?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Efficient separation of hydrophobic gangue materials such as carbonaceous minerals is paramount in minerals flotation to mitigate the industry’s carbon footprint and address negative impacts of climate changes. Despite extensive research on native biopolymers such as polysaccharides for rejecting problematic carbon, non-discriminate interactions and limited flotation selectivity persist in the minerals industry. There have also been considerable efforts to enhance biopolymers’ selectivity and depressive capacity in minerals flotation through structure modifications. However, there are no reviews conducted to scrutinize the influences of structural and functional feature alterations following biopolymer modifications. The primary objective of the review is to understand the processes underpinning selective interactions for improved carbon rejection when using diverse biopolymers. The work includes a description of structure and characterization of carbonaceous materials, particularly hydrophobic graphitic carbons as the gangue minerals of concern in industry first. In the following sections the influences of native and modified biopolymers are discussed in the context of revealing the selectivity potential when carbonaceous materials are depressed in selective flotation. In the end some possible future research directions are proposed. The review seeks to foster sustainable practices using green techniques and control carbon contamination in minerals flotation in industry.
Introduction
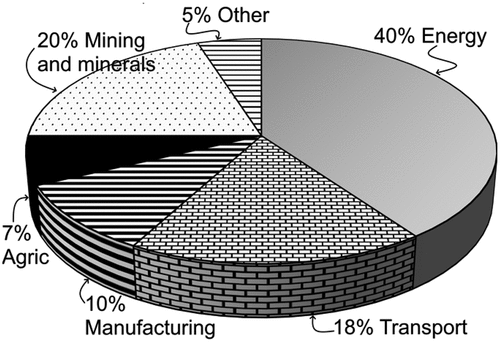
The global minerals industry continues to confront critical challenges such as declining ore grades, complex mineralogy and inefficiencies in flotation separation, leading to substantial emissions of downstream greenhouse gases (GHG) such as CO2. approximates global CO2 emissions that originate from the mining and minerals sector, attributable to different causes including energy consumption. Admittedly, estimating the exact CO2 emissions from minerals flotation is challenging, but it is certain that the presence of carbonaceous materials in the concentrates directly increases CO2 emissions of downstream processes, e.g. pyrometallurgy of valuable minerals, which is a significant contributor to climate changes. The central focus of this review is the imperative need to effectively reject carbon materials during minerals flotation.
Figure 1. Estimate of CO2 emissions according to various web-based sources (including international energy agency: www.iea.org).
During flotation, the structure and hydrophobic nature of carbonaceous materials such as graphitic carbon pose significant challenges in achieving selectivity (Sime et al. Citation2022). In particular, the hydrophobic gangue can readily attach to air bubbles along with valuable minerals with their surfaces made hydrophobic through the addition of collectors, resulting in contamination of flotation concentrates and a marked decline in product quality (Magwaneng, Haga, and Batnasan Citation2018; Saim and Darteh Citation2023; Sime et al. Citation2022). Flotation is a surface chemistry-based technique and relies on surface property differences relative to hydrophobicity and hydrophilicity to facilitate minerals separation. Thus, while this review also acknowledges the difficulties faced relative to the influence of hydrophobic nature of gangue species such as talc etc., the primary focus is on carbonaceous gangue. This is owing to its topical relevance regarding the need to adopt green strategies to mitigate CO2 emissions and climate change challenges, underscoring the requirement to achieve selectivity during flotation.
Another issue with hydrophobic carbonaceous materials is that the fines also cause a hydrophobic surface coating. This phenomenon is driven by hydrophobic interactions with both hydrophilic gangue and hydrophobic valuable minerals with natural or collector-induced hydrophobicity Tabatabaei et al. Citation2014). When this occurs, this negatively affects the selectively interactive behavior of other flotation reagents such as collectors, leading to reduced separation and increased reagents consumption.
It is worthwhile to also emphasize that the detrimental effects of elevated carbon contents in flotation concentrates include diminished metallurgical yield, reduction in strength of sinters and difficulties in controlling temperatures (thermal imbalances) during roasting in downstream processes (Konieczny et al. Citation2013). Moreover, high emissions of CO2 lead to financial penalties and economic losses and pose environmental challenges such as climate change risks.
As mitigatory strategies for rejecting the problematic hydrophobic carbon gangue, this involves pre-flotation (Magwaneng, Haga, and Batnasan Citation2018). During pre-flotation, hydrophobic gangue is floated without the addition of collectors but in the presence of frothers. However, several drawbacks of pre-flotation have already been reported, including high reagent consumption, poor carbon recovery and loss of valuable minerals. At Century Mine in Australia, significant amounts of frother MIBC is reportedly utilized to stabilize the froth in pre-flotation, yet the carbonaceous materials are not fully recovered. Furthermore, as depicted in EquationEquations (1(1)
(1) –Equation3
(3)
(3) ), valuable minerals such as chalcopyrite undergo surface oxidation, generating hydrophobic species such as CuFe1−xS2 and CuS*2 as metal deficient and sulfur rich phases. This results in the loss of valuable sulfide minerals alongside unwanted carbonaceous gangue (Chen, Liu, and Peng Citation2021; Sime et al. Citation2022).
Another common and preferred approach is the utilization of native biopolymers as depressants to induce hydrophilicity on targeted hydrophobic gangue minerals, thereby inhibiting their unwanted flotation (Saim and Darteh Citation2023). The literature extensively discusses polysaccharides such as native starch, CMC, guar gum and dextrin as particularly attractive biopolymers due to their environmentally friendly nature, biodegradability and chemical versatility (Beaussart, Mierczynska-Vasilev, and Beattie Citation2009; Chapagai, Fletcher, and Gidley Citation2022; Gupta Citation2017; Haung and Miller Citation1978; Miller, Laskowski, and Chang Citation1983; Nyamekye and Laskowski Citation1993; Pugh Citation1989; Qi, Zhang, and Laskowski Citation2000; Sime et al. Citation2022; Taner and Onen Citation2024; Wie and Fuerstenau Citation1974).
While the above-mentioned biopolymers are green and attractive, limitations such as variability and poor selectivity continue to be a concern. The latter concern happens owing to indiscriminate binding mechanisms which include hydrogen bonding, chemical complexations, acid-base interactions, hydrophobic and electrostatic or dipole interactions of the biopolymers on mineral surfaces (Steenberg and Harris Citation1984). This is at the expense of preferential affinity for the targeted hydrophobic gangue. Therefore, in the context of carbonaceous gangue, if the intention is to selectively reject carbon and reduce CO2 emissions in industry, discriminative separation activity of biopolymers should be promoted.
As such, attention has been shifting to modifying the natural biopolymers to enhance their selectivity and depressive efficacy as attractive green alternatives (Asgari et al. Citation2024; Mishra et al. Citation2023; Natarajan Citation1992). It is curious, however, that there are no detailed reviews on modifications, characterizations and applications of biopolymers with a view to enhance rejection of naturally hydrophobic gangue at scale in industry. To guide the reader in a structured way, this review consists of two main parts relative to the influence of native (unmodified) and modified biopolymers on rejecting carbon, wherein each of the sections begins by introducing the structure or chemistry of selected biopolymers. This would be followed by evaluating the performance of biopolymers in minerals flotation and explicating the associated mechanisms. Finally, some potential limitations of the biopolymers are highlighted or compared.
Therefore, this review endeavors to bridge the above-described gaps and makes comparisons of biopolymers within the context of enhancing the rejection of hydrophobic gangue, particularly carbon. In addition, it seeks to develop a clearer and deeper understanding of the mechanisms governing discriminative separation when using diverse biopolymers. In the end, a possible future research direction is articulated based on the identified gaps. Overall, the review provides a solid framework to align future research strategic goals with adopting green technologies and advance global economic and environmental sustainability in minerals industry.
Different structures and properties of carbonaceous materials
According to the literature, carbonaceous (carbon-containing) materials encompass a diverse range of organic carbon compounds, including hydrocarbons (such as shale, bitumen and coal) and organic acids as well as native or elemental carbon forms such as graphite or activated carbon (Afenya Citation1991; Chimonyo et al., Citation2020a; Gredelj, Zanin, and Grano Citation2009; Niu et al. Citation2019). In many deposits, carbonaceous materials exist in association with the primary rocks such as metamorphic, sedimentary, or igneous rocks which constitute the mineralized veins or main ore-bearing zones (Afenya Citation1991; Kuz’min et al. Citation2010; Mossman Citation1999). In the literature, different terms have also been listed to describe organic carbon matter, including kerogen, maceral, vitrinite and alginite. They are all primarily found in sedimentary rocks (Mossman Citation1999; Mossman and Thompson-Rizer Citation1993).
In the context of flotation, carbonaceous gangue refers to the unwanted carbon-containing materials (hydrophobic or hydrophilic) naturally present in ore deposits alongside valuable minerals. It is important to note that the type and form of carbonaceous materials, along with their structure and surface properties, can substantially affect flotation separation. Konieczny et al. (Citation2013) highlighted carbonaceous black shale (an example of problematic organic carbon) which causes separation issues in copper minerals flotation. A fundamental understanding of the structural characteristics and surface properties of carbonaceous materials is crucial for comprehending their flotation behaviors and the mechanisms responsible for interfacial interactions during flotation.
Structural characterization of carbonaceous materials
According to the literature, the structure of carbonaceous materials varies significantly based on the degree of graphitization, which determines crystallite sizes, molecular arrangements, configuration orientations and degrees of disorder (Gupta Citation2017; Sime et al. Citation2022).
From a structural perspective, organic acids or humic substances are characterized as complex macromolecular compounds containing hydrophilic oxygen-containing functional groups like hydroxyl (–OH) and carboxylic (–COOH) groups, ketones, quinones, phenolic groups and organic acids. Additionally, they possess a skeleton of aromatic ring structures linked by bonds, such as – CH2– and – O (Niu et al. Citation2019).
Hydrocarbons, another form of organic carbon, can be high molecular weight compounds or long carbon chain structures while elemental carbon, such as graphitic carbon, features a unique crystalline structure composed of a hexagonal lattice formed by covalently bonded carbon atoms arranged in stacked interlayers. These interlayers are connected by van der Waals forces and include face surfaces (basal planes) and edge surfaces.
Meanwhile, it has been reported that organic acids may also be predominantly constituted by significant proportions of graphitic carbon structures or elemental carbon and contribute to determining the surface properties (Niu et al. Citation2019).
Surface properties of carbonaceous materials
The structural features of carbonaceous materials play a crucial role in determining their surface properties, which in turn affect their flotation performances, especially when interacting with unmodified or modified biopolymers.
Organic acids or humic substances are generally hydrophilic due to their polar oxygen-containing functional groups. These materials also possess a negative surface charge when ionized. Consequently, organic acids can engage in electrostatic interactions with various species during interfacial interactions in addition to forming chemical complexes or chelates with minerals or other surface-active species (Pyke et al. Citation1999).
In contrast, native or elemental carbon such as graphite exhibits anisotropic properties with hydrophobic face surfaces and hydrophilic edge surfaces. According to the literature, the contact angle of graphitic carbon’s face surfaces is around 80°, while the edge surfaces have a contact angle of approximately 40° (Sime et al. Citation2022). The inherent surface hydrophobicity of graphitic carbon is primarily due to its hydrophobic face surfaces. It is problematic as it results in unwanted floatability and poses challenges to achieving selective separation (Pyke et al. Citation1999).
Graphitic carbon is typically negatively charged in flotation with an isoelectric point ranging between 2.2 and 4.5. This negative surface charge arises from oxidation at the edge surfaces, which generates oxygen-containing functional groups such as carboxyl (–COOH). The negative charge influences electrostatic interactions in solution and thus affects the adsorption behavior.
Influence of native biopolymers on carbonaceous materials depression
As earlier indicated, across the literature, owing to their green characteristics and biodegradability there is no doubt that native starch, dextrin, guar gum and cellulose (or carboxymethylcellulose (CMC)) stand out as the most reported native biopolymer depressants for hydrophobic minerals. In the following subsections, first the structure and chemistry of each of these biopolymers will be described. Then, their applications in minerals flotation mostly involving rejection of carbonaceous materials will be discussed along with the adsorption mechanisms involved.
Native starch
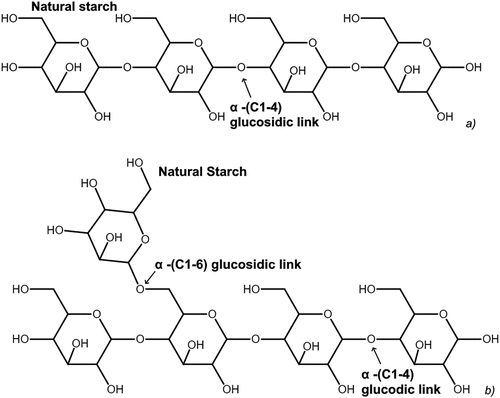
Native starch is mainly composed of two nonionic macromolecular homopolymers viz., amylose and amylopectin, both characterized by free 3 –OH groups on each of the cyclic glucose rings with an oxygen atom as part of the ring structure (Chimonyo, Fletcher, and Peng Citation2020c; Pearse Citation2005; Shrestha and Halley Citation2014). The typical ratio of amylose and amylopectin is 20–30 and 70–80%, respectively, for most starches (Bertoft Citation2004; Chimonyo, Fletcher, and Peng Citation2020c). Amylose is generally reported as a linear glucan with α, 1–4 D-glucose linkages and limited branching as shown in . On the other hand, amylopectin possesses a considerable number of non-reducing end groups and is composed of short chains with α, 1–4-D-glucosidic linkages and is highly branched owing to α, 1–6-D-glucosidic linkages (Asimi Neisiani et al. Citation2023; Pearse Citation2005). In the literature, it is also known that there are many different types of native starch such as corn, rice and wheat etc (Shrestha and Halley Citation2014).
Depending on the botanical origin of the native starch, the structural features of amylose and amylopectin differ relative to chain length and degree of branching (Shrestha and Halley Citation2014). The free 3 –OH groups cause an amphiphilic nature wherein as they rotate to one side of the glucose molecule ring they induce hydrophilicity, while the opposite side is hydrophobic due to the exposed methyl groups as well as the – CH groups (Pavlovic and Brandao Citation2003).
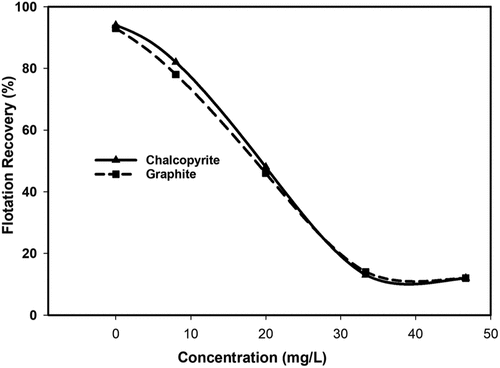
In our recent study we employed native wheat starch during flotation to separate chalcopyrite from graphite (Chimonyo, Fletcher, and Peng Citation2020a). Referring to , the results demonstrate the recovery of chalcopyrite and graphite as a function of native wheat starch concentration at pH 7.5. As can be observed, the recoveries for both chalcopyrite and graphite are about 94% in the absence of native starch and no significant recovery difference appears as starch concentration increases, demonstrating a poor selective performance.
Figure 3. Flotation recovery of chalcopyrite and graphite versus native starch concentration at pH 7.5 (adapted from Chimonyo, Fletcher, and Peng Citation2020a).
From a mechanistic perspective, the above-described poor selective performance of native starch was attributed to macromolecular aggregation and inefficient surface coverage on graphite, particularly at low concentrations (Chimonyo, Fletcher, and Peng Citation2020a). It was linked to the indiscriminate adsorption on chalcopyrite and graphite surfaces owing to an amphiphilic nature (nonpolar and polar nature) as well as the influence of the multiple – OH functionality on the macromolecular structure matrix. The adsorption mechanisms of native starch on hydrophobic mineral surfaces involve hydrophobic bonding. In addition, acid–base interactions including hydrogen bonding are also possible if the mineral surface possesses species that can act as a base, while the – OH groups of starch molecules function as acid groups (Drzymala, Kapusniak, and Tomasik Citation2003; Steenberg and Harris Citation1984).
In other studies, Shrimali et al. (Citation2018) reported corn starch as an effective graphite depressant based on some micro flotation cell experiments at a single concentration, 100 ppm. However, by comparison with the above-described work, the study lacks information on evaluating the depressive performance of corn starch at varying concentrations. Furthermore, the selectivity in the presence of valuable minerals was not explored. From , while native wheat starch showed some depression of graphite, it was illustrated that its selectivity with chalcopyrite was poor across the concentration range investigated.
In general, the limitations of native starch are inherently attributed to a complex bulky supramolecular structure and entangling homopolymers (amylose and amylopectin). In addition, intermediate fractions between amylose and amylopectin make it hard to differentiate their influences on minerals depression and link their depression roles with their specific functions/activities. Apart from the above-described poor selectivity, native starch also exhibits low solubility and high viscosity due to the complex structure and intermolecular interactions and entanglements.
Dextrin
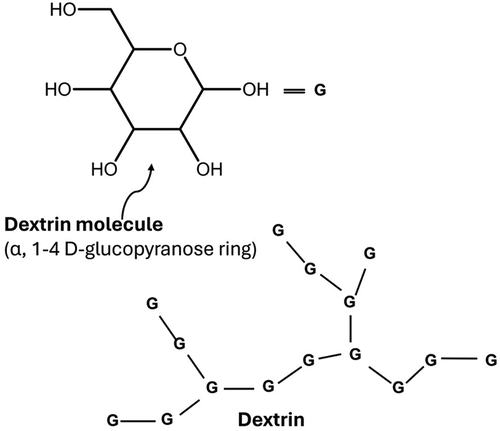
Dextrin is a nonionic, natural class of low MW biopolymer composed of dextrose monomeric units. Like native starch, dextrin is linked through α,1–4 D-glucose linkages for linear chains and α,1–6 D linkages for branched chains (Brossard, Du, and Miller Citation2008; Silva et al. Citation2014). The schematic structure of dextrin with the monomeric units labeled as (G) is illustrated in . Without derivatization of its multiple OH functional groups, dextrin is produced by partial hydrolysis and chains scissions, thus it possesses a lower degree of polymerization and lower MW compared to native starch. As a highly branched biopolymer, the high segment density and end groups composed of relatively flexible sub chains are key structural features in determining the depression properties of dextrin, which can be suited for different applications during flotation.
Figure 4. Schematic structure of dextrin with glucose monomeric units (G) linked through 1–4 glycosidic joints for straight chains and 1–6 joints for branched chains (redrawn from Brossard, Du, and Miller Citation2008).
Several studies have investigated dextrin as a depressant during the processing of inherently hydrophobic minerals viz. carbonaceous materials in pyritic gold ores, talc, graphite (carbon), sulfur, coal and molybdenite etc (Beaussart, Mierczynska-Vasilev, and Beattie Citation2009; Brossard, Du, and Miller Citation2008; Somasundaran and Wang Citation2006). In base metal sulfide minerals flotation dextrin was explored to separate, for instance, galena from chalcopyrite or other copper sulfides (Bolin and Laskowski Citation1991; Laskowski, Liu, and Bolin Citation1991). At industrial scale, some reports on dextrin for regulation of organic carbon (Corg) during segregation of copper concentrates are available in the literature (Foszcz and Drzymala Citation2011). At Mt Isa, dextrin is also utilized in rougher copper flotation to reject carbonaceous materials (Bulatovic Citation2007). Despite these studies it appears that research of convincing flotation selectivity of dextrin is still limited.
In terms of mechanisms, in a similar concept to native starch as shown earlier in , the multiple – OH groups on the glucopyranose structural unit of dextrin are preferentially projected away from the carbon (C) structure matrix and interact with hydrophobic mineral surfaces through hydrophobic interactions (Brossard, Du, and Miller Citation2008). On the other side, hydrogen bonding occurs with surrounding water molecules and imparts hydrophilicity on the mineral surface. Compared with native starch, which has a large macromolecular architecture, dextrin is a smaller and highly branched molecule with a more flexible polymeric network. It should then be reasonable to mention that because of this structure, dextrin’s conformational and amphiphilic properties would have more control of the interfacial interactions on mineral surfaces than native starch.
However, due to the structural flexibility and unmodified nonionic functionality of dextrin, the molecule tends to interact in a random or less discriminative manner, resulting in poor selectivity during minerals flotation. Additionally, depending on the native source, the extent of partial hydrolysis or dextrose equivalent, different types of dextrin exist and exhibit diverse conformational and amphiphilic properties (Bulatovic Citation2007; Silva et al. Citation2014).
Cellulose
Cellulose is another common biopolymer after starch and consists of β-1, 4 glucans when compared to α-1, 4 glucans in native starch. Meanwhile, carboxymethyl cellulose (CMC) is the main derivative of cellulose where the H atom is replaced by functional acidic groups (–COO–), forming a carboxymethyl (–CH2-COO) as illustrated in (Zhao et al. Citation2019). Depending on the degree of substitution (DS), the incorporated functional groups enhance the hydrophilic tendency of cellulose and induce an ionic or polyelectrolytic character on the structure matrix.
Figure 5. Cellulose molecular structure (redrawn from Zhao et al. Citation2019).
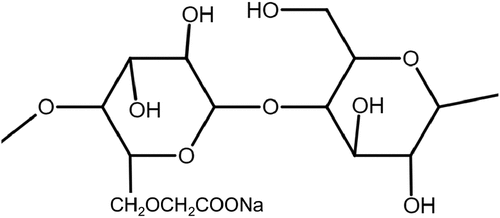
As alluded earlier, several previous studies reported CMC as a common depressant to reject hydrophobic minerals. A recent study by Solongo et al. (Citation2021) investigated graphite floatability in the presence of CMC to be significantly affected by electrolytes and pH. With a particular focus on isopropyl ethyl thionocarbamate (IPETC) used in copper sulfide minerals flotation as a collector, the effects of Ca2+ and Na+ on graphite depression in flotation were compared. According to the results shown in , as the ionic strength increased the depression of graphite increased. It also appears that Ca2+ had a more depressive effect at high pH than Na+.
Figure 6. Graphite floatability as a function of ionic strength (IS) at pH (a) 5.7 and (b) 10 with 150 mg/L CMC and 1 mM IPETC (adapted from Solongo et al. Citation2021).
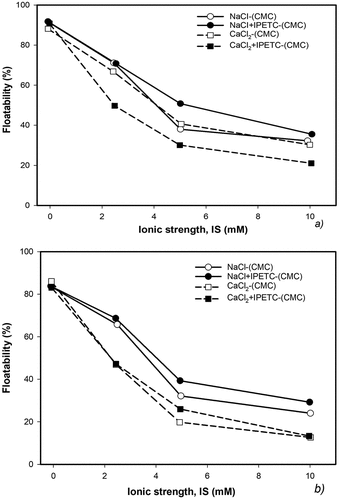
The authors reported that depending on the cation valence of the electrolytes, CMC can adsorb on graphite surfaces and surface-active centers to affect interactions with other reagents and subsequent gangue depression (Solongo et al. Citation2021). While we know that graphite floatability is generally not affected by pH, the authors asserted that based on pH, a combination of electrolytes and IEPTC influenced CMC’s adsorption behavior on the graphite surface through the formation of possible complexes from double reagent – reagent interactions.
In the literature, researchers generally accept hydrophobic interactions as the favorable mechanisms of CMC adsorption on hydrophobic gangue. However, on the contrary others reported chemical complexations involving a bidentate chelating bond between deprotonated CMC acidic functional groups and metallic sites as the major mechanism while hydrophobic interactions were complimentary (Cuba-Chiem et al. Citation2008; Wang and Somasundaran Citation2005). We can concur that it is possible that CMC’s anionic acidic functional groups can electrostatically interact with cationic species such as Na+, Ca2+ or Mg2+ in solution, which adsorb on the edge surfaces of graphite. Thus, depending on the contribution of cation valence to positive charge on graphite surfaces, it implies that the degree of CMC adsorption is controlled by electrostatic forces. As such, it is agreeable that increasing the ionic strength enhances the amount of CMC adsorbed due to charge screening which reduces electrostatic repulsion between the anionic biopolymer and negatively charged mineral surface.
Also, as a synergistic effect, since the cations can enhance the adsorption of CMC, the adsorption increases the negative charge on the mineral surface. This may electrostatically repel any approaching negatively charged bubbles, resulting in depression. Deriving from these findings, apart from pH, the electrolytes’ cation valence and the induced polyelectrolytic character or minerophilic nature of the negatively charged – COO groups in CMC impact the depression mechanisms in a less straightforward manner (Somasundaran and Wang Citation2006). As an ionic biopolymer, the selectivity of CMC may be limited by the polyelectrolytic character in the presence of valuable sulfide minerals wherein, the latter are even more sensitive to solution conditions such as pH, DO and Eh relative to surface oxidation.
Gum
Gum is a high MW natural and nonionic heteropolysaccharide biopolymer. It consists of a polymannan wherein the β-D-mannose units are linked by (1–4) linkages in the linear backbone while (C1–6) linkages with α-D-galactose units are attached as side chains (Frollini et al. Citation1995). These repeating saccharide units contain – COOH and – OH as functional groups and mannose and galactose units exist in a ratio of 2:1.
According to the literature, several different gums exist in nature such as guar, locust bean, tragacanth and xanthan gum etc (Wang et al. Citation2020). As examples, the schematic structure of guar and xanthan gum (XG) is illustrated in . Guar is an electroneutral biopolymer while XG is an anionic biopolymer produced by bacteria in nature (Gao et al. Citation2023). As illustrated in , the latter’s outer mannose units can contain pyruvate or acetyl functional groups).
Figure 7. Structure of a) guar and b) xanthan gum (XG) (redrawn from Zhao et al. Citation2019).
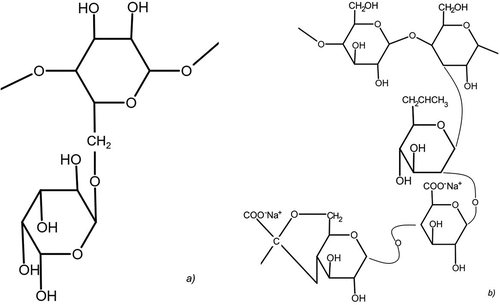
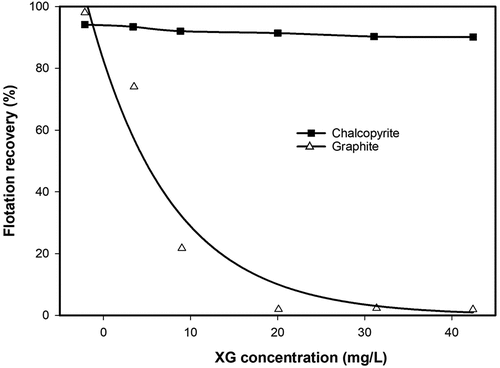
During flotation, guar is the most popular depressant to reject hydrophobic gangue such as talc, particularly, in the separation of sulfides or platinum group of minerals (Rath, Subramanian, and Laskowski Citation1997; Wang, Somasundaran, and Nagaraj Citation2005). Wei et al. (Citation2018) compared tragacanth and guar as depressants of talc and found tragacanth to be more effective and selective than guar. Xanthan gum (XG) was also reported as an effective depressant of hydrophobic minerals such as pyrophyllite (Zhao et al. Citation2019), molybdenite (Yan et al. Citation2020) and talc (Pan et al. Citation2020; Zhong et al. Citation2021). Saim and Darteh (Citation2023) investigated the separation of molybdenite and chalcopyrite using XG, while Zhang, Tian et al. (Citation2023) also evaluated its ability to depress graphite in chalcopyrite flotation. According to the flotation results in , a graphite recovery of 98.2% was achieved in the absence of XG and reduced to nearly zero at concentrations starting from 20 mg/L (Zhang, Tian et al. Citation2023). However, it should be noted that the investigated mineral system was too simplified, which makes it difficult to extrapolate the separation window to a more real or practical flotation system.
Figure 8. Flotation recovery of single chalcopyrite or graphite as a function of xanthan gum (XG) concentration at pH 7.5 (redrawn from Zhang, Tian et al. Citation2023).
In terms of the mechanisms, Ma and Pawlik (Citation2005) proposed that hydrogen bonding was the governing adsorption mechanism of guar on talc, while Wei et al. (Citation2018) reported that the adsorption of tragacanth and guar on talc was driven by hydrophobic interactions. In the latter study, hydrogen bonding also played a role. Meanwhile, in the study by Zhang, Tian et al. (Citation2023), FTIR and XPS were used to characterize XG adsorption on graphite and chalcopyrite surfaces. On the chalcopyrite surface, identical FTIR spectra were observed with no shift or formation of new peaks or changes in peak areas in the absence and presence of XG. The results implied that XG was hardly bound onto chalcopyrite. However, on the graphite surface, though no significantly visible spectral changes were observed, a physical nature of adsorption was indicated (Zhang, Tian et al. Citation2023).
As can be seen in , the XPS bulk survey spectra showing a comparison of the absence and presence of XG revealed an increase of 13.13% in oxygen (O) composition attributed to the hydrophobic interaction. Based on XPS corroborated with the FTIR studies on graphite, the possibility of a chemical interaction was ruled out, but instead a physical interaction accepted. Therefore, Zhang, Tian et al. (Citation2023) concurred that hydrophobic interaction was the main dominant driving force, wherein hydrogen bonding and electrostatic interactions played no significant roles. This was contrary to Ma and Pawlik (Citation2005) who affirmed hydrogen bonding was the main mechanism and Wei et al. (Citation2018) who reported hydrogen bonding and electrostatic interactions playing a role.
Figure 9. XPS survey spectra of graphite before and after a treatment with XG (adapted from Zhang, Tian et al. Citation2023).
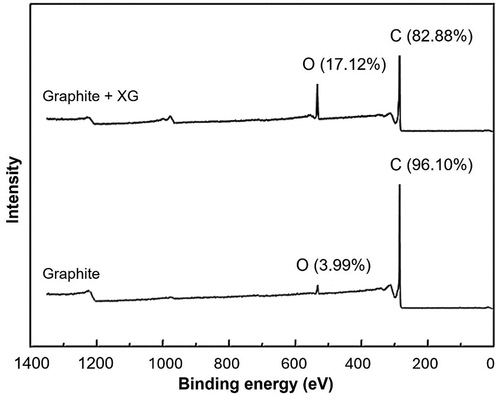
In any case, it should be reinstated that a considerable portion of FTIR or XPS spectroscopic surface signals originate from a nanoscale depth within the range of 1–2 nm, which makes it less convincing to accurately characterize organic polymers adsorbed on such surfaces due to non-surface interaction signals.
Summarily as reviewed from the literature, amongst the above-described polysaccharides, native starch possesses more advantages including low cost and ready availability, highly potentiating its use as a more attractive depressant. While CMC is more soluble and generally regarded as a more effective depressant in comparison to native starch, however, it is generally ~ 3–4 times more expensive than starch (Fletcher, Chimonyo, and Peng Citation2020). Furthermore, by comparison from a structural perspective, while starch and dextrin possess simpler structures with a smaller number of branches in the molecules, cellulose has a more twisted structure and gum is even more complex. summarizes the structural properties and highlights key applications of native starch, dextrin, cellulose and guar.
Table 1. Summary of structural properties and depressive applications of native biopolymers.
Despite all the considerable research conducted utilizing native biopolymers for rejecting hydrophobic gangue such as carbonaceous materials in valuable minerals flotation, challenges associated such as lack of selectivity and efficacy persist. These challenges are linked to the complex macromolecular architectures and nonselective polar functional features of native biopolymers. This prompts more research on the modification of native biopolymers to develop green alternatives and enhance selective depression in minerals flotation.
Influence of modified biopolymers on carbonaceous materials depression
According to the literature, some common or widely studied techniques of biopolymers modifications are oxidation, carboxymethylation and hydroxypropylation (Zhao et al. Citation2017). Apart from differences in modification techniques, the type of biopolymers and the reaction condition also influence the resulting structures and functional features of the derivatives. Thus, to provide insights into how structural and functional changes can enhance biopolymers’ selectivity, the following subsections begin by defining the purpose of each modification. Like the earlier prescribed approach, the structure and chemistry of each of the modified biopolymers are described as well as comparing their applications in flotation selectivity and the adsorption mechanisms involved.
Biopolymers oxidation
The purpose of the oxidation is to modify the biopolymers’ macromolecular structures and functionalize the derivatives with new functional groups (–C=O and – COOH). The derived multiple length scale of structure – function alterations can improve the solubility and dispersibility through opening the molecular structure and increased flexibility, steric freedom and molecular mobility of biopolymer derivatives (Chapagai et al. Citation2021). This implies that structural and functional changes in the biopolymers would result in a range of physicochemical properties, consequently leading to varying degrees of improved performances and effectiveness compared to unmodified biopolymers.
As described in the literature, the oxidation can be achieved using different chemical reagents such as ozone (O3), hydrogen peroxide(H2O2), sodium hypochlorite (NaCIO) and potassium permanganate (KMnO4) (Chimonyo et al. Citation2020a; Zhang, Xu et al. Citation2023).
Degree of oxidation
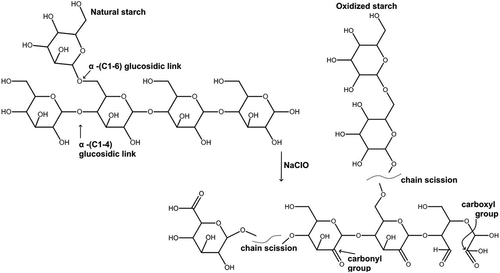
Taking native starch as an example, the degree of oxidation depends on the extent of depolymerization of amylose and amylopectin homopolymers via beta-elimination and homolytic cleavage of the C2 – C3 and opening of the glucopyranose ring structure as shown in . As a result of the oxidation, a range of molecular size distributions and structures can be derived. Additionally, the degree of oxidation also depends on the extent of substitutions of the biopolymers’ pendant – OH moieties by more reactive – C=O and – COOH functional groups. As such, the polarity ranking of the derivatives depends on the ratio of – C=O and – COOH.
Figure 10. A schematic representation of native starch oxidation using NaCIO (Chimonyo, Fletcher, and Peng Citation2020a).
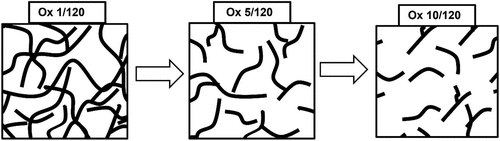
A series of oxidized starch derivatives (Ox 1/120, Ox 5/120 and Ox 10/120) were systematically generated using wheat starch with NaOCl at different concentrations (1%, 5% and 10%) with pH adjusted to 7 using dilute H2SO4 and a reaction time of 120 min (Chimonyo, Fletcher, and Peng Citation2020b; Fletcher, Chimonyo, and Peng Citation2021). During the reaction, the pH was maintained at 7 by adding dilute NaOH or HCl. At the end of the reaction, the modified starch slurry was thoroughly washed using ethanol and deionized water before filtering and drying. As described in the literature, size exclusion chromatograms (SEC) of the oxidized starches revealed the molecular structure changed and the size decreased with the degree of oxidation (Chimonyo, Fletcher, and Peng Citation2020b). presents a simple schematic of the corresponding molecular structure changes of the oxidized derivatives produced with increasing the degree of oxidation. In addition, the ratio of [–COOH]: [–C=O] characterized by titration increased according to NaOCI concentration (Chimonyo, Fletcher, and Peng Citation2020a). It was interesting to observe that the zeta potential was −12 mV, −26 mV and −42 mV for Ox 1/120, Ox 5/120 and Ox 10/120, respectively (Chimonyo, Fletcher, and Peng Citation2020b). The decrease in zeta potential implied that increasing the degree of oxidation induced a corresponding electronegative nature of the oxidized starch derivatives.
Figure 11. A schematic diagram of molecular structures of differently oxidized starches adsorbed on chalcopyrite surfaces (adapted from Chimonyo, Fletcher, and Peng Citation2020b).
During the flotation of graphite and chalcopyrite, a non-linear relationship between the degree of oxidation viz. ([–COOH]: [–C=O]) and flotation recovery was attained as shown in . A marked separation window of ~37% was observed when Ox 5/120, the intermediately oxidized biopolymer, was used with graphite recovery at around 42%. This demonstrated a remarkable potential of the oxidized starch to reject graphite in chalcopyrite flotation. However, a further increase in degree of biopolymer oxidation proved detrimental to selective depression.
Figure 12. Recovery of graphite and chalcopyrite as a function of COOH:C=O at pH 7.5 (based on data drawn from Chimonyo, Fletcher, and Peng Citation2020c, Citation2020b).
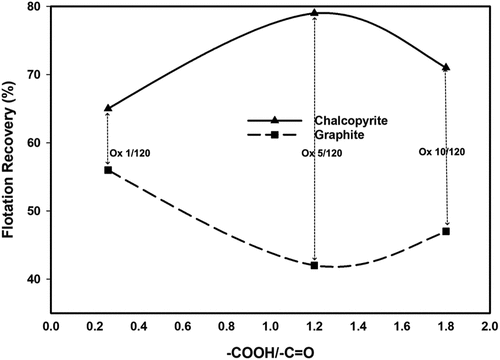
Based on the literature, a well-ordered and structured chain-like surface morphology with distinguishable junctions or conformations observed on graphite was attributed to the impressive depression efficacy to reject graphite (Chimonyo, Fletcher, and Peng Citation2020a; Chimonyo, Fletcher, and Yongjun Citation2021). Meanwhile, in the case of chalcopyrite, a restricted adsorption of Ox 5/120 on chalcopyrite was observed when compared to Ox 1/120 and Ox 10/120 with other degrees of starch oxidation and was correlated to the modest chalcopyrite depression. At the ratio of [–COOH]: [–C=O] being around 1.2 with the hydrodynamic radius Rh being 6.5 nm, the derived structure and functional features in Ox 5/120 possessed some balance of steric and electrostatic hindrances. The latter arguably inhibited extensive biopolymer adsorption and induction of hydrophilicity on the chalcopyrite surface.
In both mineral cases, increasing the degree of oxidation was found to diminish the selective effectiveness. This was speculated to occur because increasing the proportion of active functional groups would promote possible interactions with the heterogenous chalcopyrite surface while the ruptured biopolymer chains lacked a capacity to sustain strong binding on the electroneutral graphite surface. As earlier mentioned, considering the starch oxidation procedure that involved filtering and thoroughly washing the starch residue with deionized water and ethanol before drying, there was no expectation that the oxidizer would impact flotation and adsorption experiments.
As a summary, even though the oxidized starch structures demonstrated attractive potential for selectivity, the extent of adsorption on chalcopyrite was still substantial. On the other hand, the graphite recovery observed circa 42% was still relatively high, demonstrating some limitations. The idea that the oxidized starch structure instrumental to drive conformational preferences was speculative. This is because the underlying selective or discriminative influences relative to the range of derived structure features and functional groups are notoriously difficult to pinpoint, and therefore remain unclear.
Oxidant type
Chapagai, Fletcher, and Gidley (Citation2022) empolyed different oxidants including H2O2 (Perox 2/4, Perox 5/4 and Perox 10/4) and NaOCI (Ox 2/4, Ox 5/4 and Ox 10/4) to generate oxidized wheat starch biopolymer derivatives. The purpose was to assess the effectiveness of biopolymer derivatives oxidized with various types of oxidants in carbon depression. In addition, the work was also motivated by the unclear specific influence of oxidized biopolymer derivatives on polymer-mineral interactions as alluded earlier.
shows [–COOH] and [–C=O] compositions obtained during the oxidation of wheat starch biopolymers at fixed pH 4 (Chapagai, Fletcher, and Gidley Citation2022). As described in the literature [–COOH] and [–C=O] were characterized using titration method. Chapagai, Fletcher, and Gidley (Citation2022) showed no representation of the possibly derived molecular structures; however, they managed to determine molecular size distributions and structure changes characterized by SEC. Based on SEC the authors mentioned that the severity of the starch oxidation on the molecular structure was greater with NaOCI than H2O2. Regarding functionality, it should however be noted that H2O2 generally possesses a preponderance of generating more [–C=O] groups which are relatively hydrophobic over [–COOH] groups when compared to NaOCI. This suggests a wide potential variation in the structures and hydrophobic or hydrophilic characteristics of the derivatives.
Table 2. Carbonyl (-C = O) and carboxyl (–COOH) contents of oxidized starches (Chapagai, Fletcher, and Gidley Citation2022).
During flotation, the depressive performances of NaOCl and H2O2 (Perox)-oxidized biopolymer derivatives were compared. shows the recovery of graphite obtained as a function of biopolymer dosage. As can be observed in , the Perox-oxidized starch derivatives were generally superior to depress graphite compared to the NaOCI (Ox) series. Meanwhile, it can be observed in that as the proportion of the functional groups increased, the capacity of both types of depressants decreased. This was also correlated to adsorption measurements where the adsorption densities or affinities decreased with increasing the concentration of both oxidants.
Figure 13. Flotation recovery of graphite at pH 7.5 in the presence of NaOCl and H2O2-oxidized starches (Chapagai, Fletcher, and Gidley Citation2022).
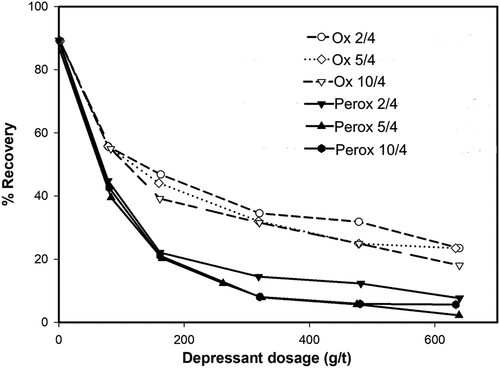
Chapagai, Fletcher, and Gidley (Citation2022) did not clarify the possible reasons behind the observed trends. One possible explanation could be that the increasing proportion of – COOH groups led to increased electronegativity and subsequent electrostatic repulsion during interactions with the negatively charged graphite surface. However, consistent with the hydrophobic interactions, it appears that more hydrophobic – C=O groups favored the inhibition of graphite flotation but the increasing proportion of – COOH was detrimental to depression. As such, one can deduce that Perox – oxidized starches probably adsorbed more strongly on graphite than NaOCI-oxidized starches.
Another important aspect worth discussing is the effect of the structures and molecular size distributions. Chapagai, Fletcher, and Gidley (Citation2022) proposed that the molecular size distribution had no systematic effects on graphite depression compared to the functional groups. The authors attempted to verify this hypothesis by incorporating dextrin in flotation tests and comparing it with oxidized starch. According to the results, dextrin proved a depressive effect on graphite intermediate between NaOCI and H2O2-oxidized starches. It was conjectured that despite the wide range of molecular size distributions of oxidized starches within either of the NaOCI or H2O2-oxidized starches, there was no systematic depression differences of graphite. This led to the assertion that the ratio of – C=O to – COOH had a notable impact on graphite depression compared to variations in molecular size. While the influence of functional properties relative to the derivatives’ polarity rankings is a sound argument, we contend that the influence of the molecular structural changes would be inconclusive to discern without characterizing the morphology on the mineral surface. Also, because of the absence of valuable minerals flotation it is hard to comment on the selectivity of the investigated NaOCI and H2O2-oxidized starches.
In the work by Fletcher, Chimonyo, and Peng (Citation2021) the authors also investigated the effect of H2O2-oxidized starch (Perox 3/30) and NaOCI-oxidized starch (Ox 5/120). Perox 3/30 was generated with 3% H2O2 after 30 min of reaction at pH 7. The work investigated gangue minerals with disparate surface properties (hydrophobic versus hydrophilic) with graphite as the hydrophobic gangue and Cu-activated pyrite as the hydrophilic gangue in copper sulfide flotation. The incorporating of the gangue minerals with disparate surface properties was intentional to expand the scope to evaluate the performance of oxidized biopolymers.
Referring to the lower MW structure of Ox 5/120, Fletcher, Chimonyo, and Peng (Citation2021) reported it more effective at depressing graphite while the larger MW structure of Perox 3/30 better to depress Cu-activated pyrite. For both mineral systems, a selective potential was identified since the oxidized starches allowed a decent recovery of chalcopyrite. Contrarily, if the comparison of performance is based on the size of molecular structures, appears to indicate that Perox 5/4 generated at 5% H2O2 (implying a large MW structure) was the most effective to depress graphite than the lowest MW structure attained at the lowest oxidation degree.
Noting the different experiment conditions by comparing the above findings, one can conclude that beyond impacting functional properties, different oxidants can result in a spectrum of performances following modifications. As such, it appears that there are uncertainties regarding the governing features relative to the molecular structures and functional groups required for achieving an enhanced depressive efficacy.
Biopolymer type
During modifications, the influence of different biopolymer types on the derived properties is closely linked to the various levels of granular, crystalline and macromolecular structures. Khoso et al. (Citation2021) oxidized potato starch using H2O2 to form tricarboxylate sodium starch (TCSS). The hypothesis was that biopolymer-derived depressants exhibit greater affinity toward hydrophobic minerals which have greater amounts of metal ions and metal – OH species on the surface. As a negatively charged derivative, TCSS’ performance was significantly affected by pH during sulfide minerals flotation. For example, in the acidic pH range, it depressed pyrite to 20% while chalcopyrite recovery was around 60%. Zeta potential measurements confirmed the interaction differences on the mineral surfaces, which contributed to the flotation behaviors, particularly in the pH range of 4–6. In this acidic pH range, it is curious that the authors did not verify the role of pH on minerals surface species present and their contributions to surface hydrophobicity. The minerals surface characteristics should influence the adsorptive behavior of the modified biopolymer.
In a study by Zhao et al. (Citation2017), guar gum was oxidized using H2O2 with a reaction time of 120 min, a weight ratio of 1:5 for oxidant to guar gum and a temperature in the range of 90–120°C to obtain oxidized guar gum (OGM). The authors did not share details of the generated OGM molecular structures, or characterization of functional properties. In any case, the purpose of the oxidation was to generate a guar molecular derivative and evaluate its efficacy to depress pyrophyllite in pyrite flotation. During flotation pyrophyllite recovery was 87% in the absence of OGM, demonstrating a highly natural floatability. However, around 20% pyrophyllite recovery was achieved in the presence of OGM at a dosage of 50 mg/L. This revealed a remarkable flotation separation. As shown in , adsorption density studies also indicated a higher density of OGM on the pyrophyllite surface than the pyrite surface in corroboration with flotation outcomes.
Figure 14. Adsorption density of OGM on single mineral surfaces as a function of OGM concentration (Zhao et al. Citation2017).
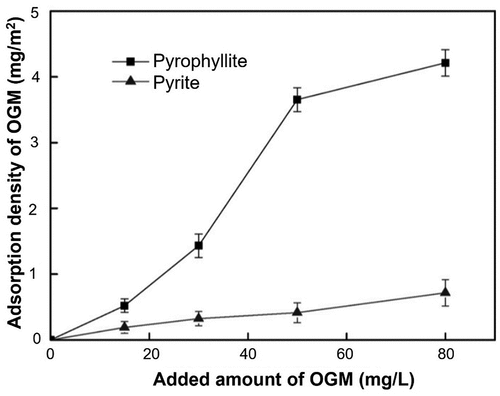
Based on FTIR characterization, Zhao et al. (Citation2017) concluded that the mechanism of OGM adsorption on pyrophyllite was a strong chemical interaction. As a hydrophobic substrate, there is no mention of hydrophobic interactions playing a role in adsorption. To emphasize what was reported earlier, relying on FTIR to infer biopolymers’ molecular adsorption may not be ideal due to possible overlaps of the surface and non-surface signals during characterization.
It should be understandable that biopolymer type plays a role in influencing the modification and corresponding depressive effect of the modified derivatives. However, there are currently scarce studies comparing different oxidants and biopolymer types, for instance, types of starch (such as potato, corn and wheat etc.) whose ratios of amylose and amylopectin vary. This may influence the mechanisms of selective depression, particularly when carbonaceous gangue occurs in valuable sulfide minerals flotation.
Overall, while biopolymer oxidation has the potential to generate effective and selective derivatives, the underlying mechanisms are still not straightforward. This has been evidenced by conflicting assertions on the depressive actions observed in the literature.
Biopolymers carboxymethylation
Carboxymethylation is an etherification reaction using monochloroacetic acid in the presence of sodium hydroxide (Jie et al. Citation2004; Khalil, Hashem, and Hebeish Citation1990). The purpose is to introduce an enhanced anionic character using hydrophilic carboxymethyl (–CH2COOH) groups which offer a higher electronegative charge density on the biopolymer structure matrix than – OH groups. shows a molecular schematic of carboxymethyl chitosan where R = CH2COOH or CH2COONa.
Figure 15. Molecular structure of carboxymethyl chitosan (R = CH2COOH or CH2COONa) (Yuan et al. Citation2019).
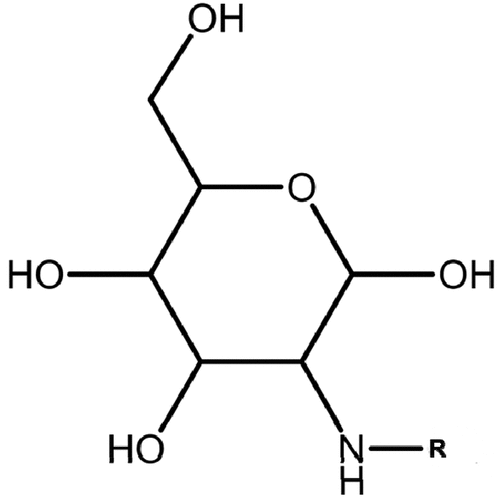
In the literature, Yuan et al. (Citation2019) developed O-Carboxymethyl chitosan using the carboxymethylation technique. Without details provided on the production, however, the degree of substitution (DS) was 95% and the degree of deacetylation was 91%. During chalcopyrite flotation, O-Carboxymethyl chitosan was utilized as a depressant of molybdenite. The authors observed that 150 ppm of the chitosan derivative was sufficient to reject molybdenite (Yuan et al. Citation2019). A large separation window was observed within a pH range of 3–11, indicating that the performance was not affected by pH.
Meanwhile, AFM morphology characterization of the molybdenite surface was also conducted (Yuan et al. Citation2019). Compared to an untreated or freshly generated molybdenite surface, treatment with 150 ppm of O-Carboxymethyl chitosan resulted in the formation of random and sparsely distributed aggregates with diameters ranging from 100 to 200 nm and heights up to 2 nm on molybdenite surface.
Noting that the biopolymer surface coverage does not always equate to a high depression effect as configuration of the adsorbed biopolymer domains on the mineral surface is a significant factor (Yuan et al. Citation2019), the authors speculated that the adsorbed chitosan derivative was irreversibly adsorbed, but on the other hand the interaction claimed was governed by hydrophobic forces (the latter implies a physical and reversible interaction).
In other studies, dextrin was modified through carboxymethylation to generate carboxymethyl (CM) dextrin. In the literature, examples of studies that mentioned the evaluation of CM dextrin derivatives to depress different hydrophobic gangue minerals include talc (Mierczynska-Vasilev, Ralston, and Beattie Citation2008) and molybdenite (Beaussart et al. Citation2012). When compared to hydroxypropylated (HP) dextrin and normal dextrin (Dextrin TY), it was reported that among the differently functionalized dextrins, CM dextrin attracted more hydration water through hydrogen bonding because of the higher polarity ranking of the CM groups compared to the unmodified biopolymers (Mierczynska-Vasilev, Ralston, and Beattie Citation2008).
These authors argued that the absence of a correlation between surface hydrophobicity reduction and adsorption amount was because of induced hydration-repulsive forces between mineral surfaces and air bubbles. The latter seemed to play a significant role contributing to the depression of hydrophobic minerals apart from simple reduction of underlying surface hydrophobicity. Additionally, the hydration water within the adsorbed biopolymer stabilizes the thin film between particles and air bubbles and consequently affects bubble–particle interactions.
Depending on the type of functional groups of the modified biopolymers and observed discrepancies between the adsorption and morphology relative to hydrophobic gangue depression, there were no comments on the influence of the same principle when dealing with diverse minerals or active surfaces to understand flotation selectivity. Nevertheless, it is agreeable that the hydration of optimal biopolymer structures (a balance of hydrophilic/hydrophobic components) and discriminative affinities of mineral surfaces would reveal more insights into the depressive surface activity.
As described, similarly for biopolymers oxidation, different etherification conditions and biopolymer types can be employed. This results in various structure-functional features being generated, which invariably influence flotation selectivity and required optimization.
Biopolymers hydroxypropylation
Hydroxypropylation is another modification technique previously described in the literature (Chimonyo, Fletcher, and Peng Citation2020c). It is also an etherification-based reaction involving propylene oxide for the introduction of bulky hydrophobic hydroxypropyl (HP) groups (–OCH2CH(OH)CH3). These large functional groups substitute the – OH groups within the biopolymer matrix, causing an increase in molecular structural openness.
In the literature, native starch is the common biopolymer reported for hydroxypropylation. From our previous article one can visualize the possible schematic structures of the HP starch ethers (Chimonyo, Fletcher, and Peng Citation2020c). Herein, with a focus on native starch, literature reports that amylose is more susceptible to hydroxypropylation compared to amylopectin, while the latter is predominantly hydroxypropylated near numerous branch points. Apart from increasing the openness through the ‘pushing apart effect’ and reduced associative forces (Jaspreet, Kaur, and McCarthy Citation2007), the incorporated HP groups also enhance the hydrophobic nature of the derivatives, which is relevant in minerals separation.
During flotation, studies by Beattie et al. (Citation2006) compared the depressive performance of a high MW hydroxypropylated (HP) starch with the DS of 5.3% to low MW biopolymers (dextrin and polyacrylamide) to depress talc. The findings revealed that while the modified high MW HP starch effectively rejected talc as the hydrophobic gangue with hydrophobic interactions recognized as the main mechanism, it also depressed the sulfide minerals compared to the low MW biopolymers, demonstrating poor selectivity. According to Langmuir adsorption modeling, HP starch was also predicted as a high affinity biopolymer derivative for all investigated mineral systems, correlating with poor selectivity. Deducing from these studies it is worth for the reader to understand that depending on the DS, an increase of MW following hydroxypropylation can enhance adsorption capacity and yet still detrimentally reduce selectivity.
In summary, even though modified biopolymers offer depressive potential compared to native counterparts, the generated diverse and hierarchically structural – functional features introduce ambiguity regarding the critical characteristics required for enhanced depressive selectivity. Therefore, there are still needs for better understanding of underlying influences of the derived structural – functional features following modifications under various conditions. Moreover, little research has been conducted on ore systems, hindering the prospects of gaining more practical insights into the selectivity of modified biopolymers, particularly between carbonaceous materials and valuable minerals.
Future research directions
What should first be agreeable is that even though there are different techniques of biopolymer modification methods, the work demonstrated that the modified structures still interacted with valuable minerals, demonstrating some limitations in the selectivity. In particular, the review clearly indicated that different techniques of modifications and conditions (pH, temperature and reaction time etc.) result in complex multiple scale changes of functional groups, molecular structures and size distributions, which are hard to delineate their specific and selective functions. As a result, more robust future research work of controlled and systematic modifications is required to optimize the derived features for impartation of selective adsorption and depressive properties.
Building on from the above point, more work is needed to evaluate the influence of biopolymer types and origins when optimizing the derived structures and functional properties. For instance, different native starch types (e.g. corn, rice and wheat), gums, or celluloses exhibit variations associated to their inherent structures and chemistry which influence their susceptibility to different biopolymer modifications. So, as the biopolymer type or origin changes, understanding and consolidating how derived structural properties are affected, and the subsequent binding mechanisms and conformation on mineral surfaces are essential. It seems that many of the studies on biopolymer oxidation explored native wheat starch modifications and hence more detailed systematic investigations of the effects of modifications of biopolymers with different origins on selective depressive mechanisms are required.
Additionally, as briefly described, there are gaps regarding the mineral systems investigated. Most of the previous studies utilized single or binary mineral mixtures and avoided the use of a more practical or real ore system. As mentioned earlier, this hinders the ability of gaining more practical insights into the selectivity potential of modified biopolymers. In a practical system, flotation reagents compete for surfaces and the resulting effects of modified biopolymer structures on selective adsorption and depression mechanisms are unknown. Thus, more investigations on minerals systems that incorporate diverse surface properties with a focus on carbonaceous materials and valuable minerals are warranted. This can enable tailored development or optimization of the desired biopolymer structure features in the quest to improve both selectivity and efficiency.
Lastly, as earlier articulated the solution chemistry is also a critical factor which influences the depressive efficacy of biopolymers in flotation. This is particularly important considering the global freshwater challenges, where many industrial plants have adopted continuous water recycling processes, which results in complex water chemistry. During the recycling of the process water, accumulation of inorganic and organic species is inevitable, which causes detrimental effects such as the reduction of flotation recoveries and grades as reported in the literature (Ikumapayi and Rao Citation2015). According to the previous reports, currently there is no consensus of the impact of solution chemistry on flotation performance as it is difficult to quantify the specific effects of water chemistry during flotation.
From the literature, it was noted that most of the previous studies utilized fresh or deionized water, overlooking the potential practical impact of solution chemistry on flotation separation using modified biopolymers. As described earlier biopolymer modifications involve some complexities, for instance, different reaction conditions or degrees of modification resulting in different structural, functional properties as well as different charges or anionic levels in the derivatives generated. Therefore, considering the lack of detailed research, it is thus reasonable to explore the impact of solution chemistry on selective depression of biopolymer derivatives.
Summary
The review offers the latest account of the influence of native and modified biopolymers on selectivity in flotation to guide adopting green technologies in minerals flotation. Compared to native biopolymers, the review has demonstrated the potential of biopolymer modifications for depressive function. However, challenges or limitations regarding selectivity still exist relative to the molecular structure and functionality attained. It seems inevitable that the modified biopolymers could still adsorb on valuable minerals and compromise selective floatability. The latter was attributed to the intricacy of the derived key biopolymer features governing depressive properties. With hindsight from the review to circumvent the described limitations, future research can be directed toward further optimizing the structures and functionalities of the modified biopolymers. Moreover, incorporating different types of biopolymers in experimental designs for comparison can help develop tailor-made green biopolymeric reagents in flotation. Lastly, solution chemistry and more practical mineral systems relative to the performance of the modified biopolymers should also be considered. This can enable ascertaining convincing potential of modified biopolymers in selective flotation of valuable minerals and reject concerning gangue such as carbonaceous materials.
Acknowledgments
The authors would like to acknowledge the ARC Centre of Excellence for Enabling Eco-Efficient Beneficiation of Minerals, grant number CE200100009 for the funding support for this work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Afenya, P. M. 1991. Treatment of carbonaceous refractory gold ores. Minerals Engineering 4 (7–11):1043–55. doi: 10.1016/0892-6875(91)90082-7.
- Asgari, K., Q. Huang, H. Khoshdast, and A. Hassanzadeh. 2024. A review on bioflotation of coal and minerals: Classification, mechanisms, challenges, and future perspectives. Mineral Processing & Extractive Metallurgy Review 45 (1):46–76. doi: 10.1080/08827508.2022.2121919.
- Asimi Neisiani, A., R. Saneie, A. Mohammadzadeh, D. G. Wonyen, and S. Chehreh Chelgani. 2023. Biodegradable hematite depressants for green flotation separation – an overview. Minerals Engineering 199 (November 2022):108114. doi: 10.1016/j.mineng.2023.108114.
- Beattie, D. A., L. Huynh, G. B. N. Kaggwa, and J. Ralston. 2006. The effect of polysaccharides and polyacrylamides on the depression of talc and the flotation of sulphide minerals. Minerals Engineering 19 (6–8):598–608. doi: 10.1016/j.mineng.2005.09.011.
- Beaussart, A., A. Mierczynska-Vasilev, and D. A. Beattie. 2009. Adsorption of dextrin on hydrophobic minerals. Langmuir: The ACS Journal of Surfaces and Colloids 25 (9):9913–21. doi: 10.1021/la9010778.
- Beaussart, A., L. Parkinson, A. Mierczynska-Vasilev, and D. A. Beattie. 2012. Adsorption of modified dextrins on molybdenite: AFM imaging, contact angle, and flotation studies. Journal of Colloid and Interface Science 368 (1):608–15. doi: 10.1016/j.jcis.2011.10.075.
- Bertoft, E. 2004. Analysing starch structure. Starch in Food: Structure, Function and Applications 57–96. doi: 10.1016/B978-1-85573-731-0.50006-2.
- Bolin, N. J., and J. S. Laskowski. 1991. Polysaccharides in flotation of sulphides. Part II. Copper/lead separation with dextrin and sodium hydroxide. International Journal of Mineral Processing 33 (1–4):235–41. doi: 10.1016/0301-7516(91)90055-N.
- Brossard, S. K., H. Du, and J. D. Miller. 2008. Characteristics of dextrin adsorption by elemental sulfur. Journal of Colloid and Interface Science 317 (1):18–25. doi: 10.1016/j.jcis.2007.09.003.
- Bulatovic, S. M. 2007. Handbook of flotation reagents: Chemistry, theory and practice, flotation of sulphide ores. Elsevier. doi: 10.1016/B978-0-444-53082-0.00023-8.
- Chapagai, M. K., B. Fletcher, and M. J. Gidley. 2022. Adsorption and depression effects of native starch, oxidized starch, and dextrin on graphite. Minerals Engineering 181 (March):107549. doi: 10.1016/j.mineng.2022.107549.
- Chapagai, K. M., B. Fletcher, T. Witt, S. Dhital, B. M. Flanagan, and M. J. Gidley. 2021. Multiple length scale structure-property relationships of wheat starch oxidized by sodium hypochlorite or hydrogen peroxide. Carbohydrate Polymer Technologies and Applications 2 (June):100147. doi: 10.1016/j.carpta.2021.100147.
- Chen, X., S. Liu, and Y. Peng. 2021. A new approach to selectively reject naturally hydrophobic gangue in the flotation of base metal sulphide minerals. Mining, Metallurgy & Exploration 38 (2):713–20. doi: 10.1007/s42461-020-00365-9.
- Chimonyo, W., B. Fletcher, and Y. Peng. 2020a. The differential depression of an oxidized starch on the flotation of chalcopyrite and graphite. Minerals Engineering 146 (November 2019):106114. doi: 10.1016/j.mineng.2019.106114.
- Chimonyo, W., B. Fletcher, and Y. Peng. 2020b. The effect of oxidized starches on chalcopyrite flotation. Minerals Engineering 165:106749. doi: 10.1016/j.mineng.2020.106749.
- Chimonyo, W., B. Fletcher, and Y. Peng. 2020c. Starch chemical modification for selective flotation of copper sulphide minerals from carbonaceous material: A critical review. Minerals Engineering 156 (May):106522. doi: 10.1016/j.mineng.2020.106522.
- Chimonyo, W., B. Fletcher, and P. Yongjun. 2021. Adsorption and morphology of oxidized starches on graphite. Minerals Engineering 168 (January):106936. doi: 10.1016/j.mineng.2021.106936.
- Cuba-Chiem, L. T., L. Huynh, J. Ralston, and D. A. Beattie. 2008. In situ particle film ATR-FTIR studies of CMC adsorption on talc: The effect of ionic strength and multivalent metal ions. Minerals Engineering 21 (12–14):1013–19. doi: 10.1016/j.mineng.2008.03.007.
- Drzymala, J., J. Kapusniak, and P. Tomasik. 2003. Removal of lead minerals from copper industrial flotation concentrates by xanthate flotation in the presence of dextrin. International Journal of Mineral Processing 70 (1–4):147–55. doi: 10.1016/S0301-7516(02)00156-4.
- Fletcher, B., W. Chimonyo, and Y. Peng. 2020. A comparison of native starch, oxidized starch and CMC as copper-activated pyrite depressants. Minerals Engineering 156 (September):106532. doi: 10.1016/j.mineng.2020.106532.
- Fletcher, B., W. Chimonyo, and Y. Peng. 2021. The potential of modified starches as mineral flotation depressants. Mining, Metallurgy & Exploration 38 (2):739–50. doi: 10.1007/s42461-021-00379-x.
- Foszcz, D., and J. Drzymala. 2011. Differentiation of organic carbon, copper and other metals contents by segregating flotation of final polish industrial copper concentrates in the presence of dextrin. Physicochemical Problems of Mineral Processing 47:17–26.
- Frollini, E., W. F. Reed, M. Milas, and M. Rinaudo. 1995. Polyelectrolytes from polysaccharides: Selective oxidation of guar gum - a revisited reaction. Carbohydrate Polymers 27 (2):129–35. doi: 10.1016/0144-8617(95)00021-X.
- Gao, Y., G. Zhang, M. Wang, and L. Zhao. 2023. Selective separation of carrollite and chalcopyrite at low alkalinity with xanthan gum as a depressant. Colloids and Surfaces A, Physicochemical and Engineering Aspects 675 (June):132003. doi: 10.1016/j.colsurfa.2023.132003.
- Gredelj, S., M. Zanin, and S. R. Grano. 2009. Selective flotation of carbon in the Pb-Zn carbonaceous sulphide ores of century mine, zinifex. Minerals Engineering 22 (3):279–88. doi: 10.1016/j.mineng.2008.08.005.
- Gupta, N. 2017. Evaluation of graphite depressants in a poly-metallic sulfide flotation circuit. International Journal of Mining Science and Technology 27 (2):285–92. doi: 10.1016/j.ijmst.2017.01.008.
- Haung, H. H., and J. D. Miller. 1978. Kinetics and thermochemistry of amyl xanthate adsorption by pyrite and marcasite. International Journal of Mineral Processing 5 (3):241–66. doi: 10.1016/0301-7516(78)90022-4.
- Ikumapayi, F., and K. H. Rao. 2015. Recycling process water in complex sulfide ore flotation: Effect of calcium and sulfate on sulfide minerals recovery. Mineral Processing & Extractive Metallurgy Review 36 (1):45–64. doi: 10.1080/08827508.2013.868346.
- Jaspreet, S., L. Kaur, and O. J. McCarthy. 2007. Factors influencing the physico-chemical, morphological, thermal and rheological properties of some chemically modified starches for food applications-a review. Food Hydrocolloids 21 (1):1–22. doi: 10.1016/j.foodhyd.2006.02.006.
- Jie, Y., C. Wen-Ren, R. M. Manurung, K. J. Ganzeveld, and H. J. Heeres. 2004. Exploratory studies on the carboxymethylation of cassava starch in water-miscible organic media. Starch/staerke 56 (3–4):100–07. doi: 10.1002/star.200300239.
- Khalil, M. I., A. Hashem, and A. Hebeish. 1990. Carboxymethylation of maize starch. Starch ‐ Stärke 42 (2):60–63. doi: 10.1002/star.19900420209.
- Khoso S. A., Y. Hu, M. Tian, Z. Gao, and W. Sun. 2021. Evaluation of green synthetic depressants for sulfide flotation: Synthesis, characterization and floatation performance to pyrite and chalcopyrite. Separation and Purification Technology 259:118138. doi: 10.1016/j.seppur.2020.118138.
- Konieczny, A., W. Pawlos, M. Krzeminska, R. Kaleta, and P. Kurzydlo. 2013. Evaluation of organic carbon separation from copper ore by pre-flotation. Physicochemical Problems of Mineral Processing 49 (1):189–201. doi: 10.5277/ppmp130117.
- Kuz’min, M. I., Y. P. Troshin, S. M. Boiko, E. A. Razvozzhaeva, L. D. Zorina, and D. K. Martikhaeva. 2010. Carbonaceous matter in sulfide-quartz veins at the kurultyken base-metal deposit, Eastern Transbaikal Region, Russia. Geology of Ore Deposits 52 (3):252–59. doi: 10.1134/S1075701510030050.
- Laskowski, J. S., Q. Liu, and N. J. Bolin. 1991. Polysaccharides in flotation of sulphides. Part I. Adsorption of polysaccharides onto mineral surfaces. International Journal of Mineral Processing 33 (1–4):223–34. doi: 10.1016/0301-7516(91)90054-M.
- Magwaneng, S., K. Haga, and A. Batnasan. 2018. Investigation for removal of organic carbon from carbonaceous copper sulphide ore and improving the recovery of copper through flotation. Characterization of Minerals, Metals, and Materials 2018:343.
- Ma, X., and M. Pawlik. 2005. Effect of alkali metal cations on adsorption of guar gum onto Quartz. Journal of Colloid and Interface Science 289 (1):48–55. doi: 10.1016/j.jcis.2005.03.035.
- Mierczynska-Vasilev, A., J. Ralston, and D. A. Beattie. 2008. Adsorption of modified dextrins on talc: Effect of surface coverage and hydration water on hydrophobicity reduction. Langmuir 24 (12):6121–27. doi: 10.1021/la8003382.
- Miller, J. D., J. S. Laskowski, and S. S. Chang. 1983. Dextrin adsorption by oxidized coal. Colloids and Surfaces 8 (2):137–51. doi: 10.1016/0166-6622(83)80081-X.
- Mishra, S., S. Panda, A. Akcil, and S. Dembele. 2023. Biotechnological avenues in mineral processing: Fundamentals, applications and advances in bioleaching and bio-beneficiation. Mineral Processing & Extractive Metallurgy Review 44 (1):22–51. doi: 10.1080/08827508.2021.1998043.
- Mossman, D. J. 1999. Carbonaceous substances in mineral deposits: Implications for geochemical exploration. Journal of Geochemical Exploration 66 (1–2):241–47. doi: 10.1016/S0375-6742(99)00015-1.
- Mossman, D. J., and C. L. Thompson-Rizer. 1993. Toward a working nomenclature and classification of organic matter in precambrian (and phanerozoic) sedimentary rocks. Precambrian Research 61 (3–4):171–79. doi: 10.1016/0301-9268(93)90111-E.
- Natarajan, K. A. 1992. Bioprocessing for enhanced gold recovery. Mineral Processing & Extractive Metallurgy Review 8 (1–4):143–53. doi: 10.1080/08827509208952683.
- Niu, H., H. Yang, L. Tong, S. Zhong, and Y. Liu. 2019. Spectral study of humic substance extract from pressurized oxidizing slag of carlin-typed gold deposit. Journal of Physics 1347 (1):012027. doi: 10.1088/1742-6596/1347/1/012027.
- Nyamekye, G. A., and J. S. Laskowski. 1993. Adsorption and electrokinetic studies on the dextrin-sulfide mineral. Journal of Colloid and Interface Science 157 (1):160–67. doi: 10.1006/jcis.1993.1171.
- Pan, G., Q. Shi, G. Zhang, and G. Huang. 2020. Selective depression of talc in chalcopyrite flotation by xanthan gum: Flotation response and adsorption mechanism. Colloids and Surfaces A, Physicochemical and Engineering Aspects 600 (March):124902. doi: 10.1016/j.colsurfa.2020.124902.
- Pavlovic, S., and P. R. G. Brandao. 2003. Adsorption of starch, amylose, amylopectin and glucose monomer and their effect on the flotation of hematite and Quartz. Minerals Engineering 16 (11):1117–22. doi: 10.1016/j.mineng.2003.06.011.
- Pearse, M. J. 2005. An overview of the use of chemical reagents in mineral processing. Minerals Engineering 18 (2 SPEC. ISS):139–49. doi: 10.1016/j.mineng.2004.09.015.
- Pugh, R. J. 1989. Macromolecular organic depressants in sulphide flotation-a review, 1. Principles, types and applications. International Journal of Mineral Processing 25 (1–2):101–30. doi: 10.1016/0301-7516(89)90059-8.
- Pyke, B., R. Johnston, and P. Brooks. 1999. The characterisation and behaviour of carbonaceous material in a refractory gold bearing ore. Minerals Engineering 12 (8):851–862. doi: 10.1016/S0892-6875(99)00073-4.
- Qi, L., Y. Zhang, and J. S. Laskowski. 2000. The adsorption of polysaccharides onto mineral surfaces: An acid/base interaction. International Journal of Mineral Processing 60 (3–4):229–45. doi: 10.1016/S0301-7516(00)00018-1.
- Rath, R. K., S. Subramanian, and J. S. Laskowski. 1997. Adsorption of dextrin and guar gum onto talc. A comparative study. Langmuir 7463 (14):6260–66. doi: 10.1021/la970518p.
- Saim, A. K., and F. K. Darteh. 2023. Eco-friendly and biodegradable depressants in chalcopyrite flotation: A review. Mineral Processing & Extractive Metallurgy Review 44 (7):492–510. doi: 10.1080/08827508.2022.2091558.
- Shrestha, A. K., and P. J. Halley. 2014. Starch modification to develop novel starch-biopolymer blends: State of art and perspectives. Starch Polymers: From Genetic Engineering to Green Applications. doi: 10.1016/B978-0-444-53730-0.00022-1.
- Shrimali, K., V. Atluri, X. Wang, and J. D. Miller. 2018. Adsorption of corn starch molecules at hydrophobic mineral surfaces. Colloids & Surfaces A 546:194–202. doi: 10.1016/j.colsurfa.2018.03.001.
- Silva, D. M., C. Nunes, I. Pereira, A. S. P. Moreira, M. R. M. Domingues, M. A. Coimbra, and F. M. Gama. 2014. Structural analysis of dextrins and characterization of dextrin-based biomedical hydrogels. Carbohydrate Polymers 114:458–66. doi: 10.1016/j.carbpol.2014.08.009.
- Sime, F. M., J. Jin, X. Wang, C. D. Wick, and J. D. Miller. 2022. Characterization and simulation of graphite edge surfaces for the analysis of carbonaceous material separation from sulfide ores by flotation. Minerals Engineering 182 (January):107590. doi: 10.1016/j.mineng.2022.107590.
- Solongo, S. K., A. Gomez-Flores, S. Ilyas, and H. Kim. 2021. Roles of solution chemistry and reagent–reagent interaction on carboxymethylcellulose adsorption onto graphite and implications on its floatability. Minerals Engineering 167 (July 2020):106873. doi: 10.1016/j.mineng.2021.106873.
- Somasundaran, P., and D. Wang. 2006. Chapter 5 application of flotation agents and their structure-property relationships. Developments in Mineral Processing. doi: 10.1016/S0167-4528(06)17005-2.
- Steenberg, E., and P. J. Harris. 1984. Adsorption of carboxymethylcellulose, guar gum, and starch onto talc, sulfides, oxides, and salt-type minerals. South African Journal of Chemistry 37 (3):85–90.
- Tabatabaei R. H., D. Nagaraj, S. M. S. M. Vianna, T. J. Napier-Munn, B. Gorain. 2014. The effect of non-sulphide gangue minerals on the flotation of sulphide minerals from Carlin-type gold ores. Minerals Engineering 60:26–32. doi: 10.1016/j.mineng.2014.02.004.
- Taner, H. A., and V. Onen. 2024. Environmentally friendly alternative depressants in chalcopyrite flotation. Separation Science and Technology (Philadelphia) 59 (2):257–67. doi: 10.1080/01496395.2024.2315617.
- Wang, J., and P. Somasundaran. 2005. Adsorption and conformation of carboxymethyl cellulose at solid-liquid interfaces using spectroscopic, AFM and allied techniques. Journal of Colloid and Interface Science 291 (1):75–83. doi: 10.1016/j.jcis.2005.04.095.
- Wang, J., P. Somasundaran, and D. R. Nagaraj. 2005. Adsorption mechanism of guar gum at solid-liquid interfaces. Minerals Engineering 18 (1):77–81. doi: 10.1016/j.mineng.2004.05.013.
- Wang, Z., H. Wu, J. Yang, Z. Tang, L. Luo, K. Shu, Y. Xu, and L. Xu. 2020. Selective flotation separation of bastnaesite from calcite using xanthan gum as a depressant. Applied Surface Science 512 (December 2019):145714. doi: 10.1016/j.apsusc.2020.145714.
- Wei, G., F. Bo, P. Jinxiu, Z. Wenpu, and Z. Xianwen. 2018. Depressant behavior of tragacanth gum and its role in the flotation separation of chalcopyrite from talc. Journal of Materials Research and Technology 8 (1):697–702. doi: 10.1016/j.jmrt.2018.05.015.
- Wie, J. M., and D. W. Fuerstenau. 1974. The effect of dextrin on surface properties and the flotation of molybdenite. International Journal of Mineral Processing 1 (1):17–32. doi: 10.1016/0301-7516(74)90024-6.
- Yan, H., B. Yang, M. Zeng, P. Huang, and A. Teng. 2020. Selective flotation of Cu-Mo sulfides using xanthan gum as a novel depressant. Minerals Engineering 156 (January):106486. doi: 10.1016/j.mineng.2020.106486.
- Yuan, D., K. Cadien, Q. Liu, and H. Zeng. 2019. Flotation separation of Cu-Mo sulfides by O-Carboxymethyl Chitosan. Minerals Engineering 134 (September 2018):202–05. doi: 10.1016/j.mineng.2019.02.007.
- Zhang, M., C. Tian, J. Ding, X. Shi, L. Qiu, W. Yao, and R. Fan. 2023. Effective flotation separation of chalcopyrite and graphite using eco-friendly xanthan gum as depressant. Mineral Processing & Extractive Metallurgy Review 1–9. doi: 10.1080/08827508.2023.2196072.
- Zhang, M., Z. Xu, Q. Zhang, Z. Dan, H. Fu, and W. Yao. 2023. Properties and potential application of ozone-oxidized starch for enhanced reverse flotation of fine hematite. Minerals Engineering 198 (April):108084. doi: 10.1016/j.mineng.2023.108084.
- Zhao, K., X. Wang, W. Yan, G. Gu, C. Wang, Z. Wang, L. Xu, and T. Peng. 2019. Depression mechanism of pyrophyllite by a novel polysaccharide xanthan gum. Minerals Engineering 132 (November 2018):134–41. doi: 10.1016/j.mineng.2018.11.047.
- Zhao, K., W. Yan, X. Wang, B. Hui, G. Gu, and H. Wang. 2017. The flotation separation of pyrite from pyrophyllite using oxidized guar gum as depressant. International Journal of Mineral Processing 161:78–82. doi: 10.1016/j.minpro.2017.02.015.
- Zhong, C., H. Wang, L. Zhang, M. Guo, and B. Feng. 2021. Flotation separation of molybdenite and talc by xanthan gum. Powder Technology 388:158–65. doi: 10.1016/j.powtec.2021.04.080.