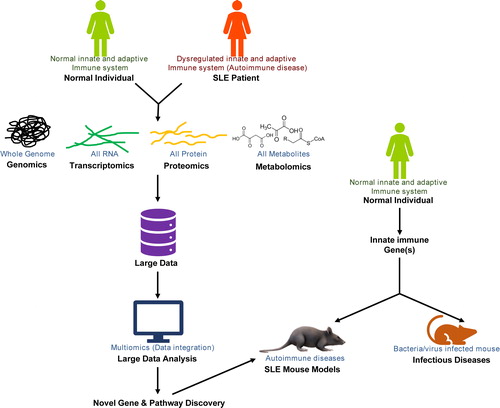
The regulated and dynamic interactions of immune cells and its soluble and cellular factor constitute the host immunity, which manages microbial challenges and maintains homeostasis. The onset of autoimmune diseases is triggered via breaching immune regulatory switches, leading to wide range of immuno-pathogenesis and is influenced by genetic and environmental factors. The immuno-pathogenesis of autoimmune diseases results from a complex interplay of various intra and extra cellular innate and adaptive immune signaling pathways. Most autoimmune diseases are managed through reducing the severity of symptoms using various drugs rather than directly treating the cause of disease. Systemic Lupus Erythematous (SLE) is an inflammatory autoimmune disease caused by a generation of antibodies against self-DNA and nuclear antigen that effects the skin, different blood cell types, and vital organs such as the brain, heart, kidney, and lungs. To understand SLE, this issue of International Reviews of Immunology describes the ex vivo and in vivo tools for the fundamental understanding of SLE that may help in better management of SLE as well as other autoimmune diseases. The issue also discusses the biology of an innate immune molecule and its therapeutic modulation in infectious and noninfectious diseases ().
Technological advancements and a fundamental understanding of major molecules of cells such as protein, nucleic acids, and its modification provide an excellent opportunity to understand the various molecular events of the cells. The investigation of all proteins or transcripts (the messenger RNA) or metabolic products generated by various metabolic pathways is known as proteomics, transcriptomics, or metabolomics, which are respectively and collectively known as multiomics. Integration of mutiomics data by bioinformatics approaches gives a deeper insight of the cellular process in various conditions, including diseased conditions. The first review article in this issue by Song et al. discusses such an approach to understand the basic biology of SLE that is also useful for the discovery of early disease biomarkers or prognostic markers. Additionally, these fundamental studies also provide potential therapeutic targets to control or manage such a complex disease.Citation1 The article will be beneficial to the cellular and molecular immunologist or bioinformatician working with immunologists or disease related fields ().
Cellular level studies provide a closer view on the molecular mechnism of the celluar processes. However, in vitro and ex vivo studies do not always match with physiological conditions due to complex multi-organ level interactions. Therefore, it is important to validate the fundamental studies in physiological conditions similar to humans. In vivo studies in humans are almost impossible due numerous ethical issues; however, conducting studies in small animals such as hamsters, mice, and rats are relatively easy to perform due to the ethical requirements for animals and better physiological understanding in in vivo condition. To gain physiological insight closer to humans for validation of fundamental discoveries, it is imortant to exploit these animal models. The second review in this issue by Halkom et al. discusses various mouse models developed for investigating various aspects of SLE biology. This article also discusses the use of various models for testing potential drug candidates for SLE.Citation2 The article will be interesting to broad readers of immunology and rheumatology or researchers involved in drug discovery for SLE (Figure-1).
The innate immune defense of the host is mediated through families of receptors or sensors known as pattern recognition receptors (PRRs). The cascade of the innate immune signaling pathway triggers through receptor-adaptor-kinases-transcription factors, which result in the transcription of appropriate genes for the effector responses. The cascade of signaling is modulated at various levels by various regulators to modulate the appropriate responses and to ensure pathogen clearance without destabilizing the host homeostasis. The last article in this issue by Dantas et al. discusses the biology of innate molecules known as triggering receptors that are expressed on myeloid cells-1 (TREM-1) and its role in infectious and noninfectious diseases. The article also discusses the extrinsic molecules that modulate the function of TREM-1 and highlight its therapeutic potential.Citation3 This article will be useful to broad readers of innate immunobiology ().
References
- Advances in applying of multi-omics approaches in the research of systemic lupus erythematosus (1736058)
- Contribution of mouse models in our understanding of lupus (1742712)
- Triggering receptor expressed on myeloid cells-1 (TREM-1) as a therapeutic target in infectious and non-infectious disease: a critical review (1762597)