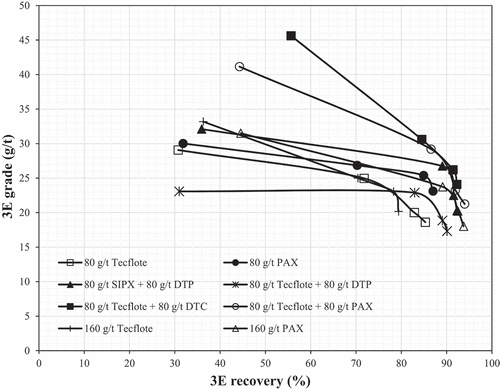
and show that at low dosages of 80 g/t, Tecflote and PAX as single collectors achieved relatively low but similar 3E, Ni and Fe recoveries, and thus demonstrate similar affinities for pentlandite and pyrrhotite. However, PAX produced better selectivity and a 3E concentrate grade of 23 g/t, i.e., 4 g/t higher than that of the Tecflote. This was to be expected as a result of the much longer chain length of the Tecflote compared to the PAX (). However, the Tecflote yielded 4.5% more mass than the PAX, accompanied by a 12% increase in Cu recovery which demonstrates that its affinity for chalcopyrite is stronger than for pentlandite, and that it is a more powerful and selective Cu collector than PAX. Similar findings were made at a laboratory scale by Lewis et al. (Citation2018) and Lewis and Lima (Citation2018), comparing the Tecflote S11 to PAX, substantiated by TOF-SIMS studies that showed S11 on chalcopyrite in the concentrate and not on that in the tailings.
Doubling the dosage of Tecflote to 160 g/t resulted in reduced 3E and Cu recoveries and increased Ni and Fe recoveries with little effect on the metal grades. On the other hand, doubling the dosage of PAX improved the 3E, Ni and Fe recoveries with no effect on Cu recovery. However, this was accompanied by loss in PGM and BMS grades due to increased mass pull. further shows that at higher dosages, PAX outperformed Tecflote in terms of 3E, Cu, Ni and Fe recoveries with a minimal effect on the metal grades.
and further show that additional 3E and Ni recovery increases were only observed when PAX was used as a single collector at a high dosage and when the Tecflote was used in a 50:50 mass% combination with PAX, DTP and DTC. The highest 3E recovery of around 94% was attained when PAX was used as a single collector and as a co-collector to Tecflote. However, the Tecflote–PAX combination achieved superior Ni and Cu recoveries than PAX as a single at a high dosage. These results suggest that Tecflote requires the backing of a strong thiol-based collector in order to achieve superior metallurgical performance.
For a fixed hydrocarbon chain length, the reactivity of thiol collectors generally decreases in the following order: DTC > Xanthate > DTP (Somasundaran and Nagaraj Citation1984; Taguta, McFadzean and O’Connor Citation2017). The fact that DTP is the weakest co-collector explains the lowest 3E recovery achieved by the Tecflote-DTP combination. Despite having the same hydrocarbon chain length of two carbons, DTC is more reactive than DTP hence the observed higher 3E recovery achieved by the Tecflote–DTC combination than the Tecflote–DTP combination. The reason for the superior performance of the Tecflote–PAX combination is that PAX is a strong collector due to the longest chain length of 5 carbons compared to DTP and DTC. Overall, the results show that Tecflote requires the backing of a strong thiol collector to achieve superior PGM and BMS recovery in the flotation of the Platreef PGM ore.
3.2. Tailings mineralogy
Mineralogical analysis of the tailings indicated that major PGM losses for both the Tecflote and its combination with PAX were predominantly the tellurides and arsenides and in the −15 µm grain size distribution. Furthermore, the use of the Tecflote as sole collector resulted in the loss of fully liberated PGMs as well as PGMs associated with liberated BMS as shown in , explaining the relatively low 3E and BMS recovery. However, by combining the Tecflote and PAX, the fully liberated PGMs and those associated with liberated BMS were recovered fully.
Table 5. Grain mode of occurrence in the tailings
4. Conclusions
At low dosages of 80 g/t, the Tecflote and potassium amyl xanthate (PAX) as single collectors produced similar 3E, Ni and Fe recoveries with the Tecflote proving to be the more powerful Cu collector. At higher collector dosages (160 g/t), PAX outperformed Tecflote in terms of 3E, Cu, Ni and Fe recoveries. While doubling the collector dosage was detrimental for Tecflote, it was beneficial for PAX.
A 50:50 mass% combination of the Tecflote with either diethyl dithiophosphate (DTP), diethyl dithiocarbamate (DTC) or amyl xanthate PAX achieved improved recoveries without a loss in 3E grade. The highest 3E, Cu and Ni recoveries were observed for the Tecflote–PAX combination. Mineralogical analysis of the tailings showed that the improved metallurgical performance was due to increased recovery of liberated PGMs and those associated with liberated BMS. The losses of PGMs were pronounced in the fine particle size range, even for the fully liberated PGM grains. Overall, the study demonstrated that Tecflote S11 and a strong thiol-based collector produced better overall flotation response for the ore.
Acknowledgments
The authors wish to acknowledge the financial and technical support of Mintek and approval to publish the results. The assistance of Nouryon in providing samples of Tecflote S11 is also gratefully acknowledged.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Grobler, W. A., S. Sondashi, and F. J. Chidley. 2005. Recent developments in flotation reagents to improve base metal recovery. The South African Institute of Mining and Metallurgy, Third Southern African Conference on Base Metals. Johanneburg, 185–190.
- Lewis, A., M. Svensson, M. Widell, A. Movahedi, and H. Nordberg. 2018. Nitrogen-based collectors for sulfide flotation – Tecflote™. Proceedings of the International Mineral Processing Congress (IMPC), Moscow, Russia.
- Lewis, A., and O. Lima. 2018. Tecflote - new collector chemistry for sulfide flotation. In 14th International Mineral Processing Conference. 5th International Seminar on Geometallurgy. Procemin GEOMET, 321–33, Santiago, Chile, November 28–30.
- Sahu, P., M. S. Jena, N. R. Mandre, and R. Venugopal. 2020. Platinum group elements mineralogy, benefiaciation, and extraction practices – An overview. Mineral Processing and Extractive Metallurgy Review 1–14. doi:https://doi.org/10.1080/08827508.2020.1795848.
- Somasundaran, P., and D. R. Nagaraj. 1984. Chemistry and applications of chelating agents in flotation and flocculation. Reagents in Mineral Industry. Institute of Mining and Metallurgy, London, 209–219.
- Taguta, J., B. McFadzean, and C. T. O’Connor. 2017. The effect of alkyl chain length and ligand type of thiol collectors on the heat of adsorption and floatability of sulphide minerals. Minerals Engineering 110:145–52. doi:https://doi.org/10.1016/j.mineng.2017.04.021.