?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Rare Earth (RE) elements can be used as alloying agents to improve properties such as strength, conductivity, and corrosion resistance of Al or Mg light metal (LM) alloys. The current route for producing Al/Mg-RE alloys is by melting Al or Mg with the required RE metal. However, the high price of RE metals makes the cost of the alloys expensive and, as a result limits, their application. Extensive studies have been conducted to find alternative process routes to making RE alloys with the aim of making the alloys more efficient and less costly. In this study, a comprehensive overview of alternative Al/Mg-RE alloy production methods is provided. Three alternative routes are identified and reviewed: the direct metallothermic reaction of the metal with RE compounds; a multistage reduction of RE compounds; and the electrolysis route. Recommendations for ongoing and future research into Al/Mg-RE alloys as well as industrial implications are also discussed.
1. Introduction
Aluminum and magnesium are classified as light metals (LM) because their specific gravities are relatively low (2.70 and 1.74, respectively) compared to other commonly used metals such as Fe (7.87) and Cu (8.96) (Rumble Citation2021). The high strength to weight ratio of light metals is also considered beneficial for their use in applications where low weight and high strength are important, such as in transportation (Bray Citation2020, Citation2022). Compared to commonly used steels, however, there are a number of properties of Mg that limit its application such as poor ductility, limited creep resistance, and high susceptibility to corrosion (Bian et al. Citation2022; Esmaily et al. Citation2017; Mordike and Ebert Citation2001; Tekumalla et al. Citation2014). Similarly, pure Al also has limited corrosion resistance, for example where chloride is present in significant quantities such as in coastal regions or in chemical plants, has relatively low strength, and is very flexible (Megson Citation2012; Schofield Citation2001). To improve the mechanical and corrosion properties of Al and Mg, different elements are often added as alloying agents. The most common alloying elements with Mg include Al, Zn, Mn, Si, Cu, and Zr (either alone or in combination) while Al is typically alloyed with Cu, Mg, Zn, Sn, Si, and Mn.
Alternative alloying elements that are used to improve the properties of Al and Mg are sourced from the Rare Earth (RE) elements series. The RE series consists of elements, known as lanthanides, that have atomic numbers ranging from 57 to 71 and which belong to the sixth period and IIIA subgroup of the periodic table. Two further elements, Sc (Z = 21) and Y (Z = 39), are commonly included as RE, due to their having similar chemical properties with members the lanthanide series (Connelly et al. Citation2007). RE elements are typically extracted from sources such as naturally occurring RE-rich deposits (e.g. deposits containing mineral such as bastnaesite (La,Ce)FCO3, monazite (Ce,La,Y,Th)PO4, xenotime (YPO4), or from ion-absorbed clay deposits), as by-products from other processes (e.g. from rutile, alumina, zircon, magnesia, and cassiterite processing); from electronic waste; or from coal and coal-based materials (e.g. fly ash, bottom ash, acid mine drainage, and its treatment products, and coal refuse) (Archambo and Kawatra Citation2021; Rafique Citation2023; Talan et al. Citation2023).
In recent years, addition of RE elements to Al or Mg to form RE-Al/Mg alloys has been the subject of increasing research interest because of their potential engineering applications (Sommer Citation1992). Beneficial effects of adding RE elements to either Al or Al-rich alloys include: the promotion of dehydrogenation whereby the RE decreases the H2 solubility in solid Al alloys and instead facilitates the formation of RE-hydrides; the modification of the eutectic Si phase in Al-Si alloys; the strengthening effect of alloys through grain refining, grain boundary strengthening, and solution strengthening; and; improving the corrosion resistance and electrical conductivity of the light metal (Nie et al. Citation2002, Citation2003). The addition of RE elements is also known to reduce the deteriorating effects of Cu and Fe impurities in Al alloys (Sims et al. Citation2021; Zhang et al. Citation2012a) and some Al-RE (RE=Ce/La) alloys have been shown to have superior castability properties, similar to Al-Si alloys, and can be used with minimal or without any heat treatment (Sims et al. Citation2022; Weiss et al. Citation2017). The latter alloys are particularly useful as Ce and La are considered lower-value by-products of the RE supply chain compared to other more expensive and in demand RE elements such as Nd, Pr, Dy and Sm. In addition, Al-Sc alloys are used for making sport equipment such as baseball and softball bats, bicycle frames, lacrosse sticks, and tentpoles because of their superior strength properties (Røyset and Ryum Citation2005). In more high-tech applications, NASA and Airbus are both evaluating the use of Al-Sc alloys for aircraft components (Jones et al. Citation2022; Lee and Chen Citation2004).
For Mg, the effects of adding RE elements to either pure Mg or an Mg alloy include: promoting purification of the alloy melt; improving the alloy castability; refinement of the microstructure; and; improving the mechanical and anti-oxidation properties (Paradiso et al. Citation2017; Rokhlin Citation2003; Sivashanmugam and Harikrishna Citation2020; Tekumalla et al. Citation2014). In contrast to Al alloys, the use of RE elements as alloying element for Mg alloys is relatively common and a lettering code for Mg alloys developed by the American Society of Testing and Materials (ASTM) is widely used in industry. For example, Mg alloy WE43C-T5 contains approximately 4 wt.% yttrium (W) and 3 wt.% RE (E), the letter C indicates this is the third composition of the alloy that become a standard, and T5 indicates the type of thermal treatment (Calado, Carmezim, and Montemor Citation2022; Moosbrugger and Marquard Citation2017). Due to its superior properties, the WE series of Mg alloys has been of particular interest to the automotive and aerospace industries for the development of high-performance car, helicopter, and fighter aircraft components (Calado, Carmezim, and Montemor Citation2022). Another emerging application for Mg-RE alloys is for use as biocompatible materials. Mg-RE alloys have an elastic modulus similar to the elastic modulus of natural bone, and lower than that of other materials used for implants such as 316 L stainless steel (Calado, Carmezim, and Montemor Citation2022). Stents and screws made from an Mg-Y-RE-Zr alloy have been shown to have good biocompatibility and to promote bone healing processes (Chen et al. Citation2018).
Essentially, there are two types of light metal RE alloys, the difference between the two being based on the concentration level of the RE element. The first type is referred to as ‘applied alloys’ and usually contains RE concentrations of 0.1–0.5%. The second type is known as ‘master alloys’ and has RE contents ranging from about 5–12% (Jang et al. Citation2015). In practice, the master alloys are commonly used as the precursor and feed materials for preparing the applied alloys. The production of LM-RE master and applied alloys is typically carried out by adding the RE metal directly to the molten LM or LM alloy and the RE will either be dissolved into the molten LM or form intermetallics with the LM. However, the requirement of using the RE element in the metallic form provides the biggest barrier toward commercialization of such processes because the pure RE metals are expensive (Savchenkov et al. Citation2021; Xiao et al. Citation2020). In addition, significant RE metal losses can occur during alloy preparation, they often require complex melting-casting processes, and the high differences in melting temperatures can result in segregation in the alloy (Liu et al. Citation2019b; Polat et al. Citation2018; Shtefanyuk et al. Citation2016). For example, during addition of scandium in the form of Al-2%Sc into Al to make alloys containing 0.22–0.23% Sc, normally 0.01–0.03% scandium is lost due to evaporation, oxidation, or deposition on the hearth surface (Zakharov and Fisenko Citation2017). Adding scandium directly to the alloy however, results in higher losses due to its higher melting temperature (~1540°C). In general, existing processes are inefficient.
The aim of the current study is to systematically review major alternative routes to produce LM-RE alloys. The routes explored avoid the use of expensive RE metals, instead employing significantly lower cost RE compounds (e.g. oxides or halides) as a means of introducing the required RE. A direct metallothermic reaction with RE compounds route is first described, followed by the multistage indirect reduction of RE compounds route, and lastly the electrolysis LM-RE alloy route. Finally, industrial implications and future outlooks for LM-RE production are discussed.
2. Direct metallothermic reaction with RE compounds
Rather than adding a RE metal directly to a light metal, an alternative way of producing Al/Mg-RE alloys is by direct addition of an RE compound to the light metal. In this process, the RE compound will be reduced by the Al or Mg. This direct reduction method is commonly used in RE metal production whereby an RE compound, typically a RE oxide, undergoes metallothermic reduction processes using calcium as a reductant to produce the RE metal (Rafique Citation2023).
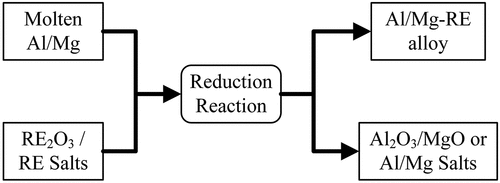
In the metallothermic reaction with RE compounds method, a simultaneous process of RE compound reduction by Al/Mg results in alloy formation between the Al/Mg and RE. This is particularly advantageous as adding a RE compound instead of a pure RE metal can significantly decrease the manufacturing costs of the alloy (e.g. the price of a RE oxide is approximately one-third of the price of the RE metal). While several RE compounds could be added to make the alloy (e.g. oxides and halides), the most studied compounds used as precursors to produce LM-RE alloys are that of RE oxides.
The overall reaction for metallothermic reduction of a general metal oxide by Al or Mg is shown in EquationEquations (1)(1)
(1) and (Equation2
(2)
(2) ), where M is the reducing metal:
A schematic of the metallothermic reaction with RE compound route is shown in .
Figure 1. Generic mechanism involved in the direct metallothermic reaction of RE compounds with a light metal.
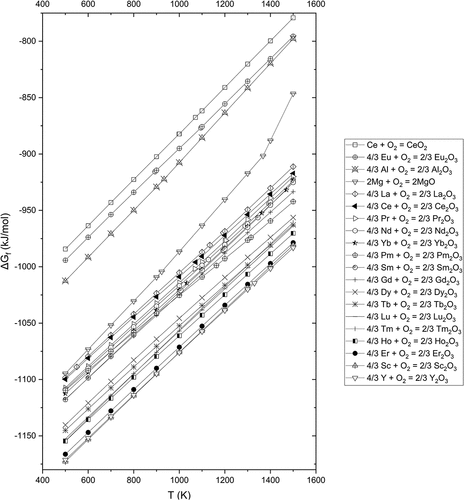
A comparison of the Gibbs free energy (ΔG°) of formation of various RE oxides and Al/Mg oxides (LMO) is presented in . The graph was constructed using the Reaction module in conjunction with the FactPS database available in the FactSage 8.2 thermochemical software package (Bale et al. Citation2016). Based on the relative ΔG° values, the metallothermic reduction of RE oxides by Al or Mg (EquationEquations (1)(1)
(1) and Equation(2))
(2)
(2) should not be favored thermodynamically, except in the case of Eu2O3, because most of RE oxides are more stable compared to Al2O3 or MgO. Note however, even though the LMOs are more stable compared to most of the REOs, the reaction will proceed if the RE metal product is soluble in the liquid Al or Mg, as represented by EquationEquation (3)
(3)
(3) :
When the amount of RE metal dissolved into the liquid Al or Mg is low, the thermodynamic activity (α) of RE metal (αRE) is very small, which results in the overall ΔG of EquationEquation (3)(3)
(3) becoming more negative, as can be seen from EquationEquation (4)
(4)
(4) , where ΔG° is the standard Gibbs free energy of the reaction, R is the gas constant and T is the reaction temperature.
Reduced REs may also form intermetallic compounds with Al/Mg which can be more stable compared to their oxides if the melting point of the intermetallic is higher than the reduction temperature (for example, in the Al-Sc2O3 system – see EquationEquation (5)(5)
(5) ).
An early study involving RE oxide addition to light metals was presented in a patent by Tarcy and Foster (Citation1988). In this patent, the RE oxide used was Sc2O3 powder that was mixed with Al and pressed to make a compact pellet. The compact was then added to a molten Al bath at temperatures of 750–950°C. A maximum alloy yield of 92.8% was reached with 0.587% Sc present in the alloy (Tarcy and Foster Citation1988). Since then, further studies have been conducted on the following systems: Al-Sc2O3 (Fujii et al. Citation2003, Citation2020; Harata et al. Citation2008), Al-Mg-CeO2 (Luna A et al. Citation2011), Mg-Al-La2O3 (Lu et al. Citation2005), Al-Cu-PrxOy (Zhao et al. Citation2008), Al-Cu-PrxOy-La2O3 (Bai et al. Citation2014; Xia et al. Citation2012; Zhao et al. Citation2011), AZ31 Alloy-La2O3 (Zhao et al. Citation2013), Mg-Sc2O3 (Kim et al. Citation2013), and A356-Y2O3 (Moussa, El-Hadad, and Khalifa Citation2019).
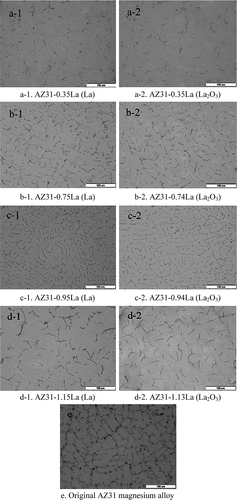
To compare the effect of adding the RE as either a metal or an oxide directly to the molten light metal (or light metal alloy), Zhao et al. (Citation2013) compared the difference in microstructure and oxidation behavior of AZ31 Mg alloyed with La or La2O3. The results showed that there was no significant difference in the microstructure of the final product when either La or La2O3 was used as the precursor, as shown in (Zhao et al. Citation2013).
Figure 3. Microstructure of AZ31 magnesium alloys prepared using both La and La2O3 additions (as indicated in the brackets) (Zhao et al. Citation2013).
Alternative techniques to make the alloys have also been tested. For example, in one study Sc2O3 and Al were mechanically alloyed and then sintered using spark plasma sintering (SPS). It was reported that the Sc2O3 was not reduced by the mechanical alloying process but was reduced during the SPS (Fujii et al. Citation2003). Calcium metal has also been used as a reductant for Sc2O3 and then molten Al used to act as a collector to form Al-Sc alloys (Harata et al. Citation2008). More recently, microwave radiation has been used to produce Al-Sc alloys, with the aim to reduce the overall energy inputs and therefore develop a more environmental-friendly process route (Fujii et al. Citation2020). Compared to the use of conventional heating, the use of microwave radiation as a heat source often results in high reaction rates, selective chemical reactions, and a smaller reactor requirement.
Although the use of RE oxides as a starting material can potentially reduce the material costs for alloy production, there are some issues which still need to be resolved. For example, alumina (Al2O3) and magnesia (MgO), are generated as by-products of the RE oxide reduction and their presence may affect the properties and the purity of the Al and Mg alloys through factors such as: a reduction in the mechanical properties; poor machinability; low surface quality; an increase in porosity and; they may exhibit a tendency to increase corrosion (Li et al. Citation2017; Lun Sin, Elsayed, and Ravindran Citation2013). Hence, these impurities present as inclusions in the alloy need to be removed. This is typically done by refining them with chloride salts (Li et al. Citation2017) although physical separation procedures such as filtration, gas bubble flotation, and settling process could also be employed to remove the inclusions (Damoah and Zhang Citation2010). It should be noted however, oxide inclusion removal processes may not be necessary particularly in the case when the initial alloy produced is the master alloy – if present the oxide inclusions may be removed when the alloy is used in the subsequent alloy-making process.
In terms of thermodynamics, based on the simulations using FactSage 8.2 (Bale et al. Citation2016), RE oxide reduction by Mg is most effective for the first half of the lanthanides series, from La up to Sm (except for Promethium for which there is a lack of thermodynamic data). Elements from the second half of the lanthanide series are much more difficult to reduce, as the oxides are more stable compared to the first half (see ). In comparison, RE oxide reduction by Al does not follow the order of the RE oxide stability. This is a result of the formation of the many intermetallic compounds (which are more stable than RE oxides) between Al and RE that can form during the process. We note however, thermodynamic simulation of these complex systems requires further study and experimental trials are needed to verify any predictions.
The use of RE compounds other than RE oxides as a precursor for allow formation has also been studied. A patent by Duyvesteyn (Citation2019) reported the use of scandium oxalate (Sc2(C2O4)3.xH2O) which decomposes to Sc2O3 when contacted with the molten metal, releasing vapor and gases. The in-situ Sc2O3 generation was suggested to reduce the burning losses (i.e. loss of Sc to the dross) because of better mixing (Duyvesteyn Citation2019). Scandium chloride may also be used to make Al-Sc alloys (EquationEquation (6))(6)
(6) .
The reaction of ScCl3 with Al to form Al-Sc alloys (EquationEquation (6))(6)
(6) is not favored thermodynamically under 1000°C, but if the partial pressure of AlCl3 is reduced to 0.01 mbar, the reaction can be reversed (Haidar Citation2014). Processing at low temperatures is preferred to help with excessive gas production and to prevent powder blowing out of the reaction zone.
summarizes these and other previous studies using direct RE oxide and RE compound addition to make RE-Al/Mg alloys.
Table 1. Summary of previous works/patents involving the direct RE compound addition route*.
3. Multistage indirect reduction of RE compounds using molten salts
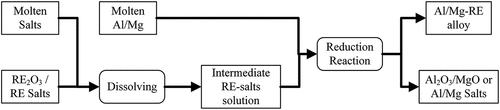
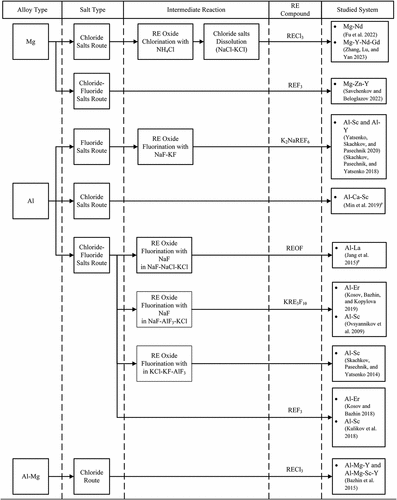
In this route, the RE oxides are first dissolved in molten salts to form an intermediate RE-salts solution. The intermediate molten salts containing the RE are then reduced via metallothermic reduction to produce the required LM-RE alloy. The generic steps of this process are presented schematically in . Compared to direct reaction between a LM with RE compounds, this process has several advantages such as better oxidation protection because of the use of salts covering the metals and typically produces higher yields in some systems. For example in the case of Er2O3 reaction with Al, adding certain salts (chloride, fluorides, etc.) can increase the yield from 0.17% to 57.65% (Kosov, Bazhin, and Kopylova Citation2019). Three different salts systems are typically used for this process, namely chloride salts, fluoride salts, and chloride-fluoride salts (and mixtures thereof). shows previously studied systems to produce LM-RE alloys through the multistage indirect reduction of RE compounds using molten salts. Detailed discussion regarding these systems is presented in the following subsections.
Figure 5. Classification of previously studied LM-RE alloy production routes via multistage indirect reduction in molten salts.
3.1. Chloride salt system
Chloride salts are usually used as a cover flux to prevent the molten alloy from oxidation, but recently they have also been used as the media involved in the actual reaction process itself. Systems involving RE chloride salts have been previously studied for the making of Mg-RE alloys however, only one study was found for the making of a Al-RE alloy using RE chloride salts (Min et al. Citation2019). This is most likely because the use of chloride salts for the Al system provides some complexities due to the possibility of AlCl3 formation. If formed, AlCl3 as a by-product must be recovered in gaseous form due to its low boiling point, thus complicating the process and equipment. In addition, the chloride may react with water vapor in the air producing alumina dust and hydrochloric acid. This does not occur in the Mg system because MgCl2 as the by product is still in the liquid form at the processing temperature used.
The RE oxide starting material is usually chlorinated first to form RECl3 by reaction with NH4Cl (EquationEquation (7))(7)
(7) (Meyer and Ax Citation1982). The RECl3 is then dissolved in a low melting point chloride salt mixture and the RE becomes reduced when it comes into direct contact with the liquid alloy, i.e. the RE3+ salt reacts with the Mg and is reduced to RE metal which then dissolves into the molten Mg (EquationEquation (8))
(8)
(8) . The ΔG° for EquationEquation (8)
(8)
(8) is positive indicating that the reaction is not favorable thermodynamically under standard conditions (i.e. 1 atm and 298.15 K). However, comparable with the process from the previous section involving direct metallothermic reaction with RE compounds, the reaction can still progress forward due to the low activities, i.e. low
and
in EquationEquation (9)
(9)
(9) , respectively (Fu et al. Citation2022; Zhang et al. Citation2023). A high ratio between the RE chloride salts and the metal is typically used in this process (i.e. msalts/malloy >1). The reason for this high ratio is to ensure that the protection given by the molten salts to the liquid magnesium alloy is adequate. When only small amounts of salts are present, for example only covering the top of the liquid Mg alloy, the relatively higher density of the molten salts compared to the Mg alloy results in the molten salts passing through the liquid Mg alloy and sinking to the bottom of the furnace. In comparison, for a high ratio, the liquid Mg alloy will be covered on all sides and stays afloat inside the molten salts (Zhang et al. Citation2023). For example, Zhang et al. reported that during the process, the alloy was reported to be moving both upward and downward in the molten salts caused by density changes through the process. Different chloride salt mixtures could potentially be used in this process, namely NaCl-KCl and NaCl-KCl-CaCl2.
It has been reported that the yields of RE could reach up to 58%, 84%, and 91%, in the case of Mg-Nd, Al-Mg-Sc, and Al-Mg-Y alloys, respectively (Bazhin et al. Citation2015; Fu et al. Citation2022).
This process has also been studied for the process of making WE43A and WE43B magnesium alloys (Zhang et al. Citation2023). In these studies, a mixture of Y2O3, Gd2O3, and Nd2O3 dissolved in KCl-NaCl was used as the RE source, with the result being an alloy containing 3.92% Y, 2.02% Nd, and 1.47% Gd. The concentrations of impurities such as Na and K were reported to be very low implying the process did not introduce any inclusions to the alloy. It should be noted however, that RECl3 dissolved in molten chloride salts may be hydrolyzed to REOCl when in contact with water vapor in an ambient atmosphere, and this would decrease the efficiency of the process as REOCl formation would contribute to RE loss and inhibit the reaction process. The RECl3 hydrolysis could be decreased by lowering the molten salt surface area (interface between molten salts and gas) and increasing the height of molten salt (Zhang et al. Citation2023). This is because the hydrolysis of RECl3 was found to be controlled by the diffusion of RECl3 to the interface between the molten salts and the ambient atmosphere.
After the alloy production process, the used chloride salt mixture will be contaminated with MgCl2 (EquationEquation (10))(10)
(10) . In order to reuse the chloride salt mixture, the MgCl2 has to be removed because it can potentially react with RE elements in the Mg-RE alloy, i.e. the reverse of EquationEquation (10)
(10)
(10) can occur. MgCl2 can be removed by reacting the spent chloride salt mixture with REF3 (EquationEquation 11
(11)
(11) ). In this case, the MgCl2 is converted into MgF3 and removed via a dilute acid leaching and filtration process (Fu et al. Citation2022; Zhang et al. Citation2023).
3.2. Fluoride salt system
In contrast to the chloride system, the fluoride salt system has been mainly used for making Al-RE alloys. There are only limited studies on the production of Mg-RE alloys using fluoride salt systems. One of the complexities associated with this route relates to the higher operating temperatures (T > 750°C) required which are not suitable for a relatively low boiling point metal such as Mg. The high temperatures are required because MgF2 forms in the fluoride system and this phase has a high melting point (~1263°C), which in turn increases the melting point of the mixed molten salts.
When fluoride salts are used to make Al-RE alloys, however, an RE oxide can be dissolved directly into the molten salts without the need of a prior (and separate) fluorination process. The RE oxide will react with the fluoride salt (usually a mixture of NaF and KF) to form a complex compound dissolved in the molten fluoride salts, K2NaREF6 (Skachkov et al. Citation2018). This intermediate complex solution then reacts with Al to form the Al-RE alloy. It has been reported that yields of 93% for Sc and 61% for Y, respectively, can be obtained in a system involving Sc2O3/Y2O3-NaF-KF-Al (Skachkov et al. Citation2018).
RE fluorides could also be used as the source of RE to be incorporated in the LM alloy. In a previous study, a mixture of KF and NaF (1:1) was mixed with YF3 (5 wt.%) and then reacted with Al (Yatsenko, Skachkov, and Pasechnik Citation2020). The resulting Al-Y alloy was reported to contain 30.5 wt.% Yttrium. The study also described a method for the enrichment of an Al-Sc alloy. The use of a mixture of Sc oxide/oxyfluoride/fluoride produced <10 wt.% Sc in the resulting alloy, however, with further processing through filtration, the average Sc concentration in the alloy was increased to 20.3 wt.% (Yatsenko, Skachkov, and Pasechnik Citation2020).
3.3. Mixed chloride-fluoride salt system
When a mixed chloride-fluoride salt system is used, RE oxides react and dissolve in the molten salts to form intermediate solutions (depending on the specific salt system used). Different molten chloride-fluoride salt mixtures that have been used include, NaF-NaCl-KCl, NaF-AlF3-KCl, NaF-KCl, and KCl-KF-AlF3.
In the NaF-NaCl-KCl-Al system, because the diameter of the O2- ions in the RE oxide and the F ions in molten salts are similar, the oxygen and fluorine may exchange with each other and transform the RE2O3 to (EquationEquation (12)
(12)
(12) ) (Jang et al. Citation2015; Xu et al. Citation2012). The formation of
increases the solubility of RE oxide in the molten salts and reacts with molten Al to form the Al-RE alloy (EquationEquation (13)
(13)
(13) ). In the case of the Al-La system, the La content in the alloy can reach up to 10.1 wt.% when La2O3 is used as precursor (Jang et al. Citation2015). The solubility of La2O3 was reported to increase with an increase in the NaF concentration. In the case of the Al-Sc system, the spent molten salts were tested and able to be reused up to three times (after mixing with fresh Sc2O3) with the yield reported to reach up to 91% in the case of Sc2O3 being used (Xu et al. Citation2012).
In the NaF-AlF3-KCl-Al system, the RE2O3 is initially fluorinated to form KRE3F10 (EquationEquation (14))(14)
(14) which is then reduced by Al (EquationEquation (15)
(15)
(15) ). AlF3 is an important component in the molten salt mixture because it can enhance the fluorinating effect on the RE oxide and it promotes the formation of KRE3F10 in the presence of KF. The KF forms through reaction between KCl and AlF3 and by high temperature ion exchange, which likely proceeds via aluminum sub-halides formation.
An incomplete fluorination can occur when the NaF is present in high concentrations. In this case, the NaF can react with KF and AlF3 to form K2NaAlF6, which reduces the available KF and AlF3 for further reaction with RE2O3 to form KRE3F10. An incomplete RE2O3 fluorination was also reported to promote the formation of a binary oxide phase of Al10Er6O24 (5Al2O3.3Er2O3) in the system NaF-AlF3-KCl-Al (Kosov, Bazhin, and Kopylova Citation2019). Therefore, to avoid incomplete fluorination, molten salts with a small Cryolite Ratio (CR) (i.e. a lower NaF concentration in the molten salt mixture) is preferred. When Er2O3 is used in the NaF-AlF3-KCl-Al system, the yield was reported to reach up to 71.43% with 6.45 wt.% Er content in the alloy (Kosov, Bazhin, and Kopylova Citation2019). KCl-KF-AlF3 mixtures could also be used as the molten salt. A yield in oxide reduction of more than 90% was reported when the Sc2O3-KCl-KF-AlF3 system was used (Skachkov, Pasechnik, and Yatsenko Citation2014).
In the NaF-KCl system, REF3 will react to form NaREF4 (EquationEquation (16))(16)
(16) and then either be reduced to the metal form and dissolved in the molten Al (EquationEquation (17)
(17)
(17) ), or form intermetallic phases, depending on the thermodynamics of the system (Kosov and Bazhin Citation2018). RE fluorides could also be used in combination with a NaCl-KCl-CaCl2 salts mixture (Savchenkov and Beloglazov Citation2022). In this case, the yield has been reported to reach up to 99.8% for the Mg-Zn-Y system.
Overall, the use of RE fluorides instead of RE oxides improves the yield of the LM-RE alloy process by 2–7% (Skachkov et al. Citation2018). This method, however, involves adding steps to the process because RE are usually extracted in the form of oxides. The fluorination of RE can also be done by reacting it with HF solutions.
shows the summary of the key previous studies in the production of the Mg/Al-RE alloys through multistage reduction using molten salts.
Table 2. Summary of previous works/patents in multistage indirect reduction in molten salts.
4. Electrolysis route for production of Mg/Al-RE alloy
The process of making RE alloys using electrolysis may be divided into several types based on the form of the cathode used, the molten salt system used, and the deposition/co-deposition of the alloying element. These processes have previously been summarized by Krishnamurthy and Gupta (Citation2001) however unlike the two previous methods which depends on the differences of chemical stability between RE compound and Al/Mg compound, the electrolytic process is not limited by chemical stability.
In the process of RE alloy production using electrolysis, the RE compounds are dissolved in the molten salts electrolyte and reduced with the application of an electrical current. The RE compound is reduced at the cathode (EquationEquation (18))(18)
(18) and carbon dioxide gas is formed at the anode (EquationEquation (19)
(19)
(19) ). The overall reaction of this electrolysis process, when a RE oxide is used, is provided in EquationEquation (20)
(20)
(20) .
An RE alloy could also be made by reducing the RE compound simultaneously with a LM compound. When a LM compound is present in the system, this compound will also be reduced at the cathode and an example of overall reaction involving a LM oxide is provided in EquationEquation (21)(21)
(21) . This co-reduction of RE and LM, especially when using Al, could potentially be conducted using a modified Hall-Héroult cell while producing Al from alumina.
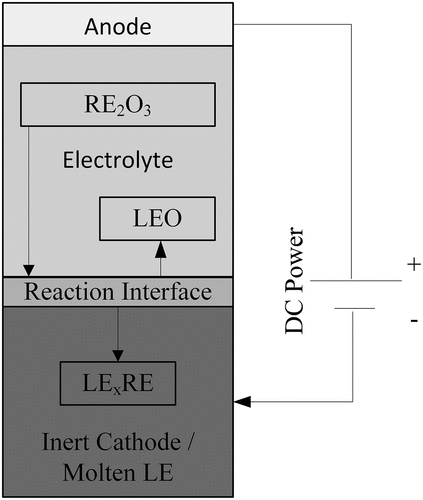
A general schematic of the electrolysis route for production of Mg/Al-RE alloys is presented in . The electrolysis process can be performed using an inert or a reactive cathode. If an inert cathode is used, the formed alloy will sink to the bottom of the electrolysis cell because the wettability of the alloy with the cathode is poor. In comparison, when a reactive cathode is used, the product will stick to the cathode surface when the processing temperature is below the melting point of the cathode, or the product will dissolve into the cathode (when the processing temperature is above the melting point of the cathode).
The LM-RE electrolysis processes may be differentiated on the basis of the molten salt system used. Chlorides, fluoride, and mixed chlorides-fluorides salts could all be used for the electrolysis. The molten salts system selection is usually based on the electrochemistry and techno-economics of the system. The electrodeposition energy of the metal halides (Mg, Al, or RE) should be lower than the salts halides.
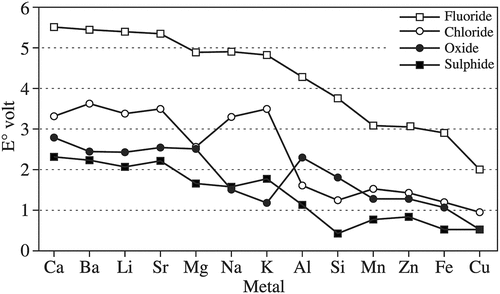
For making Mg-RE alloys, most previous studies have been conducted using chloride salts systems. This is because in order to use fluoride salts, cheap fluoride salts, such as NaF or KaF, cannot be used because their electrodeposition energies are lower than that of MgF2 () (Vignes Citation2013). Instead, more expensive LiF molten salts must be used.
Figure 7. Reversible decomposition voltages for sulfides, oxides, chlorides and fluorides at 1,000 K (from Vignes Citation2013).
For making Al-RE alloys, both chloride and fluoride salt system can be used however the trend of previous studies has been toward fluoride systems because of its similarity to the Hall-Héroult system and the alloy manufacturing process can potentially be integrated into existing Hall-Héroult cells. Also, for making Al-RE alloys in chloride salt systems, the Al2O3 needs to first be converted into AlCl3, unlike for the process for making the Al-RE alloys in the fluoride system route in which Al2O3 can be used directly. The conversion process needs Cl2 gas as a reactant which will increase the cost and complexity of the system.
The RE compounds used in the electrolysis process could also be reacted with the molten salts (the same process compared to metallothermic reduction of RE compound dissolved in the molten salts route) before being electrolyzed. When a RE oxide is used as the starting material, it will react to form an RE fluoride or a RE chloride based on EquationEquations (22)(22)
(22) to (Equation24
(24)
(24) ).
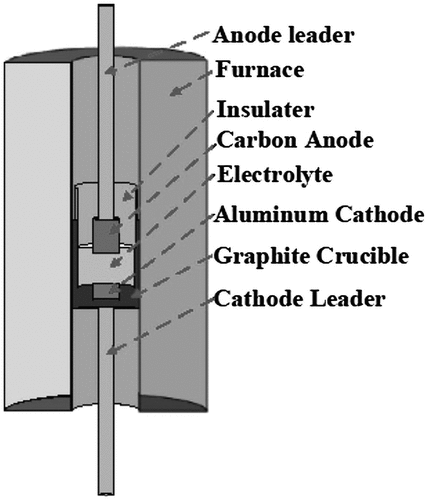
The Al-Sc system is the most studied electrolysis route to produce LM-RE alloys, because Sc is the most effective alloying element strengthener for Al alloys (Dorin et al. Citation2018). It has been reported that the current efficiency for the production of a Al-Sc alloy could reach up to 63% for a NaF/AlF3 molten salt system with a graphite crucible acting as the cathode (Martinez et al. Citation2021) and 65.2% for KF/AlF3 with a pre-wetted tungsten cathode (Nikolaev, Suzdaltsev, and Zaikov Citation2019). An example of an electrolytic cell set up for producing LM-RE alloys using a reactive cathode is presented in (Liu et al. Citation2019b). Previous studies reported segregation of intermetallics in the alloy product (Harata et al. Citation2009). Segregation in the product is also reported in the direct metallothermic reduction form RE compound route (Xiao et al. Citation2020). For the direct metallothermic reduction form RE compound route, common mixing methods such as stirring or gas blowing could be employed to reduce segregation, while for the electrolysis route, these methods cannot be employed because it will disturb the electrolysis process.
Figure 8. Typical electrolytic cell for making Mg/Al-RE alloys using a liquid Al metal cathode (Liu et al. Citation2019ba.
To improve the homogeneity of the alloy, ultrasound could potentially be applied during the process to facilitate redistribution of the intermetallics, as shown in (Liu et al. Citation2019a). However, an ultrasound method has not been commercially used. The electrolysis process may also be used to simultaneously separate an individual RE from a RE element mixture while also producing a LM-RE alloy (Wang et al. Citation2015; Zhang et al. Citation2013). For example, ErCl3 can be successfully separated from a ErCl3-PrCl3 mixture with a separation efficiency up to 95.3% while also producing a Mg-Er alloy (Wang et al. Citation2015). In comparison, for a SmCl3-DyCl3 mixture, DyCl3 was reported to reduce and form Mg-Dy alloy, while SmCl3 was not (Zhang et al. Citation2013). summarizes previous studies on the electrolysis route to produce various LM-RE alloys.
Figure 9. The mechanism of refining of the Al3Sc phase by ultrasound (Liu et al. Citation2019a).
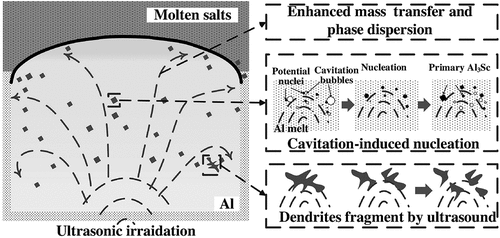
Table 3. Summary of previous works on electrolysis production of LM-RE alloys.
5. Discussion, implications, and outlook
This article has reviewed research and development of the various process routes to produce LM-RE alloys. Evidence from the literature shows that the production of LM-RE alloys using alternative routes, rather than just mixing the metals of LM and RE, is still an ongoing process. Many of the processes remain at either laboratory and/or pilot scales and further studies are required to scale up the processes to mass production.
Alternative routes to produce LM-RE alloys have the potential to be more economical compared to direct alloying with RE metal. The price of RE compounds, especially RE oxides, is approximately 50% cheaper compared to RE metals which are currently used as the raw materials for LM-RE alloy production. The direct metallothermic reaction with RE compounds appeared to be economically better from a raw material and capital cost perspective, especially if the RE oxide is used as the precursor material. Inclusion of oxides will be unavoidable, but the alloy could be refined through common industry processes such as density-based separation or filtration. The use of RE oxide as the starting material also means that this process is simplest compared to the others, indicating potential for easier scalability. Furthermore, RE elements are widely traded in the form of their oxides, which mean that additional conversion processes will not be required like other processes which require RE halides. The direct metallothermic reaction process can also be conducted without having to use large amounts of salts, which avoids the problem of post treatment of the salt by-product. Even though the processing temperature is like the other two processes (Mg system at 660–800°C and Al system at 700–975°C, respectively), a salt-free processing route means that the process does not require expensive corrosion resistance equipment. In terms of yield of the process, for the most studied system, which is the scandium-LM alloy system, studies have demonstrated that a 96.72% yield could be achieved for making a 2.9 wt.% Al-Mg-Sc alloy when using ScCl3 as a starting material (Xiao et al. Citation2020) and a 92.8% yield for making a Al-Sc alloy when using Sc2O3 as a starting material (Tarcy and Foster Citation1988). Compared to the multistage reduction of RE compounds using a molten salts method, the yield is similar with the yield for the direct metallothermic reduction form RE compound method which reaches ~96% when ScF3 with fluoride salts were used to make a Al-0.91% Sc alloy (Skachkov Citation2018) and 91% when Sc2O3 was used with cryolite for making Al-2 wt.% Sc-0.5 wt.% Y alloy.
The alloy produced from a multistage indirect reduction of RE compound route is expected to contain less oxide impurities compared to the direct metallothermic reduction route because most of the oxide is dissolved in the salts. Unreacted RE in the salts requires further processing either for recovering the RE or conditioning of the salts to be reused in the process again. A high amount of salts is required for this process and failure to reuse the salts would lead to waste problems. Most European countries are prohibiting salt cake to be deposited in landfill. It is classified as toxic and hazardous waste by the European Union (Padilla et al. Citation2022; Tsakiridis Citation2012). If disposed improperly, it can pollute ground water and when in contact with water or moisture, it can release explosive, toxic and unpleasant smelling gasses such as CH4. NH3, H2S, PH3, and H2. Molten salts at high temperature also tend to be volatile and corrosive. Hence, equipment at larger scale will need to survive those environments.
The electrolysis route will produce the highest purity of the LM-RE alloy compared to the other routes. The composition of the alloy could also be tuned by changing the process parameters, such as the electrolyte composition and the applied current density/voltage. Two different cathodes can be used, inert or reactive. The use of an inert cathode can cause the alloy sink to the bottom of the cell in the form of droplets because of its poor wettability. In contrast, a reactive cathode will react with the deposited RE and form intermetallics, which causes under-potential deposition of RE metal. This results in a lower operating temperature of the molten salts electrolysis process (Han et al. Citation2016). There is also the possibility for RE segregation in the produced alloy, however, this may potentially be reduced by applying ultrasound to the process (or other means to stir). Currently, there is no industrial size electrolysis equipment specifically tailored for producing LM-RE alloys although the modified Hall-Héroult process is a promising route, especially for Al-RE alloys. The high temperature of the process increases the volatility and corrosivity of the molten salts, thus requiring the cell to be designed to withstand these corrosive environments (Yin et al. Citation2021). Compared to the other routes, process control for the electrolysis route is much more complex, however implementation in an industrial scale is possible, with the current scale of study being a 15–20 kg bath for making Al-Sc alloy (Wang Citation2022).
6. Concluding remarks
RE elements are emerging one of the alloying elements that could be used to improve the properties of light metal alloys. Currently, most LM-RE alloys are made by the expensive route of direct melting of a LM and a RE metal. This process is expensive because RE metals are historically more expensive compared to the LM itself. Research is still needed to develop alternative low-cost and high-efficiency process which use different routes and different, lower cost RE sources.
The review has confirmed that there are three main alternative routes capable of producing LM-RE alloys. The direct metallothermic reduction of RE compound route and the electrolysis route (especially a modified Hall-Héroult route) are the most promising alternatives and should be the focus of future studies. The electrolysis route might seem to have several advantages compared to the others through the demonstrated scalability and existing infrastructure associated with Al production, but the Al-RE alloy process is more complex. The metallothermic reduction of RE compound seems to be the simplest but research still need to improve the efficiency of the process. Further studies are also still needed to scale up the process from laboratory. All routes have their own disadvantages that still need to be addressed for them to be successful at a commercial scale.
Successful development of a new process would lower the price of the LM-RE alloy, and potentially expand its application to different areas. For example, Mg-RE alloys are promising materials for making biocompatible materials as their mechanical properties could make them suitable to be used in biomedical applications. In addition, the potential of utilizing low-cost RE supply chain metals such as Ce and La as alloying elements for producing Al alloys that display similar properties to commercial Al alloys – and require minimal heat treatment – could potentially shift the industry into making more Al-RE alloys. Lightweight and strong material such as Mg-RE and Al-RE alloys will have a significant role in improving the efficiency of a process. For example, 10% weight reduction could deliver 6% fuel economy improvement and 14% increase in range for internal combustion vehicle and electric vehicle, respectively (Luo Citation2021). The increased demand for improved fuel economy will also drive the demand for light alloys.
Acknowledgments
This work is part of a PhD study by main author under the joint PhD program between Swinburne University of Technology, Australia, and the National Innovation and Research Agency (BRIN), Indonesia. The authors are grateful with the joint funding support from Swinburne University of Technology; the National Innovation and Research Agency-Indonesia (BRIN); the Commonwealth Scientific and Industrial Research Organization-Australia (CSIRO); Magontec Ltd.; Grandfield Technology Pty. Ltd.; and Platina Resources Ltd.
Disclosure statement
The author declares that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Funding
References
- Archambo, M., and S. K. Kawatra. 2021. Red mud: Fundamentals and new avenues for utilization. Mineral Processing & Extractive Metallurgy Review 42 (7):427–50. Taylor & Francis. doi:10.1080/08827508.2020.1781109.
- Bai, Z., Y. Xia, F. Qiu, Y. Liu, W. Hu, and Q. Jiang. 2014. Effects of RExOy addition on corrosion behavior of the Al–Cu alloys in 3.5wt.% NaCl solution and PH=4 acid solution. Applied Surface Science 307 (July):153–57. doi:10.1016/j.apsusc.2014.03.198.
- Bale, C. W., E. Bélisle, P. Chartrand, S. A. Decterov, G. Eriksson, A. E. Gheribi, K. Hack, I.-H. Jung, Y.-B. Kang, J. Melançon, et al. 2016. FactSage thermochemical software and databases, 2010–2016. Calphad 54 (September):35–53. doi:10.1016/j.calphad.2016.05.002.
- Bazhin, V. Y., Y. I. Kosov, O. L. Lobacheva, and N. V. Dzhevaga. 2015. Synthesis of aluminum-based scandium–yttrium master alloys. Russian Metallurgy (Metally) (7):516–20. doi:10.1134/S0036029515070034.
- Bian, M., I. Nakatsugawa, Y. Matsuoka, X. Huang, Y. Tsukada, T. Koyama, and Y. Chino. 2022. Improving the mechanical and corrosion properties of pure magnesium by parts-per-million-level alloying. Acta Materialia 241 (December):118393. Elsevier Ltd. doi:10.1016/j.actamat.2022.118393.
- Bray, E. L. 2020. Magnesium metal, mineral commodity summaries.
- Bray, E. L. 2022. Aluminum, mineral commodity summaries.
- Calado, L. M., M. J. Carmezim, and M. F. Montemor. 2022. Rare earth based magnesium alloys—A review on WE series. Frontiers in Materials 8. doi:10.3389/fmats.2021.804906.
- Cao, P., M. Zhang, W. Han, Y. Yan, S. Wei, and T. Zheng. 2011. Electrochemical behaviour of erbium and preparation of Mg-Li-Er alloys by codeposition. Journal of Rare Earths 29 (8):763–67. doi:10.1016/S1002-0721(10)60538-8.
- Castrillejo, Y., M. R. Bermejo, E. Barrado, and A. M. Martínez. 2006. Electrochemical behaviour of erbium in the eutectic LiCl-KCl at W and Al electrodes. Electrochimica Acta 51 (10):1941–51. doi:10.1016/j.electacta.2005.07.004.
- Castrillejo, Y., A. Vega, M. Vega, P. Hernández, J. A. Rodriguez, and E. Barrado. 2014. Electrochemical formation of Sc-Al intermetallic compounds in the eutectic LiCl-KCl. determination of thermodynamic properties. Electrochimica acta 118:58–66. doi:10.1016/j.electacta.2013.11.163.
- Chen, J., L. Tan, X. Yu, I. P. Etim, M. Ibrahim, and K. Yang. 2018. Mechanical properties of magnesium alloys for medical application: A review. Journal of the Mechanical Behavior of Biomedical Materials 87:68–79. doi:10.1016/j.jmbbm.2018.07.022.
- Chen, Y., K. Ye, and M. Zhang. 2010. Preparation of Mg-Yb alloy film by electrolysis in the molten LiCl-KCl-YbCl3 system at low temperature. Journal of Rare Earths 28 (1):128–33. doi:10.1016/S1002-0721(09)60065-X.
- Connelly, N. G., T. Damhus, R. M. Hartshorn, and A. T. Hutton. 2007. Nomenclature of inorganic chemistry - IUPAC recommendations 2005. Chemistry International – Newsmagazine for IUPAC 27 (6):25–26. doi:10.1515/ci.2005.27.6.25.
- Damoah, L. N. W., and L. Zhang. 2010. Removal of inclusions from aluminum through filtration. Metallurgical and Materials Transactions B 41 (4):886–907. doi:10.1007/s11663-010-9367-3.
- Dorin, T., M. Ramajayam, A. Vahid, and T. Langan. 2018. Aluminium Scandium Alloys. In Fundamentals of aluminium metallurgy, 439–94. Elsevier. doi:10.1016/B978-0-08-102063-0.00012-6.
- Duyvesteyn, W. P. C. 2019. Scandium-containing master alloys and methods for making the same. US Patent 10,450,634 B2, filed July 31, 2017, and issued October 22, 2019.
- Esmaily, M., J. E. Svensson, S. Fajardo, N. Birbilis, G. S. Frankel, S. Virtanen, R. Arrabal, S. Thomas, and L. G. Johansson. 2017. Fundamentals and advances in magnesium alloy corrosion. Progress in Materials Science 89 (August):92–193. The Author(s). doi:10.1016/j.pmatsci.2017.04.011.
- Fujii, H., H. Akiyama, J. Kaneko, M. Sugamata, and L. Blaz. 2003. Al-Sc master alloy prepared by mechanical alloying of aluminum with addition of Sc2O3. Materials Transactions 44 (5):1049–52. doi:10.2320/matertrans.44.1049.
- Fujii, S., E. Suzuki, N. Inazu, S. Tsubaki, J. Fukushima, H. Takizawa, and Y. Wada. 2020. Microwave irradiation process for al–sc alloy production. Scientific Reports 10 (1):2689. doi:10.1038/s41598-020-59664-2.
- Fu, Y., Z. Zhang, X. Lu, B. Pan, and L. Zhang. 2022. Preparation of Mg–Nd Alloys by magnesiothermic reduction in molten salt. Metallurgical and Materials Transactions B 53 (1):617–26. doi:10.1007/s11663-021-02406-0.
- Guan, C., J. Xue, J. Zhu, and Q. Liu. 2012. Preparing aluminum-scandium alloys using direct hall reduction process. In 3rd International Symposium on High-Temperature Metallurgical Processing, 243–50. Hoboken, NJ, USA: John Wiley & Sons, Inc. doi:10.1002/9781118364987.ch30.
- Guo, Z., X. Liu, and J. Xue. 2019. Fabrication of Al-Si-Sc alloy bearing AlSi2Sc2 phase using ultrasonically assisted molten salt electrolysis. Journal of Alloys and Compounds 797:883–89. doi:10.1016/j.jallcom.2019.05.133.
- Guo, R., X. J. Zhai, and T. A. Zhang. 2011. Preparation of Al-Sc Alloy by LiF-ScF3-ScCl3 molten salt electrolysis. Materials Science Forum 675–677:1125–28. doi:10.4028/www.scientific.net/MSF.675-677.1125.
- Haidar, J. 2014. Production of aluminium-scandium alloys. Australia: World Intellectual Property Organization.
- Han, W., M. Li, M.-L. Zhang, and Y.-D. Yan. 2016. Progress in preparation of rare earth metals and alloys by electrodeposition in molten salts. Rare Metals 35 (11):811–25. doi:10.1007/s12598-016-0798-0.
- Harata, M., T. Nakamura, H. Yakushiji, and T. H. Okabe. 2008. Production of scandium and Al–Sc alloy by metallothermic reduction. Mineral Processing & Extractive Metallurgy 117 (2):95–99. doi:10.1179/174328508X290876.
- Harata, M., K. Yasuda, H. Yakushiji, and T. H. Okabe. 2009. Electrochemical production of Al-Sc alloy in CaCl2-Sc2O3 molten salt. Journal of Alloys and Compounds 474 (1–2):124–30. doi:10.1016/j.jallcom.2008.06.110.
- Jang, P., H. Li, W. Kim, Z. Wang, and F. Liu. 2015. Preparation of Al-La master alloy by thermite reaction in NaF-NaCl-KCl molten salt. JOM 67 (5):1130–36. doi:10.1007/s11837-015-1392-x.
- Jang, P. N., H. M. Li, W. J. Kim, S. C. Yun, and G. H. Hwang. 2019. Electrolytic preparation of Mg-Al-La Alloys in KCl-MgCl2-AlF3 molten salts. Journal of Materials Research and Technology 8 (6):5456–63. doi:10.1016/j.jmrt.2019.09.013.
- Jiang, T., N. Wang, S.-M. Peng, M. Li, W. Han, and M.-L. Zhang. 2016. Electrochemical formation of Mg–Lu alloy and alloy layer in molten LiCl–KCl. Journal of Alloys and Compounds 658:198–209. doi:10.1016/j.jallcom.2015.10.159.
- Jones, R., J. Cizek, O. Kovarik, A. Ang, and V. Champagne. 2022. Observations on comparable aluminium alloy crack growth curves: additively manufactured scalmalloy® as an alternative to AA5754 and AA6061-T6 alloys? Additive Manufacturing Letters 2:100026. doi:10.1016/j.addlet.2022.100026.
- Kim, S. K., H. Seo, Jung, and I. K. Kim. 2013. Method of manufacturing a magnesium-scandium master alloy and method of manufacturing an aluminum alloy containing scandium. European Patent Office.
- Kosov, Y. I., and V. Y. Bazhin. 2018. Preparation of Novel Al-Er master alloys in chloride-fluoride melt. Materials Science Forum 918:21–27. doi:10.4028/www.scientific.net/MSF.918.21.
- Kosov, Y. I., V. Y. Bazhin, and T. N. Kopylova. 2019. Effect of the technological parameters of the aluminothermic reduction of erbium oxide in chloride–fluoride melts on the transition of erbium to a master alloy. Russian Metallurgy (Metally) (9):856–62. doi:10.1134/S0036029519090040.
- Krishnamurthy, N., and C. K. Gupta. 2001. Rare earth metals and alloys by electrolytic methods. Mineral Processing & Extractive Metallurgy Review 22 (2):477–507. doi:10.1080/08827509808962512.
- Kulikov, B. P., V. N. Baranov, A. I. Bezrukikh, V. B. Deev, and M. M. Motkov. 2018. Preparation of aluminum-scandium master alloys by aluminothermal reduction of scandium fluoride extracted from Sc2O3. Metallurgist 61 (11–12):1115–21. doi:10.1007/s11015-018-0614-1.
- Lee, J. A., and P. S. Chen. 2004. Aluminum-scandium alloys: Material characterization, friction stir welding, and compatibility with hydrogen peroxide. https://ntrs.nasa.gov/api/citations/20050158693/downloads/20050158693.pdf.
- Li, C., J. Li, Y. Mao, and J. Ji. 2017. Mechanism to remove oxide inclusions from molten aluminum by solid fluxes refining method. China Foundry 14 (4):233–43. doi:10.1007/s41230-017-7005-2.
- Liu, X., Z. C. Guo, J. L. Xue, Z. J. Wang, X. Li, C. W. Zhu, and P. J. Zhang. 2020. Ultrasonic refining mechanism of ternary phase in Al-Sc based alloys prepared through molten salt electrolysis. Gongcheng Kexue Xuebao/Chinese Journal of Engineering 42 (11):1465–72. doi:10.13374/j.issn2095-9389.2019.11.28.007.
- Liu, X., Z. Guo, J. Xue, and C. Zhang. 2019a. Effects of synergetic ultrasound on the Sc yield and primary Al3Sc in the Al-Sc alloy prepared by the molten salts electrolysis. Ultrasonics Sonochemistry 52:33–40. doi:10.1016/j.ultsonch.2018.09.009.
- Liu, K., Y.-L. Liu, L.-Y. Yuan, X.-L. Zhao, Z.-F. Chai, and W.-Q. Shi. 2013a. Electroextraction of gadolinium from Gd2O3 in LiCl–KCl–AlCl3 molten salts. Electrochimica Acta 109:732–40. doi:10.1016/j.electacta.2013.07.084.
- Liu, K., Y.-L. Liu, L.-Y. Yuan, X.-L. Zhao, H. He, G.-A. Ye, Z.-F. Chai, and W.-Q. Shi. 2014. Electrochemical formation of erbium-aluminum alloys from Erbia in the chloride melts. Electrochimica Acta 116:434–41. doi:10.1016/j.electacta.2013.11.093.
- Liu, X., J. Xue, Z. Guo, and C. Zhang. 2019b. Segregation Behaviors of Sc and Unique Primary Al3Sc in Al-Sc Alloys Prepared by Molten Salt Electrolysis. Journal of Materials Science and Technology 35 (7):1422–31. doi:10.1016/j.jmst.2019.02.002.
- Liu, Q., J. Xue, J. Zhu, and C. Guan. 2012. Preparing aluminium-scandium inter-alloys during reduction process in KF-ALF3-SC2O3 melts. In Light metals 2012, ed. C. E. Suarez, 685–89. Cham: Springer International Publishing. doi:10.1002/9781118359259.ch118.
- Liu, Q., J. Xue, J. Zhu, Y. Qian, and L. Feng. 2013b. Processing Al-Sc alloys at liquid aluminum cathode in KF-AlF3 molten salt. ECS Transactions 50 (11):483–89. doi:10.1149/05011.0483ecst.
- Li, M., Y. Zhang, H. Li, H. Jiang, W. Han, and Y. Sun. 2022. Electrochemical formation of Mg–La–Mn alloys by coreduction of Mg(II), La(III) and Mn(II) in LiCl+KCl molten salts. Journal of Rare Earths 40 (3):501–07. doi:10.1016/j.jre.2020.12.013.
- Luna A, J. S., A. Flores V, R. Muñiz V, A. F. Fuentes, J. Torres, N. Rodríguez R, J. C. Ortiz, and P. Orozco. 2011. Cerium extraction by metallothermic reduction using cerium oxide powder injection. Journal of Rare Earths 29 (1):74–76. doi:10.1016/S1002-0721(10)60402-4.
- Lun Sin, S., A. Elsayed, and C. Ravindran. 2013. Inclusions in magnesium and its alloys: A review. International Materials Reviews 58 (7):419–36. doi:10.1179/1743280413Y.0000000017.
- Luo, A. A. 2021. Recent advances in light metals and manufacturing for automotive applications. CIM Journal. 12 (3):79–87. Taylor & Francis. doi:10.1080/19236026.2021.1947088.
- Lu, P., J. G. Wang, J. F. Liu, and Q. C. Jiang. 2005. Effect of La2O3 addition on the microstructure of partially remelted Mg-9Al-1Zn alloy. Journal of Materials Science 40 (24):6429–32. doi:10.1007/s10853-005-1628-1.
- Martinez, A. M., K. S. Osen, H. Gudbrandsen, C. Sommerseth, Z. Wang, and O. Darell. 2018. Direct method for producing scandium metal and scandium-aluminium intermetallic compounds from the oxides. In Light metals 2018, ed. O. Martin, 1559–64. Cham: Springer International Publishing. doi:10.1007/978-3-319-72284-9_203.
- Martinez, A. M., S. Senanu, H. Gudbrandsen, K. S. Osen, A. Støre, Z. Wang, and O. Kjos. 2021. Production of Al-Sc alloy by electrolysis from cryolite melt using secondary feedstock material. In International Conference on Raw Materials and Circular Economy, 41. Basel Switzerland: MDPI. doi:10.3390/materproc2021005041.
- Megson, T. H. G. 2012. Aircraft structures for engineering Students. Aircraft structures for engineering students. Oxford: Elsevier Science & Technology. doi:10.1016/B978-0-08-096905-3.00039-5.
- Meyer, G., and P. Ax. 1982. An analysis of the ammonium chloride route to anhydrous rare-earth metal chlorides. Materials Research Bulletin 17 (11):1447–55. doi:10.1016/0025-5408(82)90231-8.
- Min, D., 민동준, B. Lee 이병필, J. Park 박진균, J. Lee 이재영, and J. Ko 고재웅. 2019. Scandium alloy and preparation method thereof. South Korea. https://patents.google.com/patent/KR101927379B1.
- Moosbrugger, C., and L. Marquard. 2017. Engineering properties of magnesium alloys. ed. Charles Moosbrugger and Liz Marquard. Materials Park, Ohio: ASM International.
- Mordike, B. L., and T. Ebert. 2001. Magnesium properties - applications - potential. Materials Science and Engineering: A 302 (1):37–45. doi:10.1016/S0921-5093(00)01351-4.
- Moussa, M. E., S. El-Hadad, and W. Khalifa. 2019. Influence of chemical modification by Y2O3 on eutectic Si characteristics and tensile properties of A356 alloy. Transactions of Nonferrous Metals Society of China 29 (7):1365–74. doi:10.1016/S1003-6326(19)65043-0.
- Nie, Z. R., T. Jin, J. Fu, G. Xu, J. Yang, J. X. Zhou, and T. Y. Zuo. 2002. Research on rare earth in aluminum. Materials Science Forum 396-402 (396–402 (3):1731–0. doi:10.4028/www.scientific.net/MSF.396-402.1731.
- Nie, Z. R., T. N. Jin, J. X. Zou, J. B. Fu, J. J. Yang, and T. Y. Zuo. 2003. Development on research of advanced rare-earth aluminum alloy. Transactions of Nonferrous Metals Society of China (English Edition) 13 (3):509–14.
- Nikolaev, A. Y., A. V. Suzdaltsev, and Y. P. Zaikov. 2019. Electrowinning of aluminum and scandium from KF-AlF 3 -Sc 2 O 3 melts for the synthesis of Al-Sc master alloys. Journal of the Electrochemical Society 166 (8):D252–D57. doi:10.1149/2.0231908jes.
- Padilla, I., M. Romero, S. López-Andrés, and A. López-Delgado. 2022. Sustainable management of salt slag. Sustainability 14 (9):4887. doi:10.3390/su14094887.
- Paradiso, V., F. Rubino, P. Carlone, and G. S. Palazzo. 2017. Magnesium and aluminium alloys dissimilar joining by friction stir welding. Procedia Engineering 183:239–44. doi:10.1016/j.proeng.2017.04.028.
- Polat, Ç., M. Erdoğan, A. S. İ̇plikçioğlu, and İ. Karakaya. 2018. Electrochemical formation of alloys of scandium in molten salts. In Light metal 2018, ed. O. Martin, 1555–58. Cham: Springer International Publishing. doi:10.1007/978-3-319-72284-9_202.
- Qian, Y., J. Xue, Q. Liu, and J. Zhu. 2016. Preparing Al-Sc-Zr alloys in aluminum electrolysis process, In B. A. Sadler.ed.Light metals 2013 1311–14. Cham.10.1007/978-3-319-65136-1_221
- Rafique, M. M. A. 2023. Pyrometallurgy and electrometallurgy of rare earths – Part A: Analysis of metallothermic reduction and its variants. Mineral Processing and Extractive Metallurgy Review :1–8. doi:10.1080/08827508.2022.2164576.
- Ricketts, N. 2021. Scandium master alloy production. US Patent 10,988,830 B2, filed January 16, 2019, and issued April 27, 2021.
- Rokhlin, L. L. 2003. Magnesium alloys containing rare earth metals. CRC Press. doi:10.1201/9781482265163.
- Røyset, J., and N. Ryum. 2005. Scandium in aluminium alloys. International Materials Reviews 50 (1):19–44. doi:10.1179/174328005X14311.
- Rumble, J. R., ed. 2021. CRC handbook of chemistry and physics. 102nd ed. Boca Raton, FL: CRC Press/Taylor & Francis. doi:10.1016/0022-2860(92)85083-S.
- Savchenkov, S., and I. Beloglazov. 2022. Features of the process obtaining of Mg-Zn-Y master alloy by the metallothermic recovery method of yttrium fluoride melt. Crystals 12 (6):771. doi:10.3390/cryst12060771.
- Savchenkov, S., Y. Kosov, V. Bazhin, K. Krylov, and R. Kawalla. 2021. Microstructural master alloys features of aluminum–erbium system. Crystals 11 (11):1353. doi:10.3390/cryst11111353.
- Schofield, M. J. 2001. Corrosion. In Plant engineer’s handbook, 961–85. Burlington: Elsevier. doi:10.1016/B978-075067328-0/50055-0.
- Shtefanyuk, Y., V. Mann, V. Pingin, D. Vinogradov, Y. Zaikov, O. Tkacheva, A. Nikolaev, and A. Suzdaltsev. 2016. Production of Al-Sc alloy by electrolysis of cryolite-scandium oxide melts. In Light metals 2015, ed. M. Hyland, 589–93. Cham: Springer International Publishing. doi:10.1002/9781119093435.ch98.
- Sims, Z. C., H. B. Henderson, M. J. Thompson, R. P. Chaudhary, J. A. Hammons, J. Ilavsky, D. Weiss, K. Anderson, R. Ott, and O. Rios. 2021. Application of Ce for scavenging Cu impurities in A356 Al alloys. European Journal of Materials. 1 (1):3–18. Taylor & Francis. doi:10.1080/26889277.2021.1974801.
- Sims, Z. C., M. S. Kesler, H. B. Henderson, E. Castillo, T. Fishman, D. Weiss, P. Singleton, R. Eggert, S. K. McCall, and O. Rios. 2022. How cerium and lanthanum as coproducts promote stable rare earth production and new alloys. Journal of Sustainable Metallurgy 8 (3):1225–34. doi:10.1007/s40831-022-00562-4.
- Sivashanmugam, N., and K. L. Harikrishna. 2020. Influence of rare earth elements in magnesium alloy - a mini review. Materials Science Forum 979:162–66. doi:10.4028/www.scientific.net/MSF.979.162.
- Skachkov, V. 2018. Application of alkaline metal fluorides for doping of aluminum. Fluorine Notes (February):3–4. doi:10.17677/fn20714807.2018.02.02.
- Skachkov, V. M., L. A. Pasechnik, and S. P. Yatsenko. 2014. Introduction of scandium, zirconium and hafnium into aluminum alloys. Dispersion hardening of intermetallic compounds with nanodimensional particles. Nanosystems: Physics, Chemistry, Mathematics 5 (4):603–12.
- Skachkov, V. M., P. A. Varchenya, B. V. Ovsyannikov, and S. P. Yatsenko. 2013. Injection of scandium-containing process powders into aluminum alloys. Tsvetnye Metally 12:81–86.
- Sommer, F. 1992. Thermodynamic properties of rare earth metal alloys. Mineral Processing and Extractive Metallurgy Review 9 (1):195–207. doi:10.1080/08827509208952704.
- Su, L.-L., K. Liu, Y.-L. Liu, L. Wang, L.-Y. Yuan, L. Wang, Z.-J. Li, X.-L. Zhao, Z.-F. Chai, and W.-Q. Shi. 2014. Electrochemical behaviors of Dy(III) and its co-reduction with Al(III) in molten LiCl-KCl salts. Electrochimica acta 147:87–95. doi:10.1016/j.electacta.2014.09.095.
- Suzdaltsev, A. V., A. A. Filatov, A. Y. Nikolaev, A. A. Pankratov, N. G. Molchanova, and Y. P. Zaikov. Extraction of scandium and zirconium from their oxides during the electrolysis of oxide–fluoride melts. Russian Metallurgy (Metally) 2018 (2):133–38. doi:10.1134/S0036029518020180.
- Talan, D., Q. Huang, L. Liang, and X. Song. 2023. Conceptual process development for the separation of thorium, uranium, and rare earths from coarse coal refuse. Mineral Processing and Extractive Metallurgy Review 44 (5):330–45. doi:10.1080/08827508.2022.2064855.
- Tang, H., Y.-D. Yan, M.-L. Zhang, X. Li, W. Han, Y. Xue, Z.-J. Zhang, and H. He. 2013a. Fabrication of Mg–Pr and Mg–Li–Pr Alloys by electrochemical co-reduction from their molten chlorides. Electrochimica Acta 107:209–15. doi:10.1016/j.electacta.2013.05.129.
- Tang, H., Y.-D. Yan, M.-L. Zhang, X. Li, Y. Huang, Y.-L. Xu, Y. Xue, W. Han, and Z.-J. Zhang. 2013b. AlCl3-aided extraction of praseodymium from Pr6O11 in LiCl–KCl eutectic melts. Electrochimica acta 88:457–62. doi:10.1016/j.electacta.2012.10.045.
- Tarcy, G. P., and P. A. Foster Jr. 1988. Method for making a light metal-rare earth metal alloy. United States. https://patents.google.com/patent/US5037608A/en.
- Tekumalla, S., S. Seetharaman, A. Almajid, and M. Gupta. 2014. Mechanical properties of magnesium-rare earth alloy systems: A review. Metals 5 (1):1–39. doi:10.3390/met5010001.
- Tian, Z., Y. Lai, K. Zhang, X. Hu, H. Zhang, and J. Li. 2016. Preliminary study on preparation of Al-Sc master alloy in Na 3 AlF 6 -K 3 AlF 6 -AlF 3 melt. In 7th International Symposium on High-Temperature Metallurgical Processing, ed. J.-Y. Hwang, T. Jiang, P. C. Pistorius, G. R. F. Alvear F, O. Yücel, L. Cai, B. Zhao, D. Gregurek, and V. Seshadri, 157–63. NJ, USA: John Wiley & Sons, Inc. doi:10.1002/9781119274643.ch20.
- Tsakiridis, P. E. 2012. Aluminium salt slag characterization and utilization – a review. Journal of Hazardous Materials 217–218:1–10. doi:10.1016/j.jhazmat.2012.03.052.
- Varchenya P. A., B. Ovsyannikov, S. Yatsenko, N. A. Sabirzyanov, and L. Pasechnik. 2009. Synthesis and properties of aluminum master-alloy with scandium, zirconium, and hafnium. In First International Congress (Non-Ferrous Metals of Siberia -2009) Part III Non-Ferrous Rare Metals Production.
- Vignes, A. 2013. Extractive metallurgy 3: Processing operations and routes. Hoboken, NJ USA: John Wiley & Sons, Inc. doi:10.1002/9781118617106.
- Vignes, A. 2013. Molten salt electrolysis operations. In Extractive metallurgy, Vol. 3, 265–91. Hoboken, NJ USA: John Wiley & Sons, Inc. doi:10.1002/9781118617106.ch9.
- Wang, X. 2022. Preparation of aluminum master alloys by electrolytic co-deposition in Hall-Héroult cells. In Light metals 2022, ed. D. Eskin, 392–401. Cham: Springer International Publishing. doi:10.1007/978-3-030-92529-1_53.
- Wang, Z., C. Guan, Q. Liu, and J. Xue. 2015. Formation of intermetallic phases in Al-Sc Alloys prepared by molten salt electrolysis at elevated temperatures. In 6th International Symposium on High-Temperature Metallurgical Processing, 215–22. Hoboken, NJ: John Wiley & Sons, Inc. doi:10.1002/9781119093381.ch28.
- Wang, J., M. Li, W. Han, Z.-Y. Liu, X.-G. Yang, Y. Sun, and M.-L. Zhang. 2022. Electrochemical co-reduction of holmium and magnesium ions in eutectic LiCl–KCl salts. Rare Metals 41 (4):1394–402. doi:10.1007/s12598-018-1157-0.
- Wang, Y., M. Li, W. Han, M. Zhang, Y. Yang, Y. Sun, Y. Zhao, and Y. Yan. 2015. Electrochemical extraction and separation of praseodymium and erbium on reactive magnesium electrode in molten salts. Journal of Solid State Electrochemistry 19 (12):3629–38. doi:10.1007/s10008-015-2989-2.
- Wang, S. D., Q. Li, X. S. Ye, Q. G. Sun, and Z. J. Wu. 2013. Effect of oxide and fluoride addition on electrolytic preparation of Mg-La alloy in chloride molten salt. Transactions of Nonferrous Metals Society of China (English Edition) 23 (10):3104–11. doi:10.1016/S1003-6326(13)62840-X.
- Weiss, D., O. Rios, Z. Sims, S. McCall, and R. Ott. 2017. Casting characteristics of high cerium content aluminum alloys. In Light metals 2017, ed. A. P. Ratvik, 205–11. Cham: Springer International Publishing. doi:10.1007/978-3-319-51541-0_28.
- Xia, Y., Z. Bai, F. Qiu, S. Jin, and Q. Jiang. 2012. Effects of multi-modification of rare earth oxides PrxOy and LaxOy on microstructure and tensile properties of casting Al–Cu alloy. Materials Science and Engineering: A 558:602–06. doi:10.1016/j.msea.2012.08.059.
- Xiao, J., W. Ding, Y. Peng, T. Chen, and K. Zou. 2020. Preparing Sc-bearing master alloy using aluminum–magnesium thermoreduction method. Metals 10 (7):960. doi:10.3390/met10070960.
- Xu, C., X. Liu, F. Ma, Z. Wang, W. Wang, and C. Ma. 2012. Preparation of Al-Sc master alloy by aluminothermic reaction with special molten salt. In ICAA13 Pittsburgh, 195–200. Cham: Springer International Publishing. doi:10.1007/978-3-319-48761-8_30.
- Yang, K., Z. Tian, X. Hu, Y. Lai, and J. Li 2019. Effects of electrolytic parameters on the preparation of Al–Sc master alloy in Na3AlF6-K3AlF6-AlF3 melt. In 10th International Symposium on High-Temperature Metallurgical Processing, ed. T. Jiang, J.-Y. Hwang, D. Gregurek, Z. Peng, J. P. Downey, B. Zhao, O. Yücel, E. Keskinkilic, and R. Padilla, 321–27. Cham: Springer International Publishing. doi:10.1007/978-3-030-05955-2_30.
- Yang, S., F. Yang, C. Liao, M. Li, and X. Wang. 2010. Electrodeposition of magnesium-yttrium alloys by molten salt electrolysis. Journal of Rare Earths 28:385–88. doi:10.1016/S1002-0721(10)60322-5.
- Yan, Y.-D., Y.-L. Xu, M.-L. Zhang, Y. Xue, W. Han, Y. Huang, Q. Chen, and Z.-J. Zhang. 2013. Electrochemical extraction of neodymium by co-reduction with aluminum in LiCl–KCl molten salt. Journal of Nuclear Materials 433 (1–3):152–59. doi:10.1016/j.jnucmat.2012.09.008.
- Yatsenko, S. P., V. М. Skachkov, and L. А. Pasechnik. 2020. Production of rich aluminum master alloys containing scandium, yttrium and zirconium for non-ferrous and ferrous metallurgy. Tsvetnye Metally (8):49–55. doi:10.17580/tsm.2020.08.06.
- Yin, T., Y. Xue, Y. Yan, Z. Ma, F. Ma, M. Zhang, G. Wang, and M. Qiu. 2021. Recovery and separation of rare earth elements by molten salt electrolysis. International Journal of Minerals, Metallurgy & Materials 28 (6):899–914. doi:10.1007/s12613-020-2228-4.
- Zakharov, V. V., and I. A. Fisenko. 2017. Alloying aluminum alloys with scandium. Metal Science and Heat Treatment 59 (5–6):278–84. doi:10.1007/s11041-017-0142-9.
- Zhang, M. L., P. Cao, W. Han, Y. De Yan, and L. J. Chen. 2012b. Preparation of Mg-Li-La alloys by electrolysis in molten salt. Transactions of Nonferrous Metals Society of China 22 (1):16–22. doi:10.1016/S1003-6326(11)61133-3.
- Zhang, L., J. Gao, L. N. W. Damoah, and D. G. Robertson. 2012a. Removal of iron from aluminum: A review. Mineral Processing and Extractive Metallurgy Review 33 (2):99–157. doi:10.1080/08827508.2010.542211.
- Zhang, Z., X. Lu, and Y. Yan. 2023. Preparation of RE-Containing magnesium alloys via molten-salt-mediated magnesiothermic reduction. Journal of Magnesium and Alloys 11 (3):981–90. doi:10.1016/j.jma.2021.05.002.
- Zhang, M., Y. Yang, W. Han, M. Li, Y. Sun, and Y. Yan. 2013. Separation of SmCl3 from SmCl3-DyCl3 system by electrolysis in KCl-LiCl-MgCl2 molten salts. Energy Procedia 39:375–81. doi:10.1016/j.egypro.2013.07.225.
- Zhao, W., H. Wang, J. Wang, Y. Li, and Q. Jiang. 2008. Simultaneously increasing the strength and ductility of the modified casting Al–Cu alloy. Journal of Materials Research 23 (4):1076–81. doi:10.1557/jmr.2008.0110.
- Zhao, H., D. Yao, F. Qiu, Y. Xia, and Q. Jiang. 2011. High strength and good ductility of casting Al–Cu alloy modified by PrxOy and LaxOy. Journal of Alloys and Compounds 509 (5):L43–L46. doi:10.1016/j.jallcom.2010.09.059.
- Zhao, S., H. Zhou, T. Zhou, Z. Zhang, P. Lin, and L. Ren. 2013. The oxidation resistance and ignition temperature of AZ31 magnesium alloy with additions of La2O3 and La. Corrosion Science 67:75–81. doi:10.1016/j.corsci.2012.10.007.