?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study explored, on a laboratory scale, how matte separates from the gangue in the black top of a PGM smelter. Two PGM concentrates, one with high sulfide content (17.3 mass%, Platreef concentrate) and the other with low sulfide content (1.3 mass%, UG-2 concentrate), were studied in the temperature range of 800°C to 1480°C, which is the temperature gradient across the black top. The results showed that effective matte separation occurs at temperatures when the matte is completely molten, and a substantial amount of liquid silicate phase has formed. The main matte separation mechanism is the coalescence of sulfide prills and their gravitational settling through the continuous path created by the liquid silicate phase. Complete matte separation occurs at lower temperatures in Platreef concentrate (1400°C in Platreef concentrate vs. 1480°C in UG-2 concentrate), due to the higher concentration of liquid silicate phase of lower viscosity, as well as the higher amount of sulfide minerals in this concentrate. PGMs already dissolve and collect in a nickel-iron-based alloy associated with the matte at temperatures as low as 900°C.
Introduction
Ores from the Bushveld Complex (South Africa) and the Great Dyke (Zimbabwe) are exploited in Southern Africa to extract their platinum-group metals (PGMs). The ore types in South Africa include the UG–2 (Upper Group Chromitite Reef No. 2) and Platreef reefs, while the most important PGM-mineralized zones of the Great Dyke in Zimbabwe are associated with the six-metal- enriched (Pt, Pd, Rh, Au, Cu and Ni) Main Sulfide Zone (Cawthorn Citation2006; Charlier et al. Citation2015). The Merensky reef in South Africa, which contained between 2 and 4 volume% base-metal sulfide (BMS) minerals (Charlier et al. Citation2015) and 3–9 g/t PGMs (Engelbrecht Citation2012; Schouwstra, Kinloch, and Lee Citation2000), has been mostly depleted (Cramer Citation2001). As a consequence, mainly UG–2 and Platreef ores are currently processed in South Africa. Ore from the UG–2 reef contains 4–7 g/t PGMs, with platinum the most abundant (Engelbrecht Citation2012; McLaren and De Villiers Citation1982; O’Connor and Alexandrova Citation2021). The UG–2 ore has a low concentration of base metal sulfides, containing the main sulfide minerals pentlandite, (Fe,Ni)9S8, (~0.9 mass %) and chalcopyrite, CuFeS2, (~0.4 mass %). Platreef ore contains 2–5 g/t PGMs (O’Connor and Alexandrova Citation2021), but a higher concentration of sulfides than the UG-2 ore (1–10 volume %). Ore from the Great Dyke contains approximately 3–4 volume % sulfide minerals, which occur as a sulfide assemblage consisting of pyrrhotite, Fe(1-x)S, pentlandite, chalcopyrite and subordinate pyrite (Mberi, Mguni, and Ntuli Citation2018; Stribrny et al. Citation2000). Oberthür (Citation2011) reported that the ore grades of current PGM mines range from 1.16 g/t (Pt+Pd) in the Musengezi subchamber (subeconomic), up to 4.8 g/t (Pt+Pd+Rh+Au) in the Hartley platinum mine.
The PGM-containing ores are concentrated by two or three stages of milling and flotation (Engelbrecht Citation2012). The produced PGM concentrates have different sulfur contents: UG–2 concentrate contains 1–5 mass% S, Platreef reef concentrate 8–13 mass % S, while Unki concentrate from the Great Dyke in Zimbabwe contains 6–7 mass% S (). PGM concentrations in the concentrate vary between 80 and 600 g/t (Ramonotsi Citation2011). The produced PGM-containing concentrates are dried and smelted to separate the gangue (in the form of a SiO2–MgO–FeO–based slag) from the PGM-containing Fe–Ni–Cu–Co-based matte. During the smelting process, a layer of unsmelted concentrate (called the ‘black top’ (BT)) floats on top of the molten slag bath. This layer limits the thermal radiation from the molten bath, thereby protecting the refractory materials in the upper parts of the furnace (Jones Citation2005; Rivera Li Kao and Garbers-Craig Citation2022). The slag produced from UG–2 concentrate contains higher concentrations of Cr2O3, MgO and SiO2 compared to Platreef concentrate (). These higher concentrations of chromium oxide (Cr2O3) and magnesium oxide (MgO) in the UG–2 concentrates increase the liquidus temperature of the slag, while the high silica content increases the viscosity of the slag. Sulfide phases start to decompose in the BT and begin to collect as matte as soon as a continuous liquid silicate phase forms in the sintered concentrate through which the matte droplets can descend (Jones Citation2005; Rivera Li Kao and Garbers-Craig Citation2022).
Table 1. Chemical compositions of the UG–2, platreef and unki concentrates (mass %) (Adams, Liddell, and Holohan Citation2011; Barnes and Newall Citation2006; Buchspies et al. Citation2017; Crundwell et al. Citation2011; Eksteen, Van Beek, and Bezuidenhout Citation2011; Gwimbi Citation2017; Jones and Kotzé Citation2004; Liddell Citation2009; Mabiza Citation2006; Muzawazi and Petersen Citation2015; Mwase, Petersen, and Eksteen Citation2012; Rivera Li Kao and Garbers-Craig Citation2022).
PGMs occur in nature as alloys, sulfides, sulfarsenides, arsenides, antimonides, bismuthides and tellurides (Mwase, Petersen, and Eksteen Citation2012), with the sulfides, arsenides and tellurides being the most common (Vermaak Citation2005). The PGMs can also occur in very low concentrations in other minerals in the form of submicroscopic particles or in solid solution (with base metal sulfides being the major repository of PGMs), making locating and identifying these PGMs very difficult (Oberthür, Weiser, and Kojonen Citation2002; Vermaak Citation2005). Pentlandite is the main Pd carrier, while pyrrhotite, pyrite and chalcopyrite contain PGMs in very low concentrations (Vermaak Citation2005). The mineralogy and distribution of the PGMs are, however, specific to the host ore body. UG–2 ores contain more PGM sulfides, such as braggite ((Pt,Pd)S), cooperate (PtS), malanite (CuPt2S4) and laurite ((Ru,Os)S2) (McLaren and De Villiers Citation1982; Vermaak Citation2005), with Pt-Pd-tellurides being dominant in the Platreef ore, although arsenides, alloys and sulfides are also present (O’Connor and Alexandrova Citation2021; Wilson and Anhaeusser Citation1998). In the Platreef ore, the platinum group minerals occur in association with base metal sulfides (enclosed in or on grain boundaries) but also have a high association with silicate minerals in some areas (Schouwstra, Kinloch, and Lee Citation2000). According to Wilson and Anhaeusser (Citation1998) the tellurides and arsenides are mostly embedded in the silicate gangue. In UG-2, the PGM-containing minerals occur in association with the base metal sulfides and silicates, with only laurite ((Ru,Os)S2) having a preferred association with the chromite grains (Schouwstra, Kinloch, and Lee Citation2000). The average size of PGM-containing minerals in the UG–2 is approximately 12 µm and rarely exceeds 30 µm and is significantly smaller than those found in the Platreef ore (40–200 µm) (Engelbrecht Citation2012; Schouwstra, Kinloch, and Lee Citation2000).
Due to the low concentrations of sulfides in PGM concentrates, autothermal smelting (as used in the copper industry where the concentrates contain 20–30 mass% S) is not feasible (Bacedoni, Moreno-Ventas, and Ríos Citation2020). The low sulfides content of the UG–2 reef (compared to Platreef, Merensky and Great Dyke concentrates) requires blending in order to facilitate matte collection during smelting. It has been reported that a matte-fall of at least 15% is required for good collection and high recovery of PGMs (Nell Citation2004). Blending also controls slag liquidus temperatures (and therefore also matte superheat) and slag viscosities (which influences entrainment and PGM losses to the slag) (Shaw et al. Citation2013).
The primary production of PGMs entails two main pyrometallurgical operations in which matte is produced: primary smelting of the PGMs concentrates to produce an iron-rich Fe-Ni-Cu-Co-based matte that contains the PGMs, and the subsequent converting of the furnace matte to produce a PGM-enriched Ni-Cu-Co-based matte. Smelting of PGM concentrates is done in electric furnaces (rectangular six-in-line electrode or circular three-electrode furnaces), while converting of the furnace matte (to remove some of the sulfur and most of the iron) is done in either Peirce Smith converters (PSC) or in the Anglo Platinum Converting Process (ACP) converter, which uses an adapted Sirosmelt process (Jones Citation2004; Nelson et al. Citation2018).
This study investigated the behavior of sulfides in UG–2 and Platreef concentrate as they descend through the BT, including the mechanisms that control matte collection and PGM recovery in the BT. This was done by performing isothermal laboratory-scale experiments.
Background
Decomposition and melting of sulfide minerals
The main sulfide minerals in PGM concentrate, pentlandite, chalcopyrite and pyrite, decompose in an inert atmosphere at temperatures ranging from 500 to 610°C. Upon decomposition, these minerals form solid sulfides (Fe,Ni)1-xS, Ni3±xS2 (heazlewoodite), Cu2S (chalcocite), Cu5FeS4 (bornite), Fe1–xS and FeS (troilite), according to the reactions given in . Both chalcopyrite and pyrite decompose with an accompanying release of sulfur gas. Prasad and Pandey (Citation1999) reported that chalcopyrite decomposes into chalcocite, troilite and sulfur, while Gaballah et al. (Citation1994) reported that chalcopyrite decomposition occurs in two stages, forming cubanite (CuFe2S3), chalcocite and sulfur in the first stage. After this, cubanite and chalcocite react to form bornite and troilite, with a further release of sulfur. Gaballah et al. (Citation1994) did not observe chalcocite as a decomposition product. The decomposition reactions of the sulfides occur at temperatures lower than those at which the first gangue mineral (talc) starts to decompose, i.e. 800°C (Rivera Li Kao and Garbers-Craig Citation2022). The pure sulfides melt at temperatures ranging from 743 to 1190°C (), while the liquidus temperature of the matte has been reported to be below 900°C (). The liquidus temperature of the matte is therefore lower than the solidus temperature of the gangue of the concentrates, as predicted by FactSage (1100°C for Platreef and 1250°C for UG–2, Rivera Li Kao and Garbers-Craig Citation2022), which implies that the matte will be fully molten before melting of the silicate gangue matrix and liquid-state sintering start. The higher solidus temperatures exhibited by the gangue constituents also imply higher liquidus temperatures associated with the slag () and therefore high superheat associated with the matte (Eksteen Citation2011; Eksteen, Van Beek, and Bezuidenhout Citation2011; Jones Citation2005; Rivera Li Kao & Garbers-Craig Citation2022; Shaw et al. Citation2013).
Table 2. Decomposition reactions of main sulfides found in PGM concentrate (*Kullerud Citation1963; #Prasad and Pandey Citation1999; ##Gaballah et al. Citation1994; ΔXu et al. Citation2019).
Table 3. Melting points of main sulfides found in PGM concentrate (#Madelung, Rössler, and Schulz Citation2000; ΔΔMineralogical Society of America Citation1974; ΔNieroda et al. Citation2020; ##Sugaki and Kitakaze Citation1998; **Ulbrich and Campos Citation2020; *Waldner and Pelton Citation2005).
Table 4. Liquidus temperature ranges of matte and slag of pure and blended UG–2, Platreef and Merensky PGM concentrates (°C) (Eksteen Citation2011; Eksteen, Van Beek, and Bezuidenhout Citation2011; Jones Citation2005; Rivera Li Kao and Garbers-Craig Citation2020; Shaw et al. Citation2013.).
Matte–slag separation
Conventional matte–slag separation is a liquid–liquid separation process in which the matte droplets within the slag bath coalesce, increase in size and then settle through the slag into the matte phase due to density differences (Guntoro et al. Citation2018). Guntoro et al. (Citation2018) studied the reaction sequences and matte–slag separation at 1300°C in air and argon atmospheres, for a mixture of synthetic fayalitic slag and chalcopyrite concentrate. They observed (under both atmospheres) that matte coagulation occurs only after a substantial amount of liquid slag is formed. Similar observations were reported by Fagerlund and Jalkanen (Citation2001), for a copper sulfide matte settling through liquid wollastonite slag.
When the concentrate moves down through the BT (which has a temperature gradient from 800°C at the freeboard–BT interface, up to 1500°C at the BT–slag interface) and reaches the solidus temperature of the gangue (~1200 °C), sintering of the concentrate bed starts to take place. At this point, several matte droplets may become entrained in the closed pores generated by the sintering process. At higher temperatures, the generation of a liquid silicate phase (LP) in the gangue promotes matte drainage through LP channels that form in the silicate matrix. The matte subsequently separates from the gangue minerals at sub-liquidus temperatures of the gangue (Rivera Li Kao and Garbers-Craig Citation2022).
In a theoretical study on South African PGM matte drainage through a concentrate bed, Eksteen (Citation2011) developed mathematical models for matte drainage that considered the mean particle size and mineralogy of the PGM concentrate. Eksteen (Citation2011) highlighted the importance of the permeability of the BT, as the sintering of the black top and the subsequent accumulation of matte in the concentrate bed above the slag bath can lead to several problems, including (1) hindering the escape of gases (CO, CO2, H2O, S2 and volatile metal halides), which then might move sideways, recondense on the copper coolers in the sidewalls of the furnace, and cause chlorine-induced sulfide attack of the copper coolers (Shaw et al. Citation2013); (2) the need to increase the ingress airflow to reduce freeboard temperatures, resulting in higher electrode consumption; (3) uneven heat transfer in the matte accumulation zones; and (4) changes in the physical properties of the BT. These factors can cause significant matte superheat, with matte temperatures above 1500°C. If the matte temperature exceeds the liquidus temperature of the freeze lining, the matte can potentially destroy the freeze lining. This destruction can then lead to catastrophic failures in the refractory and copper coolers within the furnace.
Materials and methods
Raw materials
Experiments were conducted using two PGM concentrates: a concentrate with a low-sulfide concentration (UG–2, 1.32 mass%) and a concentrate with a high-sulfide concentration (Platreef, 17.33 mass%). A significantly higher matte fall (percentage of the mass of matte collected per tonne of concentrate) can, therefore, be expected from the Platreef concentrate. The chemical and mineralogical compositions of these concentrates are reported elsewhere (Rivera Li Kao and Garbers-Craig Citation2022).
Experimental setup
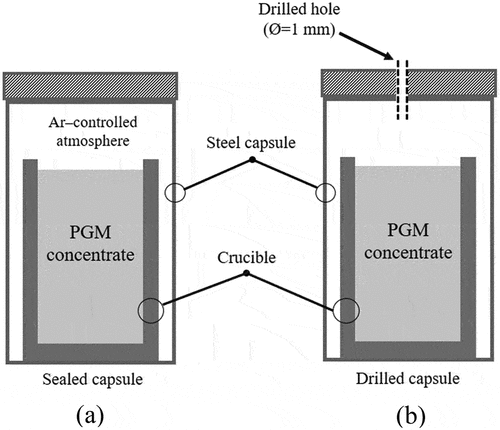
Laboratory-scale experiments simulated the thermal gradient across the BT of an industrial smelter. UG–2 and Platreef concentrates were contained in magnesia (MgO) and silica (SiO2) crucibles, respectively. In order to prevent the escape of sulfur-containing gases from the concentrate on heating, the experiments were carried out in steel capsules (), which were purged with argon before being sealed. The steel capsules were heated in an argon-controlled atmosphere in a muffle furnace. The heating process started from room temperature and gradually reached the desired temperature (800/900/1000/1100/1200/1300/1400 and 1480°C) at a heating rate of 10°C min−1. The samples were kept isothermally at the target temperature for 2 h. After reaction, the capsules were cooled down inside the furnace. The study also investigated the impact of a high sulfur pressure inside the sealed capsules of Platreef concentrate on phase relations and matte collection. This was achieved by comparing the matte drainage and phase relations in sealed capsules with those in capsules that had a 1 mm diameter hole in their lids. The hole allowed the release of excess pressure from the system (). Quench tests were also conducted on the Platreef concentrate in sealed capsules at temperatures of 1400 and 1480°C, in order to determine how cooling rates impact matte collection.
Characterization techniques
The chemical and mineralogical compositions of the bulk concentrates were determined using inductively coupled plasma–optical emission spectroscopy (ICP–OES) and a LECO sulfur analyzer (Rivera Li Kao and Garbers-Craig Citation2022). The crystalline phases were identified with X-ray diffraction (XRD), using a PANalytical X’Pert Pro powder diffractometer with Co Kα radiation (λ = 1.789 Å), employing the ICSD (Inorganic Crystal Structure Database) and X’Pert Highscore Plus software. The quantification of crystalline phases was obtained by Rietveld refinement of the XRD patterns.
The metallographic preparation of the raw and fired concentrates was carried out by embedding the samples in AKASEL epoxy resin. The resin was cured at 70°C for 24 h and the mounted samples were subsequently ground and polished using silicon carbide paper (200# to 1200#) and diamond suspensions of 9.0, 6.0 and 1.0 µm, respectively.
An Olympus BX51–M optical microscope was used for analyzing the distribution of sulfide minerals and matte in the concentrates and fired samples. The images were processed using a Fiji image processing package (Schindelin et al. Citation2012), to investigate the number of particles, their average size and circularity, and the area covered by sulfides or matte. The acquired images have resolutions of 2080 × 1544 pixels, with the magnification varying (50× and 100×) depending on the size of the analyzed sulfide particles or matte droplets.
The chemical compositions and morphologies of the observed phases were determined by using a Jeol JSM – IT300LV scanning electron microscope (SEM) coupled with an Oxford X-Max 50 Energy-Dispersive X-ray Spectrometer (EDS). The obtained EDS spectra were processed and refined individually after acquisition using standardless EDS analysis. The samples were coated using a Quantum carbon coater.
Thermochemical predictions
The solidus and liquidus temperatures of the sulfide systems were predicted (under equilibrium conditions) using FactSage® 7.3 (Bale et al. Citation2016). The equilibrium calculations were performed in the Equilib module, using the FactPS and FToxid database.
Since the samples were all reacted in closed steel capsules, it was assumed that the steel capsules would control both the oxygen and sulfur partial pressures in the capsules through the iron–wüstite (Fe/FeO) and iron–troilite (Fe/FeS) equilibria, respectively. The sulfur partial pressure was obtained from the Predom module (Fe–S–O system), for all the selected temperatures and their respective oxygen partial pressures. The oxygen partial pressures used in the FactSage calculations therefore ranged from 10−18 (800°C) to 10−9 (1480°C) bar, while the sulfur partial pressures ranged from 10−9 (800°C) to 10−3.5 (1480°C) bar. The production of sulfur gas was evidenced in the detection of sulfur-containing compounds in the walls of the steel capsules.
Results
Characterization of the sulfide phases in the PGM concentrates
The normalized sulfide mineral contents in UG–2 and Platreef concentrates obtained from the Rietveld refinement of the XRD patterns are shown in . UG–2 concentrate mainly contains a mixture of pentlandite and chalcopyrite, approximately in a 2:1 mass ratio, while the Platreef concentrate consists of pentlandite, chalcopyrite and pyrite in a 2.4 : 2.2 : 1 mass ratio. Pyrite occurs in very low concentrations in the UG-2 concentrate and, as a result, could only be detected through SEM analysis.
Table 5. Sulfides content (normalized) in UG–2 and platreef PGM concentrates.
The chemical compositions of the different sulfide minerals detected by XRD in the as-received PGM concentrates were determined through individual SEM–EDS analyses of each reported phase. The average EDS compositions (from 100 analyses) for major and trace elements in both concentrates are given in , while the calculated stoichiometries for these average EDS analyses are given in . Pentlandite in UG-2 concentrate contains lower amounts of nickel and has a lower cation/sulfur atomic ratio (i.e. is less stoichiometric) than in Platreef concentrate. The stoichiometry of chalcopyrite is similar in the UG-2 and Platreef concentrates, while pyrite contains slightly more copper in the UG-2 concentrate than in the Platreef concentrate.
Table 6. Average SEM–EDS analyses of the sulfide phases in the PGM concentrates (mass %).
Table 7. Stoichiometries of the different sulfide phases found in the UG-2 and platreef PGM concentrates.
The total chemical compositions of the sulfide mixtures were calculated using the XRD () and SEM–EDS data (). These values () were used as the basis for the thermochemical calculations in FactSage®. The most significant differences between the two types of concentrates are the higher nickel and cobalt content in UG–2 concentrate and its lower sulfur, iron and copper content compared to the Platreef concentrate (). The UG-2 concentrate has a higher bulk metal/sulfur atomic ratio than the Platreef concentrate (0.99 vs. 0.88, respectively), while the (Ni + Cu)/Σmetal atomic ratios of the two concentrates are very similar (0.49 vs. 0.42 for UG-2 and Platreef, respectively). A higher bulk metal/sulfur ratio corresponds to a lower solidus temperature of the concentrate (Ballhaus, Tredoux, and Späth Citation2001). Chromium was only detected in UG–2 concentrate (0.03 mass %).
Table 8. Calculated total chemical compositions of the sulfide mixtures in the UG–2 and platreef concentrates (mass %).
Morphologies of the sulfide phases in the as-received PGM concentrates
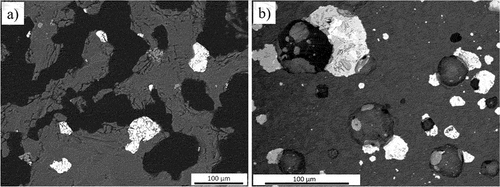
The backscattered electron images (BEIs) of the as-received PGM concentrates are presented in . Sulfides in these concentrates are present as monophase sulfides as well as sulfide assemblages consisting of pentlandite, pyrite and chalcopyrite. UG–2 sulfides exhibit a high degree of liberation, with a few microcracks in some of the particles, while Platreef sulfides exhibit a higher degree of association with gangue minerals and other sulfide phases. Generally, the sulfides exhibit sharp edges. The presence of chromite particles and the low concentration of sulfides in the UG–2 concentrate are apparent.
Figure 2. BEI images of (a) UG–2 and (b) Platreef PGM concentrates (Ccp: chalcopyrite; Pn: pentlandite; Po: pyrrhotite; Chr: chromite; Py: pyrite).
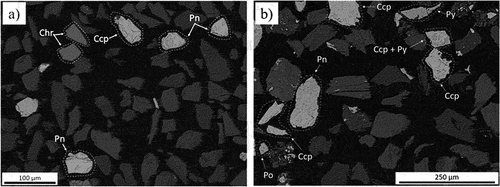
The low concentration of PGMs in the concentrates was evidenced by the detection of only one PGM mineral, michenerite ((Pd,Pt)BiTe), which was completely liberated. This palladium bismuth telluride was found exclusively in the Platreef concentrate, containing 22.0 wt% Pd, 42.5 wt% Bi, and 26.1 wt% Te.
FactSage® prediction of solidus and liquidus temperatures and liquid content of matte and silicates at different temperatures
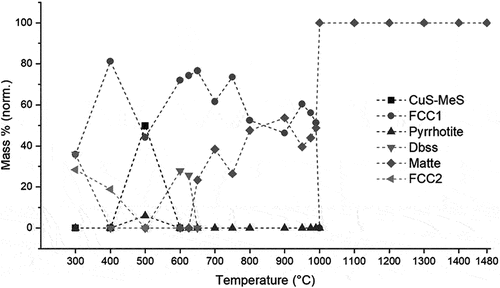
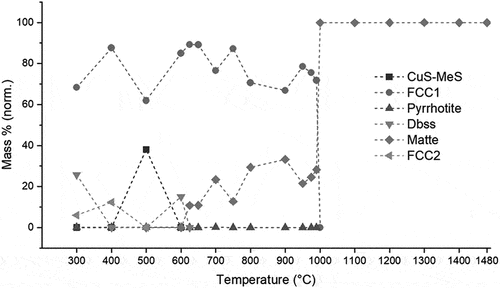
FactSage predicts that the solidus temperature of UG–2 matte is lower (623°C) than that of Platreef matte (645°C). Both concentrates have estimated liquidus temperatures of 1000°C (), with a nickel–iron solid solution alloy phase which precipitates as primary phase on cooling. Despite the earlier onset of liquid matte formation in UG–2 concentrate, the amount of liquid matte which forms in Platreef concentrate is significantly higher and contributes more to the total liquid content in the black top (compare ).
Figure 3. Mass% liquid matte, liquid silicate and total liquid in the UG-2 concentrate as a function of temperature (predicted by FactSage®).
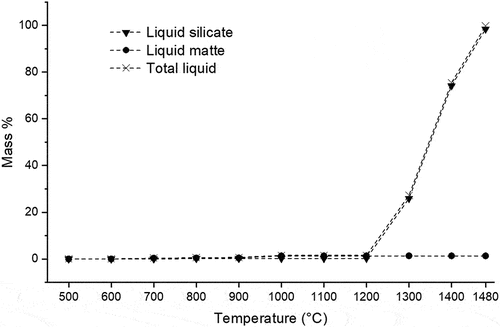
Figure 4. Mass% liquid matte, liquid silicate and total liquid in the platreef concentrate as a function of temperature (predicted by FactSage®).
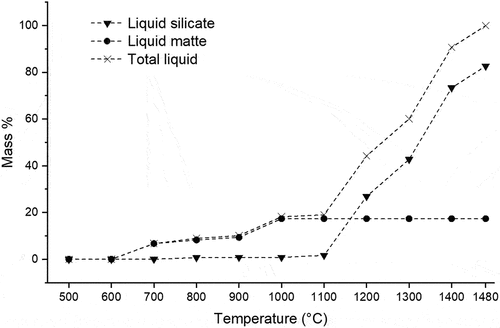
Table 9. FactSage predicted solidus and liquidus temperatures for UG–2 and platreef matte and silicates.
Coalescence of the matte
The coalescence of the matte was studied by examining the changes in the number and average size of matte droplets (sulfide assemblages) as they descend through the BT. The analysis was performed only on the Platreef concentrate due to the challenge posed by the extremely low concentration of small-sized sulfide droplets in the UG–2 concentrate.
The number of matte droplets and their average size is plotted in as a function of temperature (299643 prills were analyzed in total). A simultaneous decrease in the number of sulfide particles and an increase in their average size were observed with increasing temperature. The increase in average size becomes noticeable at a firing temperature of 1200°C, which corresponds to the formation of approximately 26% liquid silicate and 17% liquid matte, totaling approximately 43% liquid.
Figure 5. The evolution of matte prills (from platreef concentrate) in the slow-cooled experiments is depicted through: (a) the number of matte-forming assemblages and (b) average size of the matte-forming assemblages, both as a function of temperature.
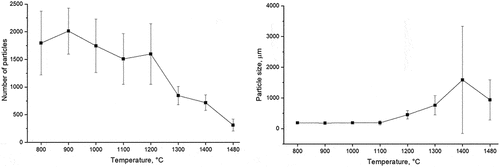
At 1200°C and higher, the LP enabled matte prills to settle due to gravitational forces, increasing the likelihood of their collision and subsequent merging. This coalescence process led to a reduction in the quantity of matte prills and an increase in their average size as the temperature increased. Elevated temperatures, which result in higher liquid concentrations, therefore create more favorable conditions for matte-gangue separation.
The large standard deviation in particle size at 1400°C can be attributed to the variation in size between the large coalesced droplets, which were attached to pores and were entrained in cavities, and the smaller droplets that were attached to pores and suspended in the liquid silicate phase. Conversely, the reduction in the particle size at 1480°C is due to the presence of large matte droplets, exceeding 1.27 mm in diameter, which were excluded from the calculation of the average particle size. The included droplets in the average particle size calculation were those entrained or suspended in the liquid silicate phase.
Phase and microstructural analysis of the sulfides and matte as a function of temperature
Crystallization sequence of the matte as predicted by FactSage®
Sulfide melts cannot be quenched to preserve their high-temperature phase relations; they invariably recrystallize, no matter the cooling rate (Ballhaus, Tredoux, and Späth Citation2001). The equilibrium crystallization paths of the Platreef and UG–2 mattes were, therefore, predicted using FactSage® (), and these phases were compared to the phases observed in the furnace-cooled sulfide assemblages ().
Figure 8. BEI of a sulfide assemblage from Platreef concentrate fired at 800°C, consisting of pentlandite (Pn), digenite–bornite solid solution (Dbss) and Ni-Fe solid solution alloy (A).
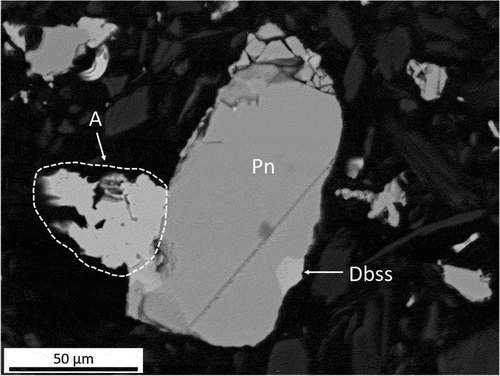
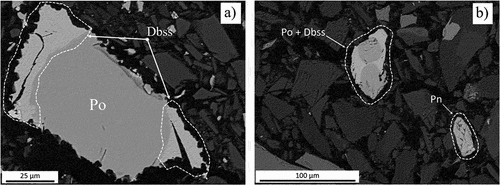
Figure 9. BEI of concentrates fired at 800°C revealing digenite–bornite solid solution (Dbss) – pyrrhotite (Po) assemblages present in both (a) Platreef concentrate and (b) UG–2 concentrate (Pn = pentlandite).
FactSage predicts that the phases that crystallize on cooling from the examined Platreef and UG-2 mattes are similar, although the proportions of these phases differ. A Ni–Fe solid solution alloy phase (FCC1) precipitates from both mattes as primary phase. FactSage® predicted a higher amount of Ni–Fe solid solution alloy in UG–2 matte compared to Platreef concentrate, due to the higher Ni concentration in the UG–2 matte (21.95 wt% Ni) compared to the Platreef matte (14.68 wt% Ni). At the solidus temperature, when the liquid matte is completely consumed, a digenite–bornite solid solution (dbss) phase (Cu9S5–Cu5FeS4), forms. The dbss phase in the Platreef has a stoichiometry of Cu4.24Fe1.88S4 at 625°C and Cu4.39Fe1.81S4 at 600°C, while in the UG-2 matte it has a stoichiometry of (Cu4.34Fe1.83S4) at 600°C. The dbss phase completely dissociates at 500°C, together with a partial consumption of the Ni–Fe solid solution phase, to form a CuS–MeS non-stoichiometric compound (Fe1.34Cu1.12Ni0.20S2) and pyrrhotite (Fe0.98Ni0.01Co0.01S). A new copper-rich (>97 wt% Cu) solid solution alloy phase (FCC2) is formed at 400°C, while the dbss phase is completely consumed at this temperature. At 300°C, two solid solution alloy phases (FCC1 and FCC2) co-exist, while the dbss phase reforms due to the partial consumption of the FCC1 alloy phase.
Microstructural analysis
The microstructures of the matte-forming components obtained in the experiments are complex mixtures of sulfide phases (pentlandite (Pn), pyrrhotite (Po), digenite–bornite solid solution (dbss, C)) and Ni–Fe solid solution alloy phases. Only the most relevant microstructural changes are reported.
Samples heated at 800 and 900 °C
After being fired at 800°C, the sulfide particles present in both concentrates showed slight softening around their edges and displayed phases different from those found in the concentrates. The first newly formed phase is a Ni–Fe solid solution alloy, the growth of which was observed preferentially at the boundaries of the sulfide assemblages. The alloy crystals mainly consist of nickel and iron in an approximate atomic ratio of 2:1, falling within the compositional field of awaruite; i.e. between Ni2Fe and Ni3Fe (Anthony et al. Citation2005), with minor amounts of dissolved copper and cobalt. A sulfide assemblage in a Platreef sample, consisting mostly of pentlandite (Pn) to which an alloy (A) is attached, as well as a small amount of a dbss phase, is shown in . The stoichiometry of the alloy is Ni2.3Fe and contains minor amounts of copper (1.6 at%) and cobalt (1.7 at%). The size of the observed dbss lamellae is too small for accurate compositional analyses (). However, an approximate stoichiometry of Cu4.20Fe1.22S4 (based on four EDS analyses) was calculated for this phase, which is associated with pyrrhotite (Fe0.92S). In the case of the UG–2, a dbss phase with Cu4.19Fe1.30S4 stoichiometry, in association with pyrrhotite (Fe0.93S, with 1.4 at% Cu and 0.4 at% Co), was observed ().
PGMs were detected in the alloy and sulfide phases of the sample fired at 900°C (). The PGM particles in the sulfide matrix (bright spots, ) are enriched in palladium (30.6 at%), copper (22.3 at%) and lead (19.8 at%), while the alloy (phase A, ) has a stoichiometry of Ni2.3Fe and contains 3.3 at% Pd. Therefore, the collection of PGMs by the Ni–Fe-based alloy takes place at sub-liquidus temperatures of the matte.
Samples heated at 1000°C and 1100 °C
At a reaction temperature of 1000°C, the sulfide assemblages are more spherical and the alloy crystals bigger in size, suggesting that the sulfides were molten at this temperature. The sphericity of the sulfides increased as the firing temperature changed from 1000°C to 1100°C (compare ). The alloy crystals in the UG–2, 1100°C sample are enriched in PGMs, although no PGMs could be detected in the Platreef samples. This is presumably due to the lower PGM content of the Platreef concentrate. At 1100°C, increased contact between the matte-forming assemblages and the gangue constituents of the concentrates was observed, especially in the Platreef concentrate.
Sample heated at 1200°C and 1300°C
The most significant difference that could be observed in the microstructures of both concentrates heated at 1200°C is the sintering of the concentrate bed (Rivera Li Kao and Garbers-Craig Citation2022). Conversely, it can be assumed that all the matte-forming components were in the liquid state, given that the temperature had reached the highest melting point of the pure sulfide constituents, i.e. pyrrhotite, at 1190°C ().
After firing at 1300°C, the low amount of LP formed in the UG–2 concentrate matrix was still insufficient to facilitate the coalescence of matte prills. The sulfides remained mostly entrained within the cavities generated after sintering, thereby forcing the molten sulfides into irregular shapes (). However, the lower liquidus temperature of the gangue components in the Platreef concentrate allowed the formation of more LP in this concentrate at 1300°C, thereby reducing the overall porosity. Small matte droplets could be distinguished in the LP, with larger matte prills mainly associated with pores of irregular shapes (). The higher sulfide mineral content of the Platreef concentrate, compared to the UG–2 concentrate (17.3 vs. 1.3 mass%, Rivera Li Kao and Garbers-Craig Citation2022) and the predicted larger concentrations of liquid silicate in the Platreef concentrate (51.7% for Platreef concentrate and 26.2% for UG–2) promoted the partial separation of matte from gangue at this temperature.
Samples heated at 1400°C and 1480°C (slow-cooled, sealed capsules)
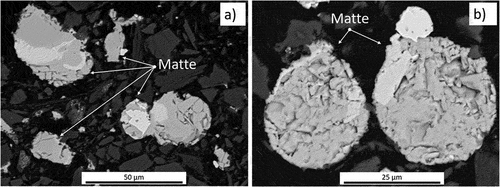
Effective matte-gangue separation could only be observed in Platreef concentrate, due to the high amount of total liquid (liquid silicate + liquid matte) that formed at 1400°C (~91%, ) (). The settling of matte prills through the liquid silicate was almost complete, with only a low concentration of small sulfide droplets (Φ <90 µm) remaining in the slag, even though the gangue components did not exhibit complete melting.
Figure 13. Optical micrograph of platreef concentrate fired at 1400°C, showing the collection of a matte button at the bottom of the crucible (P: pore).
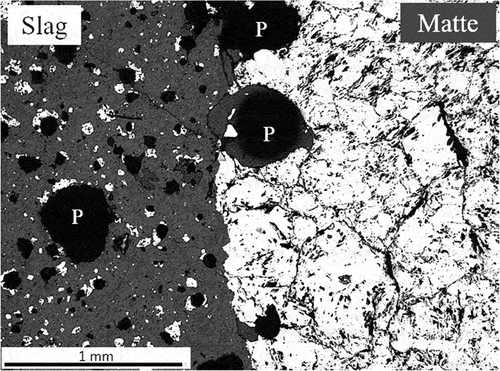
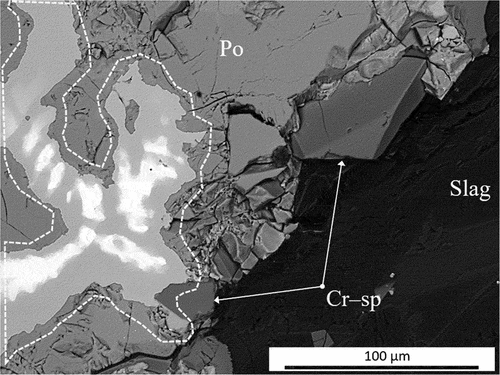
Only after heating UG–2 concentrate at 1480°C was the concentration of the liquid silicate phase sufficient to allow the matte prills to settle and collect at the bottom of the crucible. The matte button consisted of sulfides, PGM-containing alloys and faceted FeCr2O4 spinel crystals at the matte boundary (). The Ni:Fe atomic ratio of the alloy varies between 1.26:1 and 0.87:1, while the bright areas are primarily composed of iron and nickel, but also contains copper, cobalt and PGMs (54.3–55.4 atomic % Fe; 29.9–38.8 atomic % Ni; ~1.3 atomic % Cu; ~1.6 atomic % Co; max 9.2 atomic % Pt; max 1.8 atomic % Ru; max 0.8 atomic % Rh; max 0.6 atomic % Ir and max 0.2 atomic % Pa). The PGM-containing alloy is hosted in a pyrrhotite substrate with a stoichiometry of Fe0.94Ni0.03Cu0.03Co0.01S.
Comparison between slow-cooled (sealed and drilled capsules) and quenched platreef samples (1400 °C)
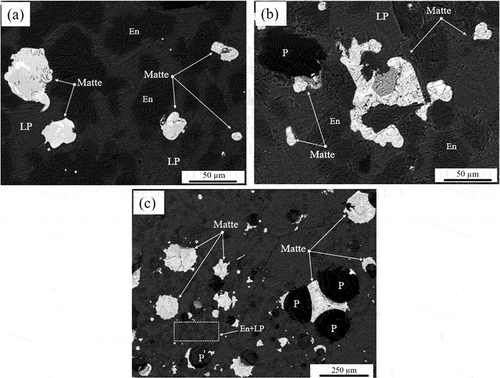
BEI images of the Platreef concentrate fired at 1400°C and slow-cooled (sealed and drilled capsules) versus quenched, are presented in . The microstructures obtained are similar, with matte prills entrained within the LP, matte attachment to pores, and a mixture of enstatite and LP as dominant gangue phases. The quench test, as expected, impacted the microstructures of the gangue phases, exhibiting finer enstatite crystals in the LP matrix compared to the slow-cooled sample (dashed area, .
Figure 15. BEI of the platreef concentrate fired at 1400 °C: (a) slow-cooled, sealed capsule (b) slow-cooled, drilled capsule (c) quenched.
The quantification of the crystalline matte phases in the three tests is presented in . Bornite in the slow-cooled experiments, as well as dbss and alloy in the drilled experiments, was not detected by XRD. However, the SEM–EDS analyses confirmed the coexistence of these phases as matte-forming assemblages. Slow-cooled samples (both sealed and drilled capsules) exhibited the presence of FeCr2O4 spinel crystals at the boundaries of the matte droplets, while the quenched test only contained very small crystals (Φ~10 µm), randomly distributed within the matte prills.
Table 10. Major crystalline phases found in the mattes (1400°C).
Comparison between slow-cooled (sealed and drilled capsules) and quenched platreef samples (1480°C)
Porosity observed microstructurally at lower temperatures virtually disappeared at 1480°C. In both the slow-cooled samples (sealed and drilled capsules), matte was pushed to higher positions in the concentrate bed when columnar enstatite crystals crystallized (, 1480°C). Conversely, the quenched test exhibited clear signs of complete melting. This sample consisted of a glassy slag with a few matte droplets suspended in the liquid silicate phase, and others attached to some remaining pores ().
Figure 16. BEI of the platreef concentrate fired at 1480 °C: (a) slow-cooled, sealed capsule (b) slow-cooled, drilled capsule (c) quenched.
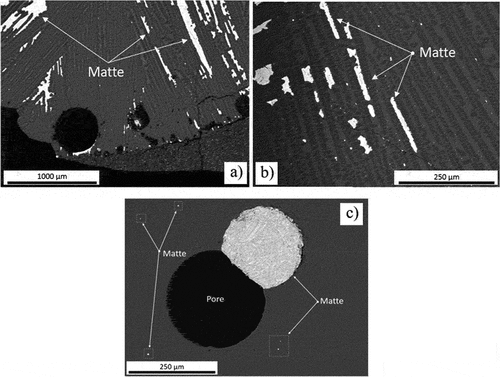
The matte-forming assemblages observed in the 1480°C tests are similar to those found after firing at 1400°C (compare ). Euhedral FeCr2O4 spinel crystals could be detected again on the boundaries of the matte prills in the slow-cooled samples (sealed and drilled capsule), and small spinel crystals (ϕ~10 µm) were homogeneously dispersed in the quenched matte prills.
Table 11. Major crystalline phases found in the mattes (1480°C).
Discussion
The temperature gradient across the BT ranges from 800°C at the BT-freeboard interface to 1500°C at the BT-slag interface. At 800°C, the sulfides in the concentrates have already decomposed, while some of them have already melted. The onset of melting of the sulfides (UG-2 concentrate at 640°C and Platreef concentrate at 648°C) was reflected by the spherification of a few nickel sulfide particles in both concentrates after firing at 900°C. As the temperature increased from 900 to 1100°C and higher, the microstructural analysis displays the complete spherification of the matte-forming assemblages, with the exception of some single pyrrhotite crystals, which have the highest melting point of the sulfides (~1196°C). This is consistent with the FactSage predicted liquidus temperatures of 1000°C for the sulfide assemblages of both the Platreef and UG–2 mattes.
At 1200°C, noticeable sintering of the concentrate bed occurred, which is also the temperature at which all sulfide minerals have either decomposed or melted. These molten sulfide droplets are squeezed into the porosity created by the sintered silicate bed, but matte did not segregate from the gangue. With a further increase in temperature to 1300°C, a decrease in the number of sulfide particles in the Platreef concentrate and the simultaneous increase in their average size is associated with the merging, coalescence and settling of sulfide prills through the silicate liquid phase from the gangue. At this temperature (1300°C), partial matte separation takes place in the Platreef concentrate when 52% liquid silicate is present, forming a continuous path along which the sulfide droplets can settle through gravitation. In contrast, the low liquid silicate content of 26% in the UG-2 concentrate is not sufficient to facilitate coalescence, with the consequence that most of the sulfide droplets remain entrained in the porosity associated with the gangue. At 1400°C, the 88.7% liquid silicate phase present facilitates effective matte–gangue separation in the Platreef concentrate, while this does not occur in the UG–2 concentrate when 75% liquid silicate is present. Matte–gangue separation in the UG–2 concentrate takes place at 1480°C when virtually all the gangue is molten, although a very low concentration of sulfides is present.
PGM collection occurred through the incorporation of PGMs in the Ni–Fe–based solid solution alloy phase, forming PGM-enriched crystals from 900°C up to 1100°C (UG–2 concentrate). In the PGM-bearing sulfides, the majority of PGMs occur in solid solution with pyrrhotite and pentlandite and, to a lesser extent, as Pd antimonides and arsenides in some pyrite, chalcopyrite and millerite (Smith, Holwell, and McDonald Citation2014). Therefore, the complete melting of the PGM-bearing minerals (after firing at 1200°C and above) promotes the liquid-state diffusion of the PGMs in the liquid matte.
The morphology of the sulfides that crystallized after firing is dominated by exsolution textures, which coarsen with increased cooling times. However, the observed resulting phases do not agree well with the thermochemical FactSage predictions, presumably due to equilibrium not being maintained during cooling. FactSage predicted the presence of two FCC alloys, a nickel-rich and a Cu-rich alloy phase, while only a Ni-rich Ni-Fe alloy phase was found in the cooled mattes. Pentlandite was observed in the majority of cooled matte samples, while this phase was not predicted by FactSage. Ni–Fe solid solution alloy particles, pentlandite, iron sulfides (pyrrhotite and troilite) and dbss were detected as major sulfide phases in the crystallized mattes between 800 and 1300°C. This observation is consistent with the literature, which reports the association of pentlandite [(Fe,Ni)9S8] with dbss – pyrrhotite [(Fe,Ni)1-xS] phase assemblages in recrystallized low-temperature Fe-Ni-Cu-S-based mattes (Andrews and den Hoed Citation2011; Du Toit, Cromarty, and Garbers-Craig Citation2016; Eksteen Citation2011; Govender Citation2006; Zhu et al. Citation2021). Exsolution of pentlandite from the monosulfide solid solution phase (mss), which exhibits complete solid solution between the FeS and NiS end – members above 610°C, was also reported by Wang (Citation2006). The coexistence of alloy, pentlandite and pyrrhotite is also common in crystallized industrial mattes from PGM nickel industries (Chen et al. Citation2017; Lamya and Lorenzen Citation2006; Page Citation1982; Snyders et al. Citation2018) and in natural environments (Hudson and Travis Citation1981; Thyse Citation2014).
Conclusions
The phase evolution of sulfides in UG–2 and Platreef concentrates, as well as matte drainage, over a temperature range from 800 to 1480°C was examined on a laboratory scale. The following conclusions can be drawn:
At 800°C, the sulfides in the concentrates have already decomposed, while some of them have already melted.
When sintering of the concentrate bed commences (1200°C), matte droplets are trapped in the porosity of the bed, but do not separate from the gangue.
Coalescence of sulfide particles and droplets takes place after the formation of a considerable amount of liquid silicate: 42.7% (1300°C) for partial separation and 73.3% (1400°C) and 82.6% (1480°C) for complete separation in the Platreef concentrate; 99.73% for complete separation in the UG–2 concentrate at 1480°C.
Matte–gangue separation starts at temperatures above the solidus temperature of the gangue but below its liquidus temperature. The liquid silicate phase that forms acts as a pathway for the matte droplets, enabling them to coalesce and settle through the black top.
Increased temperatures increase the concentration of the liquid silicate phase and decrease its viscosity, thereby further facilitating the coalescence and collection of matte.
Higher concentrations of sulfide minerals in the concentrate enhance the probability of matte prills colliding, merging into larger droplets, and separating from the gangue.
When blending concentrates in a PGM smelter, the solidus temperature of the gangue, the viscosity of the liquid silicate phase that forms, and the total concentration of sulfide minerals in the concentrate will directly impact its effective matte separation.
At temperatures as low as 900°C, PGMs are already collected in the Ni–Fe-based alloy that forms.
Acknowledgments
The authors gratefully thank Anglo American for their financial and technical support, as well as Ms Wiebke Grote and Professor Johan de Villiers their assistance with the XRD analysis. The assistance from the staff of the Centre for Pyrometallurgy, Department of Materials Science and Metallurgical Engineering, University of Pretoria is also greatly acknowledged.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Adams, M., K. Liddell, and T. Holohan. 2011. Hydrometallurgical processing of platreef flotation concentrate. Minerals Engineering 24 (6):545–50. doi:10.1016/j.mineng.2010.09.009.
- Andrews, A., and P. den Hoed. 2011. Mineralogical solutions for pyrometallurgical problems. In Southern African pyrometallurgy 2011, ed. R. T. Jones and P. den Hoed, 199–207. Johannesburg, South Africa: Southern African Institute of Mining and Metallurgy.
- Anthony, W., R. Bideaux, K. Bladh, and M. Nichols. 2005. Handbook of mineralogy. Chantilly, VA: Mineralogical Society of America.
- Bacedoni, M., I. Moreno-Ventas, and G. Ríos. 2020. Copper flash smelting process balance modeling. Metals 10 (9):1–19. doi:10.3390/met10091229.
- Bale, C. W., E. Bélisle, P. Chartrand, S. A. Decterov, G. Eriksson, A. E. Gheribi, K. Hack, I. H. Jung, Y. B. Kang, J. Melançon, et al. 2016. FactSage thermochemical software and databases, 2010–2016. Calphad: Computer Coupling of Phase Diagrams and Thermochemistry 54:35–53. doi:10.1016/j.calphad.2016.05.002.
- Ballhaus, C., M. Tredoux, and A. Späth. 2001. Phase relations in the Fe–Ni–Cu–PGE–S system at magmatic temperature and application to massive sulphide ores of the Sudbury igneous complex. Journal of Petrology 42 (10):1911–26. doi:10.1093/petrology/42.10.1911.
- Barnes, A. R., and A. F. Newall. 2006. Spinel removal from PGM smelting furnaces. In Southern African pyrometallurgy 2006, ed. R. T. Jones, 77–88. Johannesburg, South Africa: South African Institute of Mining and Metallurgy.
- Buchspies, B., L. Thormann, C. Mbohwa, and M. Kaltschmitt. 2017. PGE production in Southern Africa, part II: Environmental aspects. Minerals 7 (11):225. doi:10.3390/min7110225.
- Cawthorn, R. G. 2006. Centenary of the discovery of platinum in the bushveld complex. Platinum Metals Review 50 (3):130–33. doi:10.1595/147106706X119746.
- Charlier, B., O. Namur, R. Latypov, and C. Tegner.2015. Layered intrusions, ed. B. Charlier, O. Namur, R. Latypov, & C. Tegner. Springer Science+Business Media. doi:10.1007/978-94-017-9652-1.
- Chen, G. J., J. M. Gao, M. Zhang, and M. Guo. 2017. Efficient and selective recovery of Ni, Cu, and Co from low-nickel matte via a hydrometallurgical process. International Journal of Minerals, Metallurgy and Materials 24 (3):249–56. doi:10.1007/s12613-017-1402-9.
- Cramer, L. A. 2001. The extractive metallurgy of South Africa’s platinum ores. JOM 53 (10):14–18. doi:10.1007/s11837-001-0048-1.
- Crundwell, F. (F. K. Crundwell, M. S. Moats, V. Ramachandran, T. G. Robinson. 2011. Extractive metallurgy of nickel and cobalt, ed. W. G. Davenport. Elsevier Ltd. https://www.sciencedirect.com/book/9780080968094/extractive-metallurgy-of-nickel-cobalt-and-platinum-group-metals.
- Du Toit, J., R. D. Cromarty, and A. M. Garbers-Craig. 2016. Matte– tap-hole clay – refractory brick interaction in a PGM smelter. The Journal of the Southern African Institute of Mining and Metallurgy 116 (4):339–42. doi:10.17159/2411-9717/2016/v116n4a6.
- Eksteen, J. J. 2011. A mechanistic model to predict matte temperatures during the smelting of UG2-rich blends of platinum group metal concentrates. Minerals Engineering 24 (7):676–87. doi:10.1016/j.mineng.2010.10.017.
- Eksteen, J. J., B. Van Beek, and G. A. Bezuidenhout. 2011. Cracking a hard nut: An overview of Lonmin’s operations directed at smelting of UG2-rich concentrate blends. Journal of the Southern African Institute of Mining and Metallurgy 111 (10):681–90.
- Engelbrecht, J. U. 2012. Potential changes in the physical beneficiation processes that can improve the recovery grade or costs for the platinum group metals. In Proceedings of the Fifth International Platinum Conference: A Catalyst for Change, Sun City, South Africa, September 17–21, 2012, 343–59. Southern African Institute of Mining And Metallurgy.
- Fagerlund, K. O., and H. Jalkanen. 2001. Microscale simulation of settler processes in copper matte smelting. Metallurgical and Materials Transactions B: Process Metallurgy and Materials Processing Science 32 (3):555–57. doi:10.1007/s11663-001-0040-8.
- Gaballah, I., E. Allain, M.-C. Meter-Joly, and K. Malau. 1994. Thermal treatment of complex sulfide ores in N2 and H2 atmospheres: A new approach for the extraction of their valuable elements. Metallurgical and Materials Transactions B: Process Metallurgy and Materials Processing Science 25 (2):193–205. doi:10.1007/BF02665202.
- Govender, S. 2006. Micro PIXE analyses of furnace and converter mattes for the determination of trace element distribution and concentrations. MSc thesis, University of Zululand.
- Guntoro, P. I., A. Jokilaakso, N. Hellstén, and P. Taskinen. 2018. Copper matte - slag reaction sequences and separation processes in matte smelting. Journal of Mining and Metallurgy, Section B: Metallurgy 54 (3):301–11. doi:10.2298/JMMB180214021G.
- Gwimbi, P. 2017. Monitoring SO2 emission trends and residents’ perceived health risks from PGM smelting at Selous metallurgical complex in Zimbabwe. International Journal for Equity in Health 16 (1):1–11. doi:10.1186/s12939-017-0696-6.
- Hudson, D. R., and G. A. Travis. 1981. A native nickel-Heazlewoodite-ferroan trevorite assemblage from Mount Clifford, Western Australia. Economic Geology 76 (6):1686–97. doi:10.2113/gsecongeo.76.6.1686.
- Jones, R. T. 2004. JOM world nonferrous smelter survey, part II: Platinum group metals. JOM 56 (12):59–63. doi:10.1007/s11837-004-0238-8.
- Jones, R. T. 2005. An overview of South African PGM smelting, Nickel and Cobalt 2005: Challenges in extraction and production. In 44th Annual Conference of Metallurgists, Calgary, Canada, August 21–24, 147–78. CIM (Canadian Institue of Mining, Metallurgy and Petroleum).
- Jones, R. T., and I. J. Kotzé. 2004. DC arc smelting of difficult PGM-containing feed materials. In International Platinum Conference ‘Platinum Adding Value’, Sun City, South Africa, 33–36. The Southern African Institute of Mining and Metallurgy.
- Kullerud, G. 1963. Thermal stability of pentlandite. The Canadian Mineralogist 7 (3):353–66.
- Lamya, R. M., and L. Lorenzen. 2006. Atmospheric acid leaching of nickel- copper matte froM impala platinum refineries. The Journal of the South African Institute of Mining and Metallurgy 106:385–96.
- Liddell, K. 2009. 25 Years of UG2 concentrators… Mintek 75 - a celebration of technology conference. Randburg, South Africa: Southern African Institute of Mining and Metallurgy.
- Mabiza, L. 2006. An overview of PGM smelting in zimbabwe - zimplats operations. In Southern African pyrometallurgy 2006, ed. R. T. Jones, 63–75. Johannesburg, South Africa: South African Institute of Mining and Metallurgy.
- Madelung, O., U. Rössler, and M. Schulz, Eds. 2000. Chalcopyrite (CuFeS2) crystal structure, lattice parameters, density, melting point: Datasheet from Landolt-Börnstein - group III condensed matter · volume 41e: “ternary compounds, organic semiconductors”, springermaterials. Berlin Heidelberg: Springer-Verlag. doi:10.1007/10717201_225.
- Mberi, T., L. L. Mguni, and F. Ntuli. 2018. Effect of frother and depressant interaction on flotation of great Dyke PGM ore. Journal of the Southern African Institute of Mining and Metallurgy 118 (1):65–69. doi:10.17159/2411-9717/2018/v118n1a8.
- McLaren, C. H., and J. P. R. De Villiers. 1982. The platinum-group chemistry and mineralogy of the UG-2 chromitite layer of the bushveld complex. Economic Geology 77 (6):1348–66. doi:10.2113/gsecongeo.77.6.1348.
- Mineralogical Society of America. 1974. Short course notes, sulfide mineralogy. Mineralogical Society of America. http://www.minsocam.org/msa/collectors_corner/arc/scn1.htm.
- Muzawazi, C., and J. Petersen. 2015. Heap and tank leaching of copper and nickel from a platreef flotation concentrate using ammoniacal solutions. Canadian Metallurgical Quarterly 54 (3):297–304. doi:10.1179/1879139515y.0000000017.
- Mwase, J. M., J. Petersen, and J. J. Eksteen. 2012. Assessing a two-stage heap leaching process for platreef flotation concentrate. Hydrometallurgy 129–130:74–81. doi:10.1016/j.hydromet.2012.09.007.
- Nell, J. 2004. Melting of platinum group metal concentrates in South Africa. Journal of the South African Institute of Mining and Metallurgy 104 (7):423–28.
- Nelson, L. R., G. A. Georgalli, K. L. Hines, and R. J. Hundermark. 2018. Converter processing of platinum group metals. Mineral Processing & Extractive Metallurgy 128 (1–2):134–59. doi:10.1080/25726641.2018.1506272.
- Nieroda, P., J. Leszczyński, A. Mikuła, K. Mars, M. J. Kruszewski, and A. Koleżyński. 2020. Thermoelectric properties of Cu2S obtained by high temperature synthesis and sintered by IHP method. Ceramics International 46 (16):25460–66. doi:10.1016/j.ceramint.2020.07.016.
- O’Connor, C., and T. Alexandrova. 2021. The geological occurrence, mineralogy and processing by flotation of platinum group minerals (PGMs) in South Africa and Russia. Minerals 11 (1):54. doi:10.3390/min11010054.
- Oberthür, T. 2011. Platinum-group element mineralization of the main sulfide zone, Great Dyke, Zimbabwe. Reviews in economic geology magmatic Ni-Cu and PGE deposits: Geology, geochemistry, and genesis, ed. Li, C. and Ripley, E.M. Society of Economic Geologists 17:329–49. doi:10.5382/Rev.17.12.
- Oberthür, T., T. W. Weiser, and K. Kojonen. 2002. Local variations and regional trends in PGE geochemistry and mineralogy in the main sulfide zone of the great Dyke, Zimbabwe. https://www.researchgate.net/publication/237570915.
- Page, M. L. 1982. A mineralogical study of nickel mattes from the Kalgoorlie nickel smelter, Kalgoorlie, Western Australia. Metallurgical Transactions B 13B (June):141–52. doi:10.1007/BF02664571.
- Prasad, S., and B. D. Pandey. 1999. Thermoanalytical studies on copper-iron sulphides. Journal of Thermal Analysis and Calorimetry 58 (3):625–37. doi:10.1023/A:1010108729034.
- Ramonotsi, M. 2011. Characterisation of the effect of alteration on the PPM platinum ore and evaluation of selected strategies to improve metallurgical performance. MSc thesis, University of Cape Town.
- Rivera Li Kao, O., and A. Garbers-Craig. 2022. Impact of phase evolution in platreef and UG-2 concentrates on matte drainage in the black top of a platinum group metal smelter. Mineral Processing and Extractive Metallurgy Review 43 (3):326–38. doi:10.1080/08827508.2020.1861613.
- Schindelin, J., I. Arganda-Carreras, E. Frise, V. Kaynig, M. Longair, T. Pietzsch, S. Preibisch, C. Rueden, S. Saalfeld, B. Schmid, et al. 2012. Fiji: An open-source platform for biological-image analysis. Nature Methods 9 (7):676–82. doi:10.1038/nmeth.2019.
- Schouwstra, R. P., E. D. Kinloch, and C. A. Lee. 2000. A short geological review of the bushveld complex. Platinum Metals Review 44 (1):33–39. doi:10.1595/003214000X4413339.
- Shaw, A., L. P. V. S. De Villiers, R. J. Hundermark, J. Ndlovu, L. R. Nelson, B. Pieterse, R. Sullivan, N. Voermann, C. Walker, F. Stober, et al. 2013. Challenges and solutions in PGM furnace operation: High matte temperature and copper cooler corrosion. Journal of the Southern African Institute of Mining and Metallurgy 113 (3):251–61.
- Smith, J. W., D. A. Holwell, and I. McDonald. 2014. Precious and base metal geochemistry and mineralogy of the grasvally norite–pyroxenite–anorthosite (GNPA) member, northern bushveld complex, South Africa: Implications for a multistage emplacement. Mineralium Deposita 49 (6):667–92. doi:10.1007/s00126-014-0515-6.
- Snyders, C. A., G. Akdogan, G. Thompson, S. M. Bradshaw, and A. P. Van Wyk. 2018. Investigating the behaviour of PGEs during first-stage leaching of a Ni-Fe- Cu-S converter matte. The Journal of the Southern African Institute of Mining and Metallurgy 118:353–60. doi:10.17159/2411-9717/2018/v118n4a4.
- Stribrny, B., F. W. Wellmer, K. P. Burgath, T. Oberthür, M. Tarkian, and T. Pfeiffer. 2000. Unconventional PGE occurrences and PGE mineralization in the Great Dyke: Metallogenic and economic aspects. Mineralium Deposita 35 (2–3):260–80. doi:10.1007/s001260050019.
- Sugaki, A., and A. Kitakaze. 1998. High form of pentlandite and its thermal stability. American Mineralogist 83 (1–2):133–40. doi:10.2138/am-1998-1-213.
- Thyse, E. L. 2014. Effect of iron endpoint during Peirce-Smith converting on matte mineralogy and downstream processing of base and platinum- group metals. PhD diss., University of Stellenbosch.
- Ulbrich, K. F., and C. E. M. Campos. 2020. Nanocrystalline Ni3S2 prepared by mechanochemistry and its behavior at high temperatures and high pressure. Journal of Magnetism and Magnetic Materials 493:165706. doi:10.1016/j.jmmm.2019.165706.
- Vermaak, M. K. G. 2005. Fundamentals of the flotation behaviour of palladium bismuth tellurides. PhD thesis, University of Pretoria.
- Waldner, P., and A. D. Pelton. 2005. Thermodynamic modelling of the Fe-S system. Journal of Phase Equilibria & Diffusion 26 (1):23–38. doi:10.1361/15477030522455.
- Wang, H. 2006. Isothermal kinetics of the pentlandite exsolution from mss/pyrrhotite using model-free method. Tsinghua Science and Technology 11 (3):368–73. doi:10.1016/S1007-0214(06)70202-4.
- Wilson, M. G. C., and C. R. Anhaeusser. 1998. The mineral resources of South Africa, 6th ed. Silverton, South Africa: Council of Geoscience.
- Xu, H., X. Guo, L. A. Seaman, A. J. Harrison, S. J. Obrey, and K. Page. 2019. Thermal desulfurization of pyrite: An in situ high-T neutron diffraction and DTA-TGA study. Journal of Materials Research 34 (19):3243–53. doi:10.1557/jmr.2019.185.
- Zhu, C., Y. Lei, X. Hu, Q. Xu, X. Zou, H. Cheng, and X. Lu. 2021. Effective removal of barrier layer on the surface of low-nickel matte in an FeCl3-HCl-H2O solution. Minerals 11 (11):1219. doi:10.3390/min11111219.