?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The copper (Cu) concentrators have to process high-pyrite-content ores due to rapid depletion of high-grade ores and a high demand for Cu by clean energy technologies. However, depressing high-concentration pyrite in copper flotation is challenging presently. For the first time, this study evaluated 30 wt% Cr steel, a type of high-chromium (HiCr) steel grinding media, which can effectively produce a high Cu concentrate grade in processing low-concentration pyrite ores, in treating 25% pyrite in a Cu feed at 1.7% Cu in comparison with treating 5% pyrite in a Cu feed at the same Cu grade. Flotation tests showed decreased Cu grade and Cu recovery as pyrite feed grade increased from 5% to 25%, which was attributed to the changed galvanic interaction between grinding media and sulfide minerals, supported by ethylene diamine-tetra acetic acid disodium extraction, pulp chemistry measurements, and electrochemical studies. As pyrite feed grade increased from 5% to 25%, the anodic role of 30 wt% Cr steel did not change, but the anodic role of chalcopyrite and the cathodic role of pyrite were enhanced, promoting chalcopyrite oxidation while retarding pyrite oxidation, both of which facilitated copper activation on pyrite and subsequent pyrite flotation. This study also compared 30 wt% Cr steel and traditional forged steel in depressing 25% pyrite in the feed, and forged steel poorly depressed pyrite due to a strong anodic role of forged steel and a strong cathodic role of pyrite during the galvanic interaction leading to strong copper activation on pyrite. This study points to a direction how to depress high-concentration pyrite in copper flotation.
1. Introduction
Chalcopyrite (CuFeS2) is the most crucial copper (Cu) mineral in the world as it is involved in at least 70% of global Cu production (de Melo Silva Cheloni et al. Citation2023; Li et al. Citation2013; Schlesinger et al. Citation2011). Chalcopyrite in Cu ores is diluted with various sulfide and non-sulfide gangue minerals, and usually a Cu ore only contains 0.3–1.7% Cu (Schlesinger et al. Citation2011). Hence, froth flotation is a typical enrichment process employed to separate chalcopyrite in Cu ores from other gangue minerals prior to the pyrometallurgical process (Córdoba et al. Citation2008; Xiao et al. Citation2022). In recent years, the global Cu demand has reached a record high, but the Cu head grade in ores has been reducing continuously attributable to rapid depletion of high-grade ores (Asgari et al. Citation2022; Peng and Bhambhani Citation2021). With the reduction of Cu head grades, it has been observed that pyrite (FeS2) content in Cu ores is increasing (Bakalarz Citation2021; Lee, Chen, and Peng Citation2022). To meet the growing Cu production, treating low-grade and high-pyrite-content Cu ores is a foreseeable trend (Ndoro and Witika Citation2017).
Generally speaking, pyrite flotation is poor at alkaline pH (Aghazadeh, Kamal Mousavinezhad, and Gharabaghi Citation2015; Hu, Sun, and Wang Citation2009). Thus, Cu flotation is usually carried out under alkaline conditions when pyrite is to be rejected (Bicak et al. Citation2007). However, the separation between chalcopyrite and pyrite can still be challenging as cupric (Cu2+) ions emanating from Cu minerals can interact with pyrite, forming cuprous (Cu+)-sulfide species (Chandra and Gerson Citation2009; Peng, Wang, and Gerson Citation2012; Weisener and Gerson Citation2000). The presence of Cu+-sulfide species on the pyrite surface makes this surface resemble a surface of copper sulfides, on which a thiol collector can adsorb even in alkaline conditions to render pyrite flotation. Furthermore, Cu flotation behavior tends to differ with pyrite feed content. As reported by Lee, Chen, and Peng (Citation2022), Cu concentrate grade from the flotation of chalcopyrite mixed with quartz at pH 9.0 decreases when 5% pyrite is introduced to the mixture, and it drops more significantly when 25% pyrite is introduced to the mixture despite the Cu feed grade remaining the same. During grinding and flotation, a galvanic interaction occurs between chalcopyrite and pyrite, which promotes the oxidation of chalcopyrite, producing more Cu2+ ions (Ekmekçi and Demirel Citation1997; Finkelstein Citation1997). When the pyrite proportion becomes more significant, the cathodic surface area in the chalcopyrite–pyrite (Cpy-Py) couple increases, leading to a stronger galvanic current and consequently promoting a greater extent of pyrite activation (Lee, Chen, and Peng Citation2022). This results in more pyrite flotation and a lower Cu concentrate grade accordingly. In flotation plants, the operators often have to sacrifice Cu recovery to accommodate the Cu concentrate grade required by the downstream pyrometallurgy process.
To effectively depress pyrite in copper flotation, a conventional approach is to substitute forged steel grinding media with high-chromium (HiCr) steel grinding media. It has been documented that HiCr steel provides better chalcopyrite selectivity against pyrite in flotation than forged steel under alkaline conditions (Mu, Cheng, and Peng Citation2020; Peng et al. Citation2003). This is because HiCr steel is more resistant to wear and corrosion; therefore, it produces less iron contamination, favoring chalcopyrite flotation, and at the same time, the higher pulp potential created by HiCr steel inhibits Cu activation on the pyrite surface (Huang and Grano Citation2005; Mu, Cheng, and Peng Citation2020; Peng et al. Citation2003). The oxidizing condition produced by HiCr steel also oxidizes pyrite to produce iron hydroxides on the surface to prevent the diffusion of Cu ions (Peng et al. Citation2003; Yang, Mu, and Peng Citation2021). Thus, HiCr steel has been a popular option for upgrading Cu concentrate via improved copper sulfide flotation and pyrite depression.
It is important to note that in the previous studies, single-mineral or one-to-one pyrite to chalcopyrite surface area ratio (Py/Cpy) equivalent to 5% pyrite in the feed was investigated. With increasing pyrite proportions in copper feeds, higher Py/Cpy ratios are expected, and whether HiCr steel grinding media effectively depresses high-concentration pyrite is a question to be answered. Thus, this work compared the pyrite depression ability of HiCr steel grinding media in the flotation of low- and high-pyrite-concentration feeds. A comparison was also made between HiCr and traditional forged steel in depressing high-concentration pyrite. Grinding and flotation tests were performed on a tri-mineral system containing chalcopyrite, pyrite and quartz with Cu feed content remaining the same while changing the ratio of pyrite to quartz. And 30 wt% Cr steel was chosen to represent the ‘maximum ability’ of HiCr steel grinding media in pyrite depression. Ethylene diamine-tetra acetic acid disodium (EDTA) extraction and pulp chemistry measurements were employed to understand the reactions taking place during grinding. Unlike the traditional two-dimensional galvanic couple, a novel type of electrode arrangement and configuration was developed in this study to probe into grinding media–chalcopyrite–pyrite (GM-Cpy-Py) galvanic interactions under various cathodic surface areas through linear sweep voltammetry (LSV) and Tafel polarization measurements.
2. Material and methods
2.1. Materials and reagents
Sulfide minerals (chalcopyrite and pyrite) and quartz samples were obtained from GEO-Discoveries, Australia, and Nuway Landscape supplies Paver & Walls Westerns, Australia, respectively. The purity of all mineral samples in this work is greater than 95%, confirmed by semi-quantitative X-ray diffraction (XRD) and metal inductively coupled plasma (ME-ICP). According to XRD, the main impurity in sulfide mineral samples was quartz which electrochemically inert. A few bulk pieces of chalcopyrite and pyrite were chosen for manufacturing mineral electrodes used in electrochemical measurements, and the rest mineral samples were individually dry crushed with jaw crushers followed by roll crushers and then screened to + 0.6–3.35 mm particle size fraction. The collected mineral particles were homogenized via mixing and then split by a rotary splitter into even lots. The evenly distributed samples were sealed in polyethylene bags to avoid contamination. Chalcopyrite and pyrite samples were placed in a freezer to minimize oxidation. And 30 wt% Cr steel and forged steel grinding media used in mineral grinding were supplied by Vega Industries Ltd.
A number of reagents were used in this study. Flotation collector and frother were FT-1234 (i-propyl ethyl thionocarbamate, (CH3)2CHOCSNHC2H5, purchased from Flottec) and W34 polyfroth (alcohol ethoxylates, R(OCH2CH2), obtained from Huntsman), respectively. Sodium hydroxide (NaOH) solution (0.1 M) was used as a pH modifier during flotation. AR grade EDTA purchased from Chem-Supply Pty Ltd was used to prepare a 3 wt% EDTA solution for EDTA extraction experiments. NaOH solution was added to the EDTA solution to achieve pH 7.5. For electrochemical studies, an electrolyte solution with 0.1 M of borax (sodium tetraborate decahydrate, Na2H20B4O17) and potassium chloride (KCl) was used after the pH adjustment with 32 wt% hydrochloric acid (HCl) to obtain solution pH 9.0 to match the flotation pH. The selected electrolyte concentration minimizes the effect of potential drop and solution resistance in electrochemical measurements (Hampton, Plackowski, and Nguyen Citation2011; Huai, Plackowski, and Peng Citation2017). All solutions were prepared with 15 MΩ deionized water.
2.2. Mineral grinding and flotation
The head grade of chalcopyrite in all feed samples was fixed at 4.9% (corresponding to a Cu content of 1.7%) and chalcopyrite was mixed with either 5% or 25% pyrite in relation to the feed, to represent a low- or high-pyrite head grade, which was balanced with 90.1 or 70.1% quartz representing the primary non-sulfide gangue mineral. The chosen 1.7% Cu and 25% pyrite head grades were based on a high-pyritic Cu ore in Australia. The 5% pyrite head grade represented the low pyrite head grade used in the previous studies (Chen, Peng, and Bradshaw Citation2014; Ekmekçi and Demirel Citation1997; Peng et al. Citation2003) where a one-to-one Py/Cpy ratio was employed.
In grinding and flotation tests, a chalcopyrite–pyrite–quartz (Cpy-Py-Q) mixture, weighted at 611 g, was added into a laboratory stainless steel rod mill with Brisbane tap water to make up a pulp density of approximately 67%. The mixture was ground with seven 30 wt% Cr steel or forged steel rods (6.3–6.4 kg), and the grinding time varied between 20 and 22 min in different test conditions to eventually produce a P80 value of 106 µm for all mill discharges. After grinding, the pulp potential (Eh), pH, and dissolved oxygen (DO) were measured and recorded by TPS multi-probes. The mill discharge was then transferred into a 1.5 L JK batch flotation cell. Brisbane tap water was again used to clean the mill and make up the flotation pulp at around 32% pulp density. The agitator was operated at 1000 rpm to mix the pulp, and the pulp pH was adjusted to 9.0 with 0.1 M NaOH solution. The dosage of collector FT-1234 and frother W34 added during the conditioning stage was 16.4 and 3.3 g/t, respectively. Flotation concentrates and tailings were analyzed by Australian Laboratories Services (ALS) to obtain elemental assays of Cu and Fe. This service at ALS is certified by the Australian National Association of Testing Authorities (NATA). Two replicates were performed for grinding and flotation tests, and the flotation results were presented with error bars to indicate the reproducibility of the tests.
2.3. EDTA extraction
Mineral surfaces can be characterized using X-ray photoelectron spectroscopy (XPS) but only for a single mineral system. This is because XPS cannot identify the origin of surface species within a mixed mineral system. EDTA solution dissolves metal and sulfur (S) oxidation species but not the sulfide species. Hence, Fe, Cu, and S oxidation species formed during grinding were quantified by EDTA extraction in this study. EDTA extraction solution at pH 7.5 or above was found to have the best extraction efficiency and the extraction process developed intents to extract all the oxidation products formed in a pulp (Rumball and Richmond Citation1996). The total amount of oxidation products formed could explain the flotation behavior at pH 9.0 as can be found from other studies in the literature (Lee, Chen, and Peng Citation2022; Tayebi-Khorami et al. Citation2018; Xu et al. Citation2022). In general, a higher amount of hydrophilic oxidation products formed in the pulp leads to a poorer mineral flotation behavior. Samples used in EDTA extraction were freshly prepared by repeating the grinding tests. 10 mL of the pulp from each test after grinding was collected and frozen in liquid nitrogen immediately. The frozen samples were stored in a freezer to minimize further oxidation. The detailed procedure of EDTA extraction can be found elsewhere (Lee, Chen, and Peng Citation2022). The reacted EDTA filtrates from each run were submitted to ALS for ICP-AES analysis to acquire elemental assays of Cu, Fe, and S, and the analysis work is NATA-certified. Apart from the traditional EDTA extractable Cu (EDTACu) and Fe (EDTAFe) for quantifying metal oxidation species, the EDTA-extractable S was also determined as the total dissolved sulfur (TDS) to quantify sulfur oxidation species. Each EDTA experiment was repeated thrice, and the mean values were reported with error bars to show the data variability.
2.4. Electrochemical studies
The galvanic interaction of GM-Cpy-Py couples was investigated via electrochemical studies. Grinding media rods and bulk mineral specimens of chalcopyrite and pyrite were used to prepare the working and counter electrodes involved in the electrochemical measurements. They were cut into small pieces with an alumina saw and then embedded into non-conductive resin. Then, the embedded mineral and grinding media specimens were cut using diamond (M0D15) and alumina (50A15) cutoff wheels, respectively. The electrode-making was then finished by attaching Cu wire at the back of the electrodes with a conductive silver epoxy (Chemtronics, USA), and then sealed with non-conductive epoxy (SELLEYS, Australia) to prevent any undesired electrical contacts with electrolyte.
The electrochemical studies were carried out using a three-electrode configuration electrochemical cell in a 200 mL double-layer wall glass reactor. Electrical signals during the electrochemical studies were obtained by a CHI 920D scanning electrochemical microscope (SECM) electrochemistry workstation. The electrochemical cell consists of up to two working electrodes, one counter electrode and one reference electrode. Two working electrodes connected as a bi-potentiostat were used in some measurements. In general, three electrochemical cell configurations were employed in this study as outlined in .
Table 1. Different electrode configurations used in the electrochemical measurements in this study.
Configuration 1 was designed to investigate the individual role of grinding media and chalcopyrite in a multiphase galvanic system via LSV. Configuration 2 consists of a grinding media electrode as the working electrode and a Cpy-Py mixed hybrid electrode as the counter electrode. The schematic diagram of Configuration 2 is shown in . Configuration 2 was used to understand the galvanic interaction between grinding media and mixed sulfides. Configuration 3 was set up to investigate pyrite oxidation when coupled with grinding media at different pyrite surface areas. The same reference electrode (3 M KCl Ag/AgCl) was used in all these configurations.
Figure 1. Configuration 2 of the electrochemical cell used in the electrochemical measurements (figure not to scale).
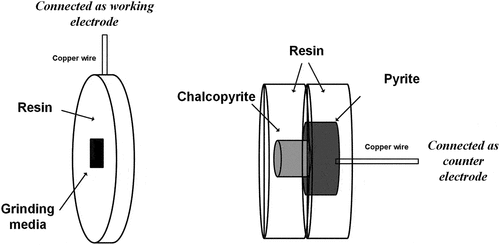
To best reflect the galvanic interactions during grinding, the surface areas of these electrodes were controlled according to their surface area ratios during grinding. The specific surface area of the mineral mixtures after grinding was measured by the Brunauer–Emmett–Teller method and found to be 0.3219 ± 0.0200 m2/g, whereas the specific surface area of the cylindrical grinding rods used for grinding was 2.03 10−5 m2/g (based on a radius of 12 mm and a length of 246 mm). Therefore, the ratios for GM-Cpy-Py mixtures at 5% and 25% pyrite during grinding were approximated as 1 to 80 to 77 and 1 to 80 to 385, respectively. Due to an unstable current captured by the electrochemistry workstation when the exposed surface of grinding media was small, the surface area of grinding media used in the electrochemical studies was fixed at 0.20 cm2, while the surface area ratios of chalcopyrite and pyrite electrodes to grinding media electrode were maintained to reflect those during grinding.
Two types of electrochemical measurements were conducted, LSV and Tafel polarization. Before each run, all electrode surfaces were ground with 300-grit silicon carbide to remove any surface passivation to create a fresh surface. Borax was used as the electrolyte with a pre-adjusted pH value of 9.0 to match the flotation pH. To confirm the relative electrochemical activity of each electrode, OCP values of each material were determined via OCP measurements using a traditional three-electrode configuration where a platinum electrode was connected as the counter electrode. The OCP values of forged steel, 30 wt% Cr steel, chalcopyrite and pyrite were found to be −83, −63, 217, and 247 mV vs standard hydrogen electrode (SHE) with the decreasing reactivity of these materials in accordance with the findings in the previous studies (Ekmekçi and Demirel Citation1997; Huang and Grano Citation2006). For LSV experiments, the current was measured at the applied potential between 0 and 0.7 V vs SHE with a positive scanning direction. The measurement commenced 600 s after the electrodes were placed in the electrolyte to ensure the electrodes reached a steady state. In Tafel polarization experiments, OCP was first obtained via Configurations 2 and 3 shown in 600 s after the electrodes were placed in the electrolyte, and the current was then measured at a positive scanning direction from 0.5 V below the OCP to 0.5 V above the OCP. The OCP of 30 wt% Cr steel and pyrite measured via Configurations 2 and 3 was −346 and 199 mV vs SHE, respectively, which was lower than the OCP obtained from the traditional configuration (−63 and 247 mV vs SHE for 30 wt% Cr steel and pyrite, respectively) due to the use of minerals/grinding media as the counter electrode.
To observe the role of anodic materials in a tri-phase galvanic system under a rapidly changing environment, such as grinding, a fast scan rate at 0.1 V/s as used in the previous studies (Herdman, Breslin, and Finnerty Citation2018; Inamuddin and Naushad Citation2014; Yi et al. Citation2007) was employed in LSV experiments to detect the instantaneous anodic current change under galvanic contacting where a low scan rate is not necessary. In Tafel polarization experiments, a much slower scan rate at 0.01 V/s was used to investigate the corrosion behavior of contacting materials to ensure the accuracy of corrosion potential (Zhou et al. Citation2022). All presented potential values in this study were converted against the SHE. Each electrochemical measurement was repeated at least two times, and all the presented findings in this study displayed a high repeatability.
3. Results and discussion
3.1. Flotation performance
Flotation tests of Cpy-Py-Q mixtures with 5% and 25% pyrite after ground by 30 wt% Cr steel, and 25% pyrite after ground by forged steel were performed. The results are shown in . displays Cu grade as a function of Cu recovery. After grinding with 30 wt% Cr steel, the flotation of the Cpy-Py-Q mixture with 5% pyrite produced a high Cu recovery of 97.2% at a high Cu grade of 12.5% at the end of flotation. With the increase in pyrite feed grade from 5% to 25%, both Cu grade and recovery decreased to 8.0% and 91.2%, respectively. Comparing the flotation performance of the Cpy-Py-Q mixture with 25% pyrite after ground by 30 wt% Cr steel over forged steel, the latter produced a slightly higher Cu recovery at 93.3% but a lower Cu grade at 6.2%. represents chalcopyrite flotation against pyrite. As the pyrite feed grade increased from 5% to 25%, chalcopyrite flotation against pyrite was improved when 30 wt% Cr steel was used as the grinding media, but this improvement was not enough to maintain a high Cu concentrate grade due to a reduced Cu recovery and more pyrite recovered. Nonetheless, 30 wt% Cr steel was still better in terms of Cpy-Py separation than forged steel in the flotation of the Cpy-Py-Q mixture with 25% pyrite.
Figure 2. Cu grade as a function of Cu recovery (a) and pyrite recovery as a function of chalcopyrite recovery (b) from the flotation of Cpy-Py-Q mixtures containing 5% (solid circle and solid line) and 25% (solid triangle and dotted line) pyrite after ground by 30 wt% Cr steel, and 25% pyrite (empty square and dotted line) after ground by forged steel.
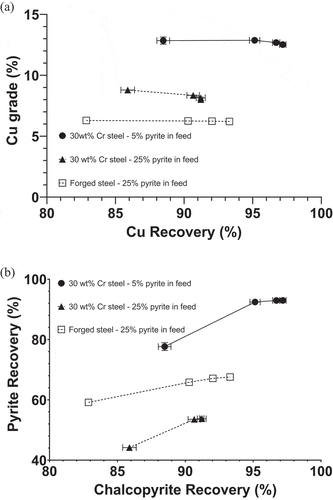
Flotation tests indicated that 30 wt% Cr steel grinding media produced a superior flotation performance with a high Cu recovery at a high Cu grade when the pyrite feed grade was 5%. When the pyrite feed grade increased to 25%, the same grinding media produced a lower Cu recovery at a lower Cu grade, although the selectivity of chalcopyrite against pyrite was improved. There was no doubt that 30 wt% Cr steel still produced a higher Cu grade than forged steel when the pyrite feed grade was 25%, owing to a better pyrite depression despite a marginal trade-off in Cu recovery. The mechanisms underpinning the different flotation behaviors of 5% and 25% pyrite feeds were investigated below via EDTA extraction, pulp chemistry measurements and electrochemical studies, which are able to provide a guide to selectively depressing high-concentration pyrite.
3.2. EDTA extraction and pulp chemistry measurements
displays the amounts of EDTAFe, EDTACu, and TDS from mill discharges with 5% and 25% pyrite after grinding with 30 wt% Cr steel, and 25% pyrite after grinding with forged steel. The amount of EDTAFe from mill discharges with 5% and 25% pyrite after grinding with 30 wt% Cr steel was similar with the error bars overlapping, meaning that the mill discharges with 5% and 25% pyrite did not have a statistically different amount of EDTAFe in pulp. In the previous studies, the total amount of EDTAFe was found to mainly originate from the oxidation of grinding media and used to quantify the iron contamination which exacerbates copper flotation as iron hydroxides (Fe(OH)2,3) coverage on copper mineral surfaces (Peng and Grano Citation2010). Based on the data shown in , the level of iron contamination in the two flotation systems after the feeds were ground by 30 wt% steel was comparable, and the decreased chalcopyrite flotation as pyrite feed grade increased from 5% to 25% should not be due to iron contamination. In terms of forged steel grinding media, the amount of EDTAFe produced from the mill discharge with 25% pyrite was more than six times greater than that produced by 30 wt% Cr steel grinding media, in line with the previous observations (Huang and Grano Citation2006; Mu, Cheng, and Peng Citation2020; Peng et al. Citation2003). Interestingly, the great increase in EDTAFe after the substitution of forged steel for 30 wt% Cr steel did not promote the depression of chalcopyrite and pyrite, implying that chalcopyrite and pyrite flotation in this case was again irrelevant to iron contamination.
Figure 3. The amounts of EDTAFe, EDTACu and TDS from mill discharges with 5% and 25% pyrite after grinding with 30 wt% Cr steel (denoted as 30Cr), and 25% pyrite after grinding with forged steel (denoted as FS).
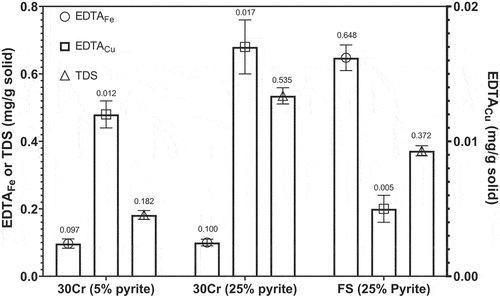
From , the amount of EDTACu in each condition varied significantly with no overlapping error bars. The amount of EDTACu increased from 0.012 to 0.017 mg per g solid as pyrite feed grade increased from 5% to 25% when 30 wt% Cr steel was used as the grinding media, corresponding to a 41.7% increment. EDTACu was only sourced from chalcopyrite; thus, the amount of EDTACu was linked to chalcopyrite oxidation according to Reactions (1) and (2) (Ekmekçi and Demirel Citation1997; Fairthorne, Fornasiero, and Ralston Citation1997). The initial oxidation of chalcopyrite produces copper sulfide (CuS) which is subject to further oxidation to produce Cu(OH)2. The increased amount of EDTACu suggested more chalcopyrite oxidation. On the other hand, the amount of EDTACu produced by forged steel from the Cpy-Py-Q mixture with 25% pyrite was the lowest (0.005 mg per g solid), indicating the weakest chalcopyrite oxidation among all the three test conditions.
As the pyrite feed grade increased from 5% to 25% when 30 wt% Cr steel was used as the grinding media, the amount of TDS increased from 0.182 to 0.535 mg per g solid. The amount of TDS represents the concentration of sulfur oxidation species in the pulp, and both chalcopyrite and pyrite can produce sulfoxyl species (e.g. thiosulfate, S2O32-, and sulfate, SO42-) via oxidation as shown in Reactions (4) and (5) (Ekmekçi and Demirel Citation1997; Tayebi-Khorami et al. Citation2018). Although the amount of TDS increased as pyrite feed grade increased from 5% to 25%, it was interesting to note that the amount only increased by 2.9 times whereas pyrite feed grade increased by five times, suggesting that pyrite oxidation per unit surface area in fact became weaker as pyrite feed grade increased from 5% to 25%. Nevertheless, the amount of TDS produced by 30 wt% Cr steel was still greater than that produced by forged steel from the same feed with 25% pyrite, implying a relatively higher sulfide oxidation.
presents the Eh, pH, and DO concentrations in mill discharges with 5% and 25% pyrite. Eh was found higher as pyrite feed grade increased from 5% to 25% when 30 wt% Cr steel was used as the grinding media, but the DO concentration decreased. Oxygen is a strong oxidant even in alkaline conditions with a standard reduction potential of +0.401 V, and hence the presence of oxygen potentially increases Eh (Bard Citation2017). The inversely proportional relationship between the changes of Eh and DO suggested that oxidants other than oxygen were present at a significant concentration in mill discharges, especially with 25% pyrite, and governed the pulp Eh. In this study, Fe3+ produced from the oxidation of grinding media, chalcopyrite and pyrite and Cu2+ produced from the oxidation of chalcopyrite were other oxidants. It is important to note that the total iron concentration produced in the two mill discharges was comparable, and the ability of Fe3+ as an oxidant was low in alkaline conditions due to the low solubility of Fe(OH)3 (Bard Citation2017), implying that the concentration of Cu2+ governed the change of pulp Eh. Moreover, a notable pH difference was also identified, and the mill discharge became more acidic as pyrite feed grade increased from 5% to 25%. Similar to chalcopyrite oxidation reactions presented in Reactions (1) and (2), pyrite oxidation also releases H+ as shown in Reaction (5) (Hu, Sun, and Wang Citation2009). According to Reactions (1) to (5), enhanced chalcopyrite and pyrite oxidation is expected to produce more H+, decreasing the pulp pH. Therefore, the lower pH in the mill discharge with 25% pyrite represented more oxidation of sulfide minerals which could be chalcopyrite, pyrite or both. In the case of forged steel, the Eh was the lowest, mainly due to the great oxygen consumption reflected in the depleted DO concentration in . The high oxygen consumption by forged steel was due to the enhanced oxygen reduction reaction (Reaction (6)) occurring on pyrite during the galvanic interaction (Rabieh, Eksteen, and Albijanic Citation2018). Sulfide oxidation as shown in Reactions (1) to (5) which produces H− was weak due to the reducing condition produced by forged steel. Therefore, less H+ and more OH− were produced during grinding with forged steel, leading to a high value of pulp pH.
Table 2. Eh, pH, and dissolved oxygen (DO) concentrations in mill discharges with 5% and 25% pyrite after grinding with 30 wt% Cr steel, and 25% pyrite after grinding with forged steel.
The electrochemical reduction of Cu2+ on the pyrite surface as shown in Reaction (7) (Peng, Wang, and Gerson Citation2012; Weisener and Gerson Citation2000) is an essential step for pyrite activation. A reducing condition has been found to promote Reaction (7), enhancing pyrite activation by Cu ions (Peng et al. Citation2003). Therefore, the low Cu grade from the flotation when forged steel was used as the grinding media was due to the promotion of pyrite activation by the reducing grinding environment. Moreover, 30 wt% Cr steel produced an oxidizing grinding environment, restricting Reaction (7) and leading to pyrite depression.
Based on the findings from EDTA extraction and pulp chemistry measurements, the mill discharges with 5% and 25% pyrite after grinding with 30 wt% Cr steel and 25% pyrite after grinding with forged steel showed different chemical characteristics, which should result from different galvanic interactions between grinding media and sulfide minerals. Hence, the following section investigated how the increased pyrite feed grade affected the galvanic interactions during grinding.
3.3. Electrochemical studies
It is known that the contact between grinding media and sulfides during grinding can trigger galvanic interactions which influence the subsequent flotation behavior. The type of grinding media (anode) significantly modifies the extent of galvanic interaction, leading to a major change in grinding pulp chemistry and thus the flotation response of valuable and gangue minerals (Bruckard, Sparrow, and Woodcock Citation2011; Huang and Grano Citation2006; Zhang, Han, and Kawatra Citation2021). Similarly, as pyrite content in the feed increased five times, it was expected that the exposed surface area of the cathode in the tri-phase GM-Cpy-Py galvanic system expanded at the same extent, which could be a decisive factor responsible for the corresponding flotation behavior. displays the anodic current of 30 wt% Cr steel and chalcopyrite electrodes as a function of applied potential after being coupled with the pyrite electrode at small and large surface areas.
Figure 4. Anodic current of (a) 30 wt% Cr steel and (b) chalcopyrite electrodes obtained from LSV measurements under Configuration 1 at pH 9.0.
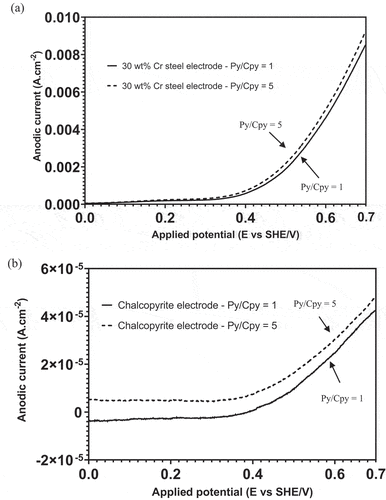
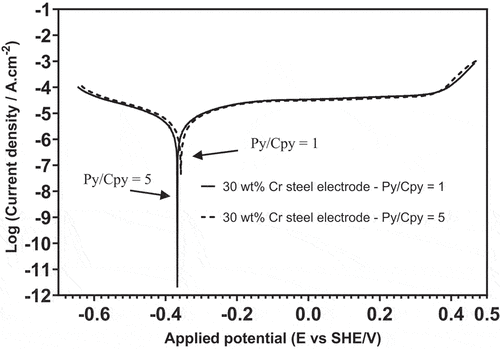
From , the anodic current detected from the 30 wt% Cr steel electrode at Py/Cpy = 1 and 5 basically overlapped between 0 and 0.4 V, and the separation was only visible at potentials higher than 0.4 V. The difference in anodic current detected from the chalcopyrite electrode at the two Py/Cpy ratios was more pronounced as shown in . Comparing the measured anodic currents from 30 wt% Cr steel and chalcopyrite at the potential region matching the pulp Eh displayed in (272–295 mV), the increased Py/Cpy ratio made almost no difference to the anodic current from 30 wt% Cr steel, whereas the anodic current from chalcopyrite increased from a negative value (−2.47 µAcm−2) to a positive value ( µAcm−2), implying a role change. Relating the LSV results to the increased amount of EDTACu presented in , it was determined that chalcopyrite oxidation became stronger as the pyrite feed grade increased from 5% to 25%. Overall, the increased pyrite surface area in the 30 wt% Cr steel–chalcopyrite–pyrite (30Cr-Cpy-Py) galvanic system tended to anodize chalcopyrite more than 30 wt% Cr steel.
Tafel polarization curve measurements with a slower scan rate were employed to observe both the cathodic and anodic behaviors of grinding media and minerals in the 30Cr-Cpy-Py galvanic system. presents the Tafel polarization curves of 30 wt% Cr steel coupled with the Cpy-Py mixed hybrid electrode. The anodic and cathodic role of 30 wt% Cr steel stayed the same although the pyrite surface increased five times, which aligned with the LSV plots obtained in . The two types of electrochemical measurements indicated the high resistance of 30 wt% Cr steel grinding media against oxidation, even in the presence of a large cathode surface area. This is also consistent with the EDTA extraction experiment where similar iron contamination levels were produced by 30 wt% Cr steel in the mill discharges with 5% and 25% pyrite.
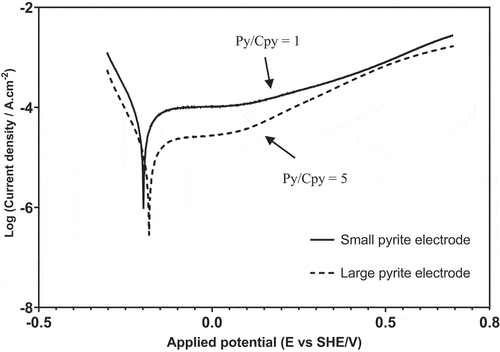
Since chalcopyrite concentration was constant, the effect of pyrite surface area on pyrite oxidation in the 30Cr-Cpy-Py galvanic system was investigated via Tafel polarization curves of pyrite coupled with 30 wt% Cr steel. The results are shown in . The corrosion potential (Ecorr) of pyrite increased slightly from −0.198 to −0.178 V in response to a five times increase in the exposed surface area, indicating a slightly more noble character of pyrite. A difference in the calculated corrosion density (icorr) from pyrite electrodes with small (63.10 µAcm−2) and large surfaces (15.84 µAcm−2) was more significant than visualization of as the y-axis was plotted in a logarithmic scale. The decrease in icorr was also consistent with the lower anodic branch of the pyrite electrode with a large surface. The findings from identified that the role of pyrite was shifted more cathodic at a greater pyrite surface area, resulting in weaker pyrite oxidation. This also agreed with the EDTA extraction experiments where a five times increment in pyrite concentration only resulted in a 2.9 times increase in the amount of TDS.
Figure 6. Tafel polarization curves of pyrite electrode coupled with 30 wt% Cr steel under Configuration 3 at pH 9.0.
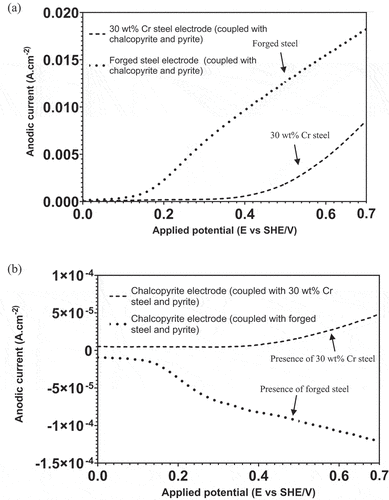
presents the anodic currents of grinding media and chalcopyrite after being coupled with pyrite under Configuration 1. The results indicated great differences in anodic current detected from the grinding media and chalcopyrite in the forged steel–chalcopyrite–pyrite (FS-Cpy-Py) and 30Cr-Cpy-Py galvanic systems. It can be seen from that the anodic current detected from forged steel was much greater than that from 30 wt% Cr steel when they were coupled with the same chalcopyrite and pyrite, which further supported the high level of iron contamination shown in . Compared to 30 wt% Cr steel, forged steel was much more electrochemically active, which was in accordance with the previous observations (Bruckard, Sparrow, and Woodcock Citation2011; Huang and Grano Citation2006). Moreover, from , the anodic current of chalcopyrite decreased from a positive to negative value as 30 wt% Cr steel was replaced by forged steel in the GM-Cpy-Py galvanic system. Such a sign switch of anodic current implied the suppression of chalcopyrite oxidation, or a more cathodic role of chalcopyrite due to the strongly anodic role of forged steel as shown in . This characteristic was also reflected in , where the total amount of EDTACu produced by forged steel in the mill discharge with 25% pyrite was significantly lower than that produced by 30 wt% Cr steel. Therefore, it is clear that the dissimilar electrochemical role of forged steel and 30 wt% Cr steel could dramatically influence the role of chalcopyrite in the GM-Cpy-Py galvanic couple at a large pyrite surface.
Figure 7. Anodic current of (a) grinding media and (b) chalcopyrite obtained from LSV measurements under Configuration 1 at pH 9.0.
Based on flotation tests, EDTA extraction, pulp chemistry measurements, and electrochemical studies, the galvanic interaction during grinding, which underpins the depression of high-pyrite concentration by HiCr grinding media in copper flotation, was proposed in . By comparing , a larger pyrite surface area in the feed did not promote the anodic character of 30 wt% Cr steel, but promoted the anodic character of chalcopyrite during grinding, leading to a higher production of Cu2+, the source of pyrite activation, via enhanced chalcopyrite oxidation as shown in Reactions (1) and (2). Despite oxygen depletion due to O2 reduction (Reaction (6)) on the pyrite surface, the high concentration of Cu2+, as an oxidant, lifted the pulp Eh. Although the grinding environment became more oxidizing as pyrite feed grade increased from 5% to 25%, this environment did not specifically oxidize pyrite. Instead, pyrite became more cathodic or less oxidized, as shown in , as the pyrite feed grade increased from 5% to 25%. Even though the more oxidizing environment produced during the grinding of 25% pyrite feed could restrict the Cu reduction as shown in Reaction (7) from going forward, the higher concentration of Cu2+ produced and less oxidized pyrite obviously pushed Reaction (7) to the right direction, leading to comparably increased pyrite activation.
Figure 8. Schematic diagram for the galvanic interaction during the grinding of the Cpy-Py-Q mixture with (a) 5% and (b) 25% pyrite in the feed by 30 wt% Cr steel, and (c) 25% pyrite in the feed by forged steel (the figure not to scale).
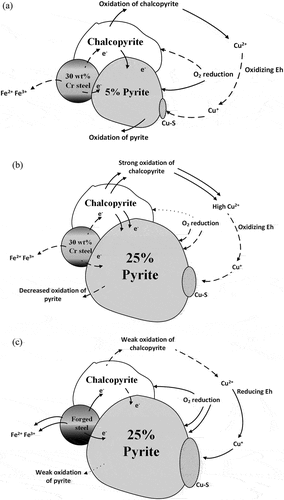
Therefore, the excessive chalcopyrite oxidation was responsible for the compromised Cu recovery in the flotation of the Cpy-Py-Q mixture with 25% pyrite after ground by 30 wt% Cr steel grinding media. As shown in Reactions (1) to (4), the oxidation of chalcopyrite produces iron, copper and sulfur oxidation species. These oxidation species are hydrophilic (Moimane, Plackowski, and Peng Citation2020), which depressed chalcopyrite flotation.
At 25% pyrite in the feed, the FS-Cpy-Py and 30Cr-Cpy-Py galvanic interactions were utterly different as shown in . The super anodic behavior of forged steel consumed almost all the oxygen, leaving a reducing pulp. Even though chalcopyrite oxidation was weak in the FS-Cpy-Py galvanic system, the conversion of Cu2+ to Cu+ in Reaction (7) was high under the reducing environment, favoring pyrite activation. At the same time, the strong cathodic role of pyrite in the FS-Cpy-Py system provided no barrier to restrict Reaction (7). Therefore, forged steel poorly depressed pyrite in the flotation of the Cpy-Py-Q mixture with 25% pyrite.
4. Conclusions
This research assessed 30 wt% Cr steel grinding media in depressing high-concentration pyrite in Cu flotation in comparison with depressing low-concentration pyrite at the same Cu feed grade. A comparison was also made between HiCr steel and forged steel in depressing high-concentration pyrite. Flotation tests indicated that 30 wt% Cr steel produced a high Cu recovery at a high Cu grade from the flotation of the Cpy-Py-Q mixture with 5% pyrite. However, both Cu recovery and concentrate grade decreased when pyrite feed grade increased from 5% to 25% although 30 wt% Cr steel was still used as the grinding media, attributed to the changed galvanic interaction between 30 wt% Cr steel and sulfide minerals during grinding. As the pyrite feed grade increased from 5% to 25%, the anodic role of 30 wt% Cr steel did not change, but the anodic role of chalcopyrite was enhanced due to the increase in cathode (pyrite) surface area. At the same time, pyrite became more cathodic. The change in the anodic role of chalcopyrite enhanced chalcopyrite oxidation, while the change in the cathodic role of pyrite retarded pyrite oxidation, both of which facilitated copper activation on the pyrite surface and subsequent pyrite flotation. The enhanced chalcopyrite oxidation was the root cause of the compromised Cu recovery due to the production of more hydrophilic oxidation species when 30 wt% Cr steel grinding media was used to depress 25% pyrite in the feed.
When forged steel grinding media was used to depress 25% pyrite in the feed, pyrite depression was poor, owing to a strong anodic role of forged steel and a strong cathodic role of pyrite in this system, facilitating copper activation on the pyrite surface.
This study identified chalcopyrite oxidation as the limiting factor in depressing high-concentration pyrite in copper flotation when 30 wt% Cr steel was used as the grinding media. This finding provides guidance for Cu concentrators to seek a strategy to avoid chalcopyrite oxidation when treating high-pyrite-containing Cu ores.
Acknowledgments
The authors would like to acknowledge the financial support to this work from Vega Industries. The first author also acknowledges the scholarship provided from the University of Queensland.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aghazadeh, S., S. Kamal Mousavinezhad, and M. Gharabaghi. 2015. Chemical and colloidal aspects of collectorless flotation behavior of sulfide and non-sulfide minerals. Advances in Colloid and Interface Science 225:203–17. doi: 10.1016/j.cis.2015.09.007.
- Asgari, K., Q. Huang, H. Khoshdast, and A. Hassanzadeh. 2022. A review on bioflotation of coal and minerals: Classification, mechanisms, challenges, and future perspectives. Mineral Processing & Extractive Metallurgy Review ahead-of-print (ahead-of-print):1–31. doi: 10.1080/08827508.2022.2121919.
- Bakalarz, A. 2021. An analysis of copper concentrate from a kupferschiefer-type ore from legnica-glogow copper basin (SW Poland). Mineral Processing & Extractive Metallurgy Review 42 (8):552–64. doi: 10.1080/08827508.2021.1971663.
- Bard, A. 2017. Standard potentials in aqueous solution. Vol. 6. 1st ed. New York: Routledge.
- Bicak, O., Z. Ekmekci, D. J. Bradshaw, and P. J. Harris. 2007. Adsorption of guar gum and CMC on pyrite. Minerals Engineering 20 (10):996–1002. doi: 10.1016/j.mineng.2007.03.002.
- Bruckard, W. J., G. J. Sparrow, and J. T. Woodcock. 2011. A review of the effects of the grinding environment on the flotation of copper sulphides. International Journal of Mineral Processing 100 (1):1–13. doi: 10.1016/j.minpro.2011.04.001.
- Chandra, A. P., and A. R. Gerson. 2009. A review of the fundamental studies of the copper activation mechanisms for selective flotation of the sulfide minerals, sphalerite and pyrite. Advances in Colloid and Interface Science 145 (1):97–110. doi: 10.1016/j.cis.2008.09.001.
- Chen, X., Y. Peng, and D. Bradshaw. 2014. The separation of chalcopyrite and chalcocite from pyrite in cleaner flotation after regrinding. Minerals Engineering 58:64–72. doi: 10.1016/j.mineng.2014.01.010.
- Córdoba, E. M., J. A. Muñoz, M. L. Blázquez, F. González, and A. Ballester. 2008. Leaching of chalcopyrite with ferric ion. Part I: General aspects. Hydrometallurgy 93 (3):81–87. doi: 10.1016/j.hydromet.2008.04.015.
- de Melo Silva Cheloni, L. M., F. L. Martins, L. Moreira Pinto, M. L. Marques Rodrigues, and V. A. Leão. 2023. Chemical and biological leaching of chalcopyrite- elemental sulfur reaction products. Mineral Processing & Extractive Metallurgy Review ahead-of-print (ahead-of-print):1–12. doi: 10.1080/08827508.2023.2181347.
- Ekmekçi, Z., and H. Demirel. 1997. Effects of galvanic interaction on collectorless flotation behaviour of chalcopyrite and pyrite. International Journal of Mineral Processing 52 (1):31–48. doi: 10.1016/S0301-7516(97)00050-1.
- Fairthorne, G., D. Fornasiero, and J. Ralston. 1997. Effect of oxidation on the collectorless flotation of chalcopyrite. International Journal of Mineral Processing 49 (1):31–48. doi: 10.1016/S0301-7516(96)00039-7.
- Finkelstein, N. P. 1997. The activation of sulphide minerals for flotation: A review. International Journal of Mineral Processing 52 (2):81–120. doi: 10.1016/S0301-7516(97)00067-7.
- Hampton, M. A., C. Plackowski, and A. V. Nguyen. 2011. Physical and chemical analysis of elemental sulfur formation during galena surface oxidation. Langmuir 27 (7):4190–201. doi: 10.1021/la104755a.
- Herdman, K. M., C. B. Breslin, and N. J. Finnerty. 2018. The aqueous deposition of a pH sensitive quinone on carbon paste electrodes using linear sweep voltammetry. Journal of Electroanalytical Chemistry (Lausanne, Switzerland) 828:137–43. doi: 10.1016/j.jelechem.2018.09.049.
- Hu, Y., W. Sun, and D. Wang. 2009. Corrosive electrochemistry of oxidation-reduction of sulphide minerals. In Electrochemistry of flotation of sulphide minerals, 1–19. Berlin, Heidelberg: Springer.
- Huai, Y., C. Plackowski, and Y. Peng. 2017. The surface properties of pyrite coupled with gold in the presence of oxygen. Minerals Engineering 111:131–39. doi: 10.1016/j.mineng.2017.06.013.
- Huang, G., and S. Grano. 2005. Galvanic interaction of grinding media with pyrite and its effect on floatation. Minerals Engineering 18 (12):1152–63. doi: 10.1016/j.mineng.2005.06.005.
- Huang, G., and S. Grano. 2006. Galvanic interaction between grinding media and arsenopyrite and its effect on flotation: Part I. Quantifying galvanic interaction during grinding. International Journal of Mineral Processing 78 (3):182–97. doi: 10.1016/j.minpro.2005.10.008.
- Inamuddin, K. A., and M. Naushad. 2014. Optimization of glassy carbon electrode based graphene/ferritin/glucose oxidase bioanode for biofuel cell applications. International Journal of Hydrogen Energy 39 (14):7417–21. doi: 10.1016/j.ijhydene.2014.02.171.
- Lee, R. L. J., X. Chen, and Y. Peng. 2022. Flotation performance of chalcopyrite in the presence of an elevated pyrite proportion. Minerals Engineering 177:107387. doi: 10.1016/j.mineng.2021.107387.
- Li, Y., N. Kawashima, J. Li, A. P. Chandra, and A. R. Gerson. 2013. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite. Advances in Colloid and Interface Science 197-198:1–32. doi: 10.1016/j.cis.2013.03.004.
- Moimane, T., C. Plackowski, and Y. Peng. 2020. The critical degree of mineral surface oxidation in copper sulphide flotation. Minerals Engineering 145:106075. doi: 10.1016/j.mineng.2019.106075.
- Mu, Y. Cheng, and Y. Peng. 2020. The interaction of grinding media and collector in pyrite flotation at alkaline pH. Minerals Engineering 152:106344. doi: 10.1016/j.mineng.2020.106344.
- Mu, Y., Y. Cheng, and Y. Peng. 2020. The interaction between grinding media and collector in pyrite flotation at neutral and slightly acidic pH. Minerals Engineering 145:106063. doi: 10.1016/j.mineng.2019.106063.
- Ndoro, T. O., and L. K. Witika. 2017. A review of the flotation of copper minerals. International Journal of Sciences: Basic and Applied Research 34 (2):145–65.
- Peng, Y., and T. Bhambhani. 2021. Preface to the MME special focus issue on managing gangue minerals. Mining, Metallurgy & Exploration 38 (2):669–71. doi: 10.1007/s42461-021-00414-x.
- Peng, Y., and S. Grano. 2010. Effect of iron contamination from grinding media on the flotation of sulphide minerals of different particle size. International Journal of Mineral Processing 97 (1):1–6. doi: 10.1016/j.minpro.2010.07.003.
- Peng, Y., S. Grano, D. Fornasiero, and J. Ralston. 2003. Control of grinding conditions in the flotation of chalcopyrite and its separation from pyrite. International Journal of Mineral Processing 69:87–100.
- Peng, Y., B. Wang, and A. Gerson. 2012. The effect of electrochemical potential on the activation of pyrite by copper and lead ions during grinding. International Journal of Mineral Processing 102-103:141–49.
- Rabieh, A., J. J. Eksteen, and B. Albijanic. 2018. Galvanic interaction of grinding media with arsenopyrite and pyrite and its effect on gold cyanide leaching. Minerals Engineering 116:46–55. doi: 10.1016/j.mineng.2017.10.018.
- Rumball, J. A., and G. D. Richmond. 1996. Measurement of oxidation in a base metal flotation circuit by selective leaching with EDTA. International Journal of Mineral Processing 48 (1):1–20. doi: 10.1016/S0301-7516(96)00010-5.
- Schlesinger, M. E., M. J. King, K. C. Sole, and W. G. Davenport. 2011. Extractive metallurgy of copper. AMSTERDAM • BOSTON • HEIDELBERG • LONDON NEW YORK • OXFORD • PARIS • SAN DIEGO SAN FRANCISCO • SINGAPORE • SYDNEY • TOKYO: Elsevier.
- Tayebi-Khorami, M., E. Manlapig, E. Forbes, M. Edraki, and D. Bradshaw. 2018. Effect of surface oxidation on the flotation response of enargite in a complex ore system. Minerals Engineering 119:149–55. doi: 10.1016/j.mineng.2018.01.024.
- Weisener, C., and A. Gerson. 2000. An investigation of the Cu (II) adsorption mechanism on pyrite by ARXPS and SIMS. Minerals Engineering 13 (13):1329–40. doi: 10.1016/S0892-6875(00)00116-3.
- Xiao, J., L. Yang, S. Liu, and G. Liu. 2022. In-situ probing the interface electrochemical properties of chalcopyrite modified by amidoxime-dithiocarbamate ester: Implications to flotation mechanism. Mineral Processing & Extractive Metallurgy Review ahead-of-print (ahead-of-print):1–9. doi: 10.1080/08827508.2022.2155153.
- Xu, S., M. Zanin, W. Skinner, and S. B. E Abreu. 2022. Influence of grinding conditions on the pulp chemistry and flotation of oxidised pyrite. Minerals Engineering 177:107385. doi: 10.1016/j.mineng.2021.107385.
- Yang, X., Y. Mu, and Y. Peng. 2021. Comparing lead and copper activation on pyrite with different degrees of surface oxidation. Minerals Engineering 168:106926. doi: 10.1016/j.mineng.2021.106926.
- Yi, Q., J. Zhang, W. Huang, and X. Liu. 2007. Electrocatalytic oxidation of cyclohexanol on a nickel oxyhydroxide modified nickel electrode in alkaline solutions. Catalysis Communications 8 (7):1017–22. doi: 10.1016/j.catcom.2006.10.009.
- Zhang, X., Y. Han, and S. K. Kawatra. 2021. Effects of grinding media on grinding products and flotation performance of sulfide ores. Mineral Processing & Extractive Metallurgy Review 42 (3):172–83. doi: 10.1080/08827508.2019.1692831.
- Zhou, H., D. Chhin, A. Morel, D. Gallant, and J. Mauzeroll. 2022. Potentiodynamic polarization curves of AA7075 at high scan rates interpreted using the high field model. Npj Materials Degradation 6 (1):1–11. doi: 10.1038/s41529-022-00227-3.