Abstract
Since its first clinical application, 120 years ago, radiotherapy evolved into a major anti-cancer treatment modality, offering high cure rates in many human malignancies. During the past ten years, the establishment of immune checkpoint inhibitors (ICIs) in cancer therapeutics has vigorously reintroduced the immune system’s role in the outcome of radiotherapy and, conversely, the role of radio-vaccination in the efficacy of immunotherapy. The knowledge and clinical experience that founded the current era of immuno-radiotherapy started alongside with the birth of radiotherapy, and evolved through exhaustive experimental work, clinical trials on active specific immunotherapy, frustrating attempts to validate the importance of cytokine administration with radiotherapy, and, finally, the encouraging ICI-based clinical trials that opened the door to a far more encouraging perspective; radio-vaccination, through its old and new methods, is rising as a research field that promises to cure, previously incurable, disease. In this critical review, we focus on the scientific knowledge gathered through more than a century of research on radiotherapy interactions with the immune system. Understanding the origins of this promising therapeutic approach will substantially contribute to developing new immuno-radiotherapy policies in the fight against cancer.
Introduction
Radiotherapy is a major anti-cancer treatment modality that offers high cure rates in the early stages of the disease, either when given as a postoperative adjuvant therapy or as a radical monotherapy offering similar efficacy to surgery and, in addition, organ preservation. Since 1898, when E. Grubbe treated the first breast cancer patient with radiotherapy [Citation1], thus after 120 years of experimental, clinical and technological developments, the cure rates achieved with radiotherapy alone or its combination with cisplatin exceed 80% in early stages of head-neck cancer, prostate and bladder cancer, or even lung tumors treated with stereotactic ablative techniques [Citation2–5]. Radiotherapy combined with chemotherapy is the standard treatment for most locally advanced inoperable tumors like lung cancer, head-neck cancer and cervical cancer, providing cure rates berween 20-60% [Citation6–8]. Proton and heavy ion irradiation is expected to further improve the radiotherapy efficacy in subgroups of tumors with increased intrinsic radioresistance or suffering from profound hypoxia, as particle irradiation produce direct lethal double-strand and cluster damage of the DNA [Citation9].
Despite the profound knowledge accumulated with regard to the molecular biology of the interactions of radiation with cells and tissues, and despite the establishment of radiotherapy as a main treatment modality of cancer, there is a lot yet to understand, discover and anticipate. The nucleo-centric puzzle of radiation-induced cell damage and death, through apoptosis and mitotic catastrophe, is gradually complemented by the encovering of the role of the cytoplasm, subcellular organells, metabolism and the autophagic machinery [Citation10]. Targeting such pathways may further enhance the radio-curability of cancer but, for the time being, such therapeutic tools are clinically unavailable.
During radiotherapy, regression of the irradiated tumor occurs, frequently resulting in radiological disapperence. Alas, remnant micro- or macro-foci of tumors, composed by the most resistant cancer cells, persevere under a, short or long lasting, equilibrium between senescent, quiescent and stem-like cells [Citation11]. Having delivered the maximum tolerable radiation dose, together with chemotherapy or targeting agents, there is nothing left but adhering to the clinical follow-up rules hoping that this balance will shift toward tumor eradication. Unfortunately, most often, this shift occurs toward tumor regeneration, even for patients declared as complete responders. For these patients, as well as those whose tumors never regressed, we can only hope for an as long as possible delay till the activation of tumor growth.
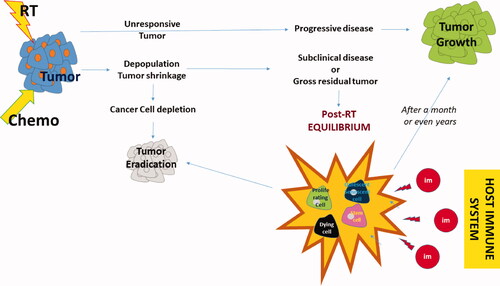
Is there something we can do to avert progression? More recently, we re-discovered the most potent ally, and enemy at the same time, of radiotherapy, along with its struggle to defeat cancer—the body itself. The immune system is gradually emerging as a decisive factor that may sustain residual tumor quiescence, shift the balance toward tumor eradication or allow progression. Activated T-cells and macrophages may eliminate tumors, while in their absence, cancer cells thrive. In fact, the immune tolerance and immune antitumor response have always been present before, during, and after radiotherapy to assist or impede our therapy’s efficacy. The recent therapeutic paradigms of impressive tumor regression after administration of immune checkpoint inhibitors and the eventual cure rates obtained in incurable non-small cell lung carcinomas treated with post-radiochemotherapy immunotherapy [Citation12] opened the door for new immuno-radiotherapy strategies. describes schematically the different outcomes of radiotherapy according to the individual tumor radioresistance, the eventual development of the post-radiotherapy equilibrium and the position of the immune system in shifting the balance between tumor progression and tumor elimination.
Figure 1. Schematic representation of the outcome of radiotherapy. Following the completion of radiotherapy or chemo-radiotherapy, tumors with high radioresistance will show no signs of regression. In a minority of cases, the tumor grows rapidly after (or during) radiotherapy. More radio-sensitive tumors will show signs of regression, even during radiotherapy, a process that continues for a couple of months. Generally, eradication of tumors occurs in about 70-90% of early and 5-40% of locally advanced stages. Persistent disease, either subclinically or clinically, enters a phase of short or long-lasting equilibrium between proliferation and death, senescence, quiescence, and stem-cell activity. The immune system plays an important role in the maintenance of this equilibrium. Overactivation may eradicate the tumor, while the development of immune tolerance (e.g., the emergence of cancer cell clones with immune checkpoint inhibitory molecule overexpression, the prevalence of regulatory immune cells that suppress cancer cell recognition of cytotoxic cells and the gradual built of an immunosuppressive microenvironment) will shift the balance toward tumor regrowth.
In this critical review we tried to retrieve fragments from the past of radiobiology and radiotherapy that heralded the recent developments in immunotherapy. It is important to keep alive the knowledge gathered from the experience of hundreds of studies performed during the past 120 years, pointing to the present and calling for recognition of the researchers that curved the path. In fact, this knowledge could prove invaluable for the steps we are invited to take in the current most promising new era of immuno-radiotherapy.
From ‘some natural means’ to ‘immune checkpoint inhibitors
In 1907, Bashford, Murray, and Crammer, published a key paper on the natural and induced resistance of mice against tumor cell transplantation [Citation13]. This paper summarized many investigators’ experience, focusing on the fact that transplanted tumors fail to grow in certain groups of mice, leading to the hypothesis that some ‘natural means’ protect a refractory group of mice against tumor growth. Besides, an interesting conclusion from the author’s experiments was that mice suffering from spontaneous tumors did not have a greater susceptibility to transplantation of other syngeneic tumors. According to Bashford et al., several parameters were involved in mice’s natural resistance against tumor transplantation. Younger mice, for example, seemed to be more susceptible to transplantation. Another parameter was the ‘tumor-forming dose,’ thus the number of cells necessary for transplantation. Larger doses of cells may result in tumor growth in refractory mice, and in this case, ‘resistance’ is translated in terms of slower tumor growth. ‘Saturation’ of the host protective mechanisms by the large amounts of cancer cells had been, therefore, postulated to explain this finding. The inoculation site also appeared important, as implantation in the axilla was more successful than the dorsal area. The authors explained this as the body area’s ability to provide the connective tissue that is important for tumor growth. Therefore, an important hypothesis was brought forward, that tumor acceptance or rejection can also be related to the host connective tissue (fibroblasts) ‘willingness’ to collaborate and support the engraftment. This brings in mind the current research attempts to clarify the role of the stroma in tumor growth and metastasis [Citation14]. The eventual role of macrophages and phagocytosis, had also been brought forward by these pioneers, although the means available to test this hypothesis were limited at that era.
In 1966, in a study by Mikulska et al., benzopyrene-induced rat fibrosarcoma cells could be successfully transplanted in all syngeneic recipients. However, when the implanted primary tumor was surgically removed, there was a high rate of failure to develop tumors once the rats were re-inoculated with the sarcoma cells [Citation15]. In vitro culturing of sarcoma cells with spleen cells obtained from rats exposed to the tumor, significantly reduced the grafting ability of sarcoma. The study suggested that the host reacts against its tumor after a certain tumor burden has been reached, but this reaction remains strong only when a low amount of cancer cells challenge the host.
In the ‘70s, lymphoblast-like cells capable of destroying cancer cells were identified [Citation16]. At the beginning of the same decade, Suit and Jurin identified the immune system as a main component of tumor regression after radiotherapy [Citation17]. Authors observed that the dose demanded to eradicate an implanted methylcholanthrene-induced synegneic sarcoma in immunocompetent mice was two-fold lower than the one demanded for tumors growing in pre-irradiated immunosuppressed mice. In 1974, A class of T-cells (expressing thymus-derived cell antigens) was recognized as effector lymphocytes, thus able to mediate cytotoxicity [Citation18]. Moreover, the recognition of a thymus-independent lymphocyte subset, the Natural Killer NK-cell, provided a rationale to explain natural antitumor immunity [Citation19]. The levels of NK-cells were positively correlated with in vivo resistance to syngeneic tumor transplants [Citation20]. In 1981, a study by Hassan et al., reported the identification of two distinct cytotoxic cell populations, following inoculation of mice with C.Parvum [Citation21]. One of them, considered activated macrophages, could kill cancer cells that were not susceptible to NK-cell killings. Once injected intraperitoneally in mice, such macrophages provided resistance to transplantation of lymphoma cells. Macrophages were capable of suppressing tumor cell proliferation and are suggested to have a role in tumor rejection [Citation22, Citation23].. In an interesting study by Schirrmacher et al., experimenting with hybrid mice between DBA/2 and B10.D2 strains (the first highly resistant to transplantation of ESb tumors, the second very susceptible), authors concluded that the ability of mice to produce Interferon and cytotoxic lymphocytes is closely linked to natural resistance to tumor cell inoculation [Citation24]. Such studies provided the basis for recognizing the immune system as the tool of natural or inducible antitumor immunity.
The discovery of interferons in 1957 opened the field for the unveiling of a group of molecules, cytokines, and growth factors, that mediate immune cell communication and activities [Citation25]. Leukocytes, fibroblasts and, also, cancer cells were soon shown to be the source of interferons (IFNs) [Citation26, Citation27]. Experimental studies subsequently showed that IFNs have antitumor properties [Citation28, Citation29], opening the field for clinical application of purified and recombinant IFNs in Oncology [Citation30–32]. The biochemical separation of Interleukin-2 (IL-2) [Citation33] and experimental studies that followed, revealed the potent therapeutic potential of this lymphokine that activates proliferation and killing activity of cytotoxic T-cells [Citation34]. Following randomized clinical trials, IFN-α was approved for the treatment of melanomas and lymphomas, and IL-2 for the treatment of renal cancer [Citation35, Citation36]. However, the overall benefit from cytokine therapy of solid tumors was far smaller than anticipated, and the frustration from immunotherapy attempts was evident in the ‘90s. The interest in tumor immunotherapy, however, had never been ceased, and this was recompensed by the encouraging results obtained during the past ten years from clinical trials with novel immune checkpoint inhibitors, and their approval for clinical use [Citation37].
Immunization studies against tumor transplants using non-tumor tissues
In 1935, MacDowell et al. reported a study on C58 mice that are 100% susceptible to transplantation of leukemia cells [Citation38]. Intraperitoneal injection of embryonic tissue from another strain of mice (StoLi), a week before inoculation of leukemic cells, induced resistance in all treated mice. In contrast, transplantation of embryonic tissue from homologous C58 strain, failed to induce any resistance. Injection of embryonic tissue from hybrid C58/StoLi mice, also induced resistance. Ernest Sturm subsequently reported similar results [Citation39]. In a series of experiments in Wistar rats, he showed that embryonic tissue and defibrinated blood (blood treated to denature fibrinogen without causing cell lysis) from the same strain or from Hooded rats, prevented the transplantation of leukemia cells (being previously induced by dibenzanthracene in a Wistar rat).
These studies confirmed the early reports by Bashford et al. [Citation13], where injection of 0.5 ml of defibrinated normal mouse blood induced resistance to tumor transplantation, even in young mice. In contrast, plasma deprived of blood cells was not able to induce resistance. This effect has been thought to resemble the spontaneous regression of choriocarcinomas, that being of hemorrhagic character induce tumor rejection on behalf of the host. Blood from other species was not able to induce a refractory state in mice. The authors also make a critical comment that although it is possible to generate mice resistance to tumor transplantation with various approaches, there is no obvious way to induce tumor rejection once the tumor grows.
In a study in Wistar rats, Murphy and Sturm implanted adrenal glands from another rat of the same strain and found a 57% reduction of susceptibility to inoculated leukemia [Citation40]. In an additional study, the above authors observed that defibrinated blood immunized rats against leukemia transplantation, but adrenalectomy reduced this immunization effect [Citation41]. The endocrine balance was therefore suggested as a component of the immune resistance against malignancy. This brings in mind the frequent development of adrenal metastasis in human carcinomas like lung cancer and triggers thoughts on an eventual teleology of this tumor behavior aiming to suppress the host resistance further.
Infusion of cancer patients with lymph node cells from other species or heterologous leukocytes has also been reported. In 1978, Symes et al. treated 24 patients with recurrent bladder cancer after radiotherapy, with intra-arterial injection of lymph node cells from pigs previously immunized by cancer cells. A remission of the disease was noted in 11/24 patients, and 3/11 remained in remission for one year [Citation42].
Immunization studies against tumor transplants using tumor material
In 1905, Clowes and Baeslack reported that mice recovering from spontaneously regressing tumors were resistant to re-inoculation of cancer cells, supporting the concept that immune mechanisms may interfere cancer growth [Citation43]. Subsequent experimental studies suggested that effective activation of anti-cancer immunity is feasible by repeated inoculation of small tumor doses (unable to form tumors) [Citation44, Citation45]. Foley EJ published a study in 1953 on the ability of methylcholanthrene-induced sarcomas to grow on rats [Citation46]. Sarcomas could easily and rapidly grow in healthy rats or rats previously transplanted with the tumor. When, however, the implanted sarcoma regressed after ligation, mice became immune to subsequent transplantation, as early as two days after ligation. Similar results were also reported by Lewis and Aptekman [Citation47]. Other early in vitro studies with various tumor types including, gastrointestinal and lung carcinomas, heptomas and sarcomas) also showed that neoplastic cells stimulate increased synthesis of DNA in lymphocytes [Citation48, Citation49], but serum factors can abolish this effect in vivo [Citation50].
In the 70’s, several attempts had been made to enhance the immunogenicity of syngeneic cancer cell transplantation, including injection of subthreshold doses of undamaged living cancer cells or tumor cells pretreated with vibrio cholera neuraminidase [Citation51]. It had been postulated that neuraminidase removes the sialic acid from the surface of tumor cells by breaking its 2-8-glucosidic linkages with mucopolysaccharides of the cell membrane, enhancing the presentation of cancer cell antigens to immune cells [Citation52, Citation53]. The intra-lymphatic administration of cancer cells gained attention, as it was thought that direct injection of cancer cells into the nodes where lymphocytes reside waiting for their activation would be a better way to immunize hosts. In 1976, Juillard et al. reported a study on the intra-lymphatic infusion of irradiated with 10 Gy and preincubated with neuraminidase tumor cells from a poorly differentiated lymphocytic lymphoma growing on dogs [Citation54]. The involved nodes showed a rapid decrease in their size that continued for about two months before starting re-growing, while a second wave of reduction was noted some weeks later.
The era of active specific immunotherapy
The above experiments opened the field of clinical experimentation with autologous cancer cell inoculation in patients (). It has been undoubtedly unethical to re-introduce cancer cells in cancer patients unless these had been rendered harmless. Heavy ex-vivo irradiation of cancer cells guaranteed cancer cells’ growth arrest and precluded any regrowth in the human body. Such attempts to immunize cancer patients against their tumors by injecting neutralized irradiated cancer cells from the tumors go back to the early 20’s, where Kelock et al. reported a ‘phase I’ study on 12 breast cancer patients, showing no side effects from the procedure [Citation55]. This type of immunotherapy is generally referred to as ‘Active Specific Immunotherapy’ (ASI). Anderson et al. published an interesting clinical study of ASI, in 1973 [Citation56]. Two groups of breast cancer patients treated with mastectomy and postoperative radiotherapy were enrolled. The experimental group comprised women who had also received an autograft of irradiated cancer cells, immediately after surgery. Lymphocytes from patients that had received the autograft were more effective in killing cancer cells in vitro. Six years after therapy, 63% of patients were alive in the autograft group vs. 30% in control [Citation57]. In 1977, Laucius et al. published a pilot study on 18 patients with metastatic melanoma, who were treated intradermally with irradiated autologous tumor cells and BCG, reporting a 22% rate of short-lasting objective remissions, mainly concerning patients with low tumor burden [Citation58]. This, however, was not confirmed in a study by Hadley et al., in 16 melanoma patients who, however, had received chemotherapy before immunotherapy [Citation59]. In a pilot study on ten patients with advanced ovarian cancer, Hudson et al. reported significant improvement of the 2-year survival when patients received irradiated cryopreserved allogeneic tumor cells and BCG [Citation60].
Table 1. Randomized trials of active specific immunotherapy.
In 1978, following the encouraging results obtained in his previous experimental studies, Juillard et al. reported a phase I study in 19 patients with advanced malignancies treated with an intra-lymphatic injection of autochthonous or allogeneic irradiated tumor cells. Objective regression of tumors was noted in 5/19 patients [Citation61]. In the year that followed, Stack et al reported a study on 52 patients with operable bronchial cancer, who were treated with an intradermal injection of irradiated autologous cancer cells and BCG, on the day of operation [Citation62]. Although the authors did not find any important survival benefit, postoperative immunological depression had been overcome, as the postoperative counts of T-cells were maintained in the immunotherapy group, while these were significantly reduced in the controls. A large number of phase II trials followed in the ‘80s, showing clinical responses or disease stabilization in 5-15% of patients, enhancement of immunity against autologous tumor-associated antigens, and/or prolonged survival, in a variety of tumor types, including gliomas, melanomas, colorectal and lung carcinomas [Citation63–76]
This large number of phase I/II studies confirming a response rate up to 20%, including complete responses, was followed by randomized trials. In a randomized study on 120 patients with renal cancer, ASI with autologous irradiated cancer cells and BCG, failed to show a benefit [Citation77]. Another randomized study by Stack et al on 84 operable lung cancer patients suggested a beneficial effect [Citation78]. Vermorken et al. reported a randomized study on 254 patients with stage II/III colon cancer treated with ASI with autologous cancer cells and BCG, showing a significantly better progression-free interval, especially for stage II disease patients [Citation79]. An Eastern Co-operative Oncology Group randomized study was published one year later, based on 412 patients with stage II/III colorectal cancer, treated with postoperative intradermal injections of irradiated cancer cells and BCG. After 7.6 years of median follow-up, a trend toward better disease-free survival (p = 0.07) was noted, especially in subgroups of patients who developed delayed cutaneous hypersensitivity after the third vaccination. The 5-year survival was 84.6% in patients who developed an induration larger than 10 mm vs. 45% in the rest of patients [Citation80].
Interesting results have also been obtained in patients with hematological malignancies treated with ASI. In 1976, Bakesi et al reported an interesting clinical study in patients with myelocytic leukemia [Citation81]. Following experimental studies in mice, where neuraminidase pretreated leukemia cells induced strong immunity and neutralized tumorigenicity, authors treated a group of patients with myelocytic leukemia, with neuraminidase treated allogeneic myeloblasts, once they had achieved complete remission with chemotherapy. Patients remained in remission for 79-132 weeks vs. 19 weeks of patients treated with chemotherapy alone. Powles et al. treated 52 patients with myelogenous leukemia with chemotherapy, while 30 of them also received immunotherapy with irradiated allogeneic myeloblastic leukemia cells. The medium survival was increased from 39 weeks to 74 weeks [Citation82].
Ohno et al. randomized 73 patients with acute myelogenous leukemia who achieved remission with chemotherapy to receive maintenance chemotherapy with or without immunotherapy with irradiated allogeneic AML cells and Nocardi-rubra cell-wall skeleton [Citation83]. A marginally significant better relapse-free survival was noted. In an EORTC randomized trial, no significant beneficial effect on disease-free survival was noted for the group of AML patients who received immunotherapy with irradiated blasts, although these patients seemed to have higher response rates to re-induction of chemotherapy after relapse [Citation84]. In a randomized study, on 64 patients with myelogenous leukemia who entered complete remission with chemotherapy, the administration of neuraminidase-treated allogenic blasts improved the median relapse-free survival from 15 to 40 months [Citation85].
ASI in combination with cytokines
The introduction in the clinical practice of IFNs and IL-2 in the 90 s triggered studies combining this immune stimulatory agents with ASI. Kirchner et al. reported a study on 208 patients with locally advanced renal cancer treated with surgery followed by injection of autologous irradiated cancer cells, that had been modified with Newcastle disease virus (NDV), in combination with low-dose rIL2 and IFNα [Citation86]. Relapse rates were half the ones expected from historical controls (9% vs. 18%). In a study by Pomer et al., 40 patients with metastatic renal carcinoma were treated with NDV-infected irradiated autologous cancer cells, concurrently with injections of rIL2 or rIFN2b/rIFNa [Citation87]. Complete and partial response rates were obtained in 5/40 and 6/40 patients, respectively, with a median survival exceeding 48 months compared to 31 months in non-responders. In 2014 Dillman et al. reported a retrospective study on 149 melanoma patients receiving IL-2 therapy, 32 of which had also received ASI. Patients in the ASI group had a significantly better median survival (39 vs. 12 months) [Citation88]. In 2017 Mordoh et al. published a phase II randomized study on a vaccine created by heavy irradiation of four melanoma cell lines. Cells were injected to 20 patients together with BCG, while rhGM-CSF was administered the days after vaccination into the vaccination site. The control group consisted of 11 patients receiving IFNa2b. The distant metastasis-free survival at 2 years was 72.8% in the ASI group vs. 27.2% in the IFN group [Citation89].
Although the published studies on ASI and its combination with cytokines were quite encouraging, the interest in such therapeutic approaches regressed under the impressive efficacy of immune checkpoint inhibitors that almost monopolized the interest of the researchers. Nevertheless, as we gradually start to understand the limitations of ICI-therapy it is anticipated that ASI and cytokines will be gradually re-embraced in future ICI-trials
Engineered cytokine-secreting or hybrid cells
Irradiated autologous melanoma cells, adenovirally transduced with GM-CSF gene, were administered in 9 patients [Citation90]. Reduction of pulmonary metastasis was confirmed in 1/9 patients. In 2004, Tani et al. published a phase I study of vaccination with irradiated retrovirally GM-CSF transduced autologous renal cancer cells, reporting enhanced antitumor immune responses and prolonged survival [Citation91]. Buchner et al. reported a study on 15 patients with metastatic renal cancer treated with vaccines formed by irradiated allogeneic RCC-26 cancer cells engineered to express CD80 and IL-2. Althouhg tumor regression was not observed the median progression free survival was 5.3 month, implying an important delay in tumor progression [Citation92]. In 2012 a phase II study was published on 60 patients with resected pancreatic cancer, receiving a vaccine composed of irradiated allogeneic cancer cells engineered to secrete GM-CSF. Patients also received 5FU and radiotherapy after immunization, and immunotherapy continued after radio-chemotherapy. The median disease-free survival obtained was 17.3 months, which is longer than the median values reported in series of patients receiving chemotherapy alone [Citation93].
The idea of fuzing irradiated cancer cells with dendritic cells or lymphocytes became also appealing in the ‘90s. Kugler et al. published a study on 11 patients with metastatic renal cancer treated with irradiated allogeneic renal cancer cells fused with unmatched activated allogeneic lymphocytes. An initial response was noted with 6/11 patients, with two complete and two partial responses [Citation94]. In 2002 Krause et al. generated autologous monocyte-derived dendritic cells and fused them with irradiated autologous melanoma cells, following incubation with polyethylene glycol. One patient out of 17 responded to therapy, and one achieved disease stabilization [Citation95]. In 2005, Homma et al. published a study of cancer patients’ vaccination with hybrid cells following fusion of irradiated cancer cells with autologous dendritic cells. Patients also received IL-12 injection at the same site of vaccination. Partial response was noted in 3/12 patients with brain tumors [Citation96]. In 2006, Wei et al. treated metastatic melanoma patients with fused dendritic cells/irradiated autologous cancer cells and low dose IL-2. A complete response was noted in 1//9 patients, and one additional patient experienced a mixed response [Citation97].
The era of cytokines
The second era of clinical immunotherapy focused on the introduction in the clinical practice of cytokines that would stimulate cytotoxic T-cell and monocyte antitumor activity. This endeavor, however, failed to produce the anticipated benefit for cancer patients for three main reasons. First, enhancement of dendritic and cytotoxic cell function could not be clinically substantiated, as cancer cells exploit immune checkpoint inhibitory pathways and microenvironmental conditions minimize the intratumoral proliferation and activity of such immune cells [Citation98]. Second, the recombinant cytokines introduced affect a broad spectrum of immune cells, enhancing also regulatory immune cells that block antitumor effectiveness [Citation99]. Third, by combining cytokines with radiotherapy, the immune suppressive effects of the latter may have masked the cytokine efficacy [Citation100].
The ‘cytokine era’ started in 1986 with the approval of IFNα for the treatment of hairy cell leukemia [Citation101]. IFNα was subsequently approved for the treatment of malignant melanoma, follicular lymphoma, and AIDs-related Kaposi sarcoma. In 1992, IL-2, another important cytokine, was approved for the treatment of renal cell cancer [Citation35]. In the context of granulocyte stimulation as support to chemotherapy therapy, GM-CSF (granulocyte-macrophage colony stimulating factor) became available in clinical practice [Citation102]. Further to its efficacy as a neutrophil stimulator, GM-CSF has important immunostimulatory effects that did not focus proper attention at that era [Citation103]. In the modern era of immunotherapy, all these cytokines are under reevaluation, as the interferon response pathways are critical for dendritic cell and cytotoxic lymphocyte activation and regulatory T-cell suppression [Citation104].
Phase I trials investigating the combination of interferons with radiotherapy appeared in 1986 [Citation105]. A randomized phase II study by Farkkila et al. in 1994 showed that intratumoral injection of IFNγ in patients with glioblastoma receiving postoperative 60 Gy of radiotherapy had no beneficial effects [Citation106]. In 2001 ECOG published a large randomized trial on 383 patients with brain tumor patients treated with chemotherapy with or without IFNα after radiotherapy [Citation107]. IFN-α did not improve the time to disease progression or survival of patients.
In 1997, a randomized trial in patients treated with chemotherapy and radiotherapy for small-cell lung cancer showed that complete resonders treated with maintenance IFN-alpha-2b had a by 5-month longer time to progression. Still, due to the small number of cases, the difference was not statistically significant [Citation108]. A similar EORTC trial, using IFNγ as maintenance therapy failed to show any benefit [Citation109].
A phase II trial in 61 patients with advanced oropharyngeal cancer treated with three cycles of induction chemotherapy with IFN-α2b followed by chemo-radiotherapy reported higher than expected cure rates and organ preservation [Citation110]. A randomized trial of postoperative and post-RT chemotherapy with or without IFN-α2b in patients with rectal cancer showed, however, no difference in efficacy and increased toxicity in the ΙFN arm [Citation111]. A randomized trial conducted in 132 patients with pancreatic cancer treated with postoperative radiotherapy combined with chemotherapy with or without IFN-α2b failed to reveal any benefit [Citation112]. In 2016 a randomized phase II trial in 209 patients with stage III cervical cancer, treated with concurrent radio-chemotherapy with IFN-α2b and retinoic acid showed inferior results compared to the group of patients receiving weekly cisplatin [Citation113].
The role of IL-2 in combination with radiotherapy has been poorly investigated. In 1997, Kimura et al. reported a randomized trial on adjuvant, post chemo-radiotherapy, immunotherapy in 174 patients with locally advanced surgically treated patients with lung cancer [Citation114]. Immunotherapy consisted of IL-2 and lymphokine-activated cell administration. No difference in survival was noted. De Stefani et al. published a randomized trial in 2002 in 202 patients with oral/oropharyngeal cancer, treated with IL-2 before surgery and radiotherapy, reporting significantly improved the 5-year overall survival rates (55% in the control group vs. 73% in the IL-2 group) and the 5-year disease free survival rates (51% vs. 64%) [Citation115]. A randomized trial in patients with non-Hodgkins lymphoma treated with Total Body Irradiation and chemotherapy, followed by maintenance IL-2 administration, did not show any survival benefit [Citation116]. An interesting recent randomized phase II trial in 44 patients with metastatic melanoma showed that IL-2 combined with stereotactic body irradiation increased the rate of objective responses (21% complet and 33% partial responses) compared to patients who received IL-2 alone (15% complete and 20% partial responses) [Citation117].
The role of GM-CSF as an immunostimulatory adjunct to radiotherapy is equally poorly investigated. GM-CSF administration for two injections every cycle of chemotherapy for patients with limited-stage small-cell lung cancer did not improve survival [Citation118]. summarizes the randomized clinical trials on radiotherapy combination with cytokines.
Table 2. Randomized trials on combinations of radiotherapy with cytokines.
The era of immune checkpoint inhibitors
Arter two decades of justified frustration spread from clinical studies with cytokines, the era of immune-checkpoint inhibitors rose in 2011 and 2015, with the approval of anti-CTLA4 and anti-PD-1 monoclonal antibody therapy, respectively, for the treatment of melanoma. Today, immune checkpoint inhibition directly challenges the position of chemotherapy as first-line therapy for metastatic disease in many human tumors.
Following a large body of experimental data, the concept of radio-vaccination has been introduced and drives the new generation of cancer immuno-radiotherapy clinical trials. The irradiated cancer cell suffers a multitude of functional changes that drastically transform its immunogenic properties. Activation of the interferon type-I response pathway in tumors receiving large radiotherapy fractions is a dominant biological response that transforms cancer cells to a potent vaccine [Citation119]. Restoration of HLA-class-I molecule expression, up-regulation of immune checkpoint molecules, the release of chemokines, and activation of dendritic cells are among the pathways involved in the radio-vaccination process [Citation120].
A large number of case reports in the literature support the enhancement of immunotherapy results and abscopal effects in patients also receiving radiotherapy [Citation121]. Grimaldi et al. treated with radiotherapy 21 patients with metastatic melanoma that had progressed during immunotherapy with ipilimumab (anti-CTLA4 MoAb) [Citation122]. Abscopal responses were noted in 52% of patients. In addition, clinical experience suggests that immunotherapy after the failure of radiotherapy to control the local disease may lead to a complete response of the tumor [Citation123]. This latter effect has been substantiated in the randomized PACIFIC study in patients with stage III non-small-cell lung cancer. Post-RT immunotherapy with anti-PD-L1 antibodies after incomplete remission to radio-chemotherapy offers an important survival advantage. Although mature data on the overall survival of patients are still awaited, this study resulted in the first-ever approval of an immunotherapeutic agent combined with radiotherapy, to enhance immunogenic cancer cell death [Citation124]. Nevertheless, the efficacy of ICIs is quite high in stage IV disease and randomized trials in stage III comparing immunotherapy to immuno-radiotherapy are demanded to confirm superiority in terms of overall survival. Moreover, the excellent results from the ADAURA trial [Citation125], showing an important increase of disease-free survival of patients with early stage operable EGFR-mutation-positive non-small cell lung cancer (NSCLC) receiving postoperative treatment with osimetrinib, urges trials to define the position of radiotherapy, immunotherapy and tyrosine-kinase inhibitors in the treatment of non-metastatic carcinomas.
The enhancement of immunotherapy efficacy through radio-vaccination has been studied in several randomized trials. A recent trial in 799 castration-resistant prostate cancer patients, pretreated with docetaxel, showed that addition of a single dose of radiotherapy (to one or more metastatic sites) to ipilimumab immunotherapy provided 2-3 fold increased overall survival rates than patients receiving ipilimumab only [Citation126]. The PEMBRO-RT randomized phase 2 trial showed that pembrolizumab (anti-PD1 MoAb) after administering 3 fractions of 8 Gy to a tumor site in patients with metastatic NSCLC resulted in a doubling of the response rates and median survival [Citation127]. The unexpected finding that RT enhanced the activity of pembrolizumab in PD-L1 negative patients demands thorough investigation. Abrogation of PD-L1+ regulatory cells in the periphery by pembrolizumab, combined with the unmasking of tumor cell recognition by radio-vaccination, may produce a clinical meaningful response even in PD-L1 negative tumors. A randomized trial in metastatic squamous cell head-neck cancer patients receiving nivolumab (anti-PD1 MoAb), however, reported no significant difference in terms of overall survival rates or abscopal effects in patients receiving stereotactic radiotherapy (3 fractions of 9 Gy) to one lesion [Citation128].
There are, however, available data that does not confirm a benefit from ICI combination with conventionally fractionated radiotherapy. Concurrent chemo-radiotherapy with avelumab in locally advanced head-neck cancer was no superior to chemo-radiotherapy alone, in an early report of the large JAVELIN randomized trial [Citation129]. Similarly, preliminary analysis of the GORTEC 2015-01 trial in patients with locally advanced head neck cancer randomized to receive pembrolizumab vs. cetuximab (anti-EGFR MoAb) combination with radiotherapy showed no significant difference in progression-free survival although immunotherapy had a better toxicity profile [Citation130]. The study of toxicities of RT combination with immunotherapy remains a main point in the on-going trials. For the time being, however, none of the published studies have provided evidence of unacceptable toxicities, and the GORTEC trial showed a better tolerance profile compared to chemo-radiotherapy [Citation130]. The JAVELIN trial reported more frequent grade 3 adverse events when immunotherapy was added to chemo-radiotherapy [Citation129], but there was no investigational arm with immuno-radiotherapy alone. Nevertheless, despite the lack of benefit when ICIs are combined with a low dose per fraction of radiotherapy, the radio-sensitization conferred appears similar to the one offered by chemotherapy. summarizes published randomized trials on combinations of radiotherapy with immune checkpoint inhibitors.
Table 3. Randomized trials on the combination of immune checkpoint inhibitors with RT.
Looking into the future
The impressive efficacy of ICIs confirmed that the human body can eradicate cancer if immune surveillance is restored. There are several directions we have to follow to achieve this endeavor. Regulatory pathways that compromise cytotoxic T-cells and monocytes should be suppressed. This is achieved by targeting immune checkpoint inhibitory molecules expressed by cancer cells, which can be effectively performed by the already available and forthcoming ICIs.
However, of equal importance is the targeting of regulatory T-cells and monocytes in the blood, bone marrow, and lymphocytes. It is imperative to realize that immuno-suppression is not a local, tumor restricted phenomenon but a generalized body status [Citation131]. Profiling and monitoring the regulatory/cytotoxic cell repertoire of patients before and during therapy can unveil targets for therapeutic interventions that would unleash immune surveillance at the write time-point of treatment. Large radiotherapy fractions can achieve enhancement of recognition of cancer cells by forcing the expression of cancer antigens (e.g., HLA-class-I antigen presentation). Radio-vaccination with ex vivo irradiation of autologous cancer cells, eventually engineered to secrete chemoattractant and activating cytokines, should be a new generation of ASI reevaluated in a combination of ICIs and local tumor radiotherapy. The immunosuppressive effects of radiotherapy and, especially, the destruction of irradiated regional lymph nodes should be strongly considered in developing immune-radiotherapy protocols. Intra-lymphatic ASI targeting lymph nodes outside the radiotherapy portals may restore the radio-ablation of regional nodes.
Cytokines specific for cytotoxic T-cell activation are awaited to enhance the radio-vaccination effect and promote tumor eradication. Given the profound lymphotoxic effect of radiotherapy, leukapheresis or specific T-cell collection before the onset of radiotherapy, ex vivo activation with cytokines and re-injection after the end of therapy is also expected to have an important role. CAR T-cell (chimeric antigen receptor Tcell) therapy is emerging as an important tool in immune-radiotherapy [Citation132].
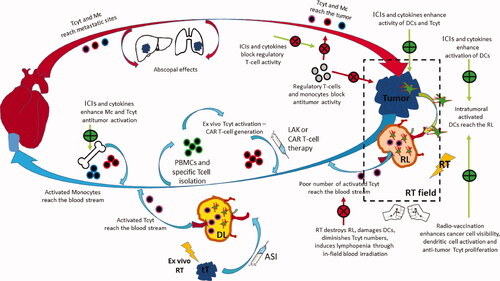
summarizes the ‘circle’ of the anti-tumor immune response and highlights the key interventions developed in the past and in the present to enhance tumor elimination by exploiting orthologically the radiovaccination effect.
Figure 2. The ‘circle’ of antitumor immune response: Tumor cells grow and dendritic cells have a poor ability to recognize them due to lack of antigenic presentation or inhibitory immune checkpoint molecule expression. RT damage induces radiovaccination, thus enhanced expression and release of tumor specific peptides, chemokine chemoatractive molecules and cytokines activating DCs and Tcells. Primed DCs carrying tumor antigens leave the tumor through peripheral lymphatic vessels and reach the RL. There these activate tumor specific Tcyt that leave the node through the central vein to reach the venous circulation. RT destroys DCs and Tcyt and the overall lymph node structure minimizing the efficacy of the radio-vaccination effect. Moreover, RT kills lymphocytes circulating in the normal tissues included in the RT fields, inducing strong lymphopenia and, further minimizing the radiovaccination effect. Extraction of autologous cancer cells, transduction to secrete cytokines and ex-vivo irradiation build a strong vaccine that can be injected in the efferent lymphatics of DLs, outside the RT portals. ASI reproduces the radiovaccination effect in undamaged lymph nodes that replace the radio-suppressed immune function of damaged RLs. Extraction of Tcells, activation with cytokines or development CAR-Tcells and re-infusion in the venous stream further boost the anti tumor immune cascade. ICIs or cytokines may shft the monocytic popolulation toward anti-tumor phenotypes that leave the bone-marrow to enrich the venous stream. Anti-tumor lymphocytes and monocytes reach metastatic sites to exert abscopal tumoricidal activity, controlling the tumor outside the RT portals. These cell will also reach the primary tumor shifting the post-RT equilibrium toward tumor eradication. ICIs further unmask cancer cells, block regulatory immune cells and allow killer cell activity. Cytokines that specifically prime cytotoxic activity and block regulatory activity may further assist the immunological elimination of the disease. (Green curved arrow: lymphatic circulation; red curved arrow: arterial circulation; blue curved arrow: venous circulation; green straight arrow: enhancement; red straight arrow: inhibition; green +: promotion; red X: blockage; RT = radiotherapy; RL = regional lymph node; DL = distal out-field lymph node; tT = transduction of Tcells; ASI = active specific immunotherapy; Tcyt = cytotoxic Tcell; DC = dendritic cell; LAK = lymphokine activated killer cells; CAR Tcell = chimeric antigen receptor Tcell; ICI = immune checkpoint inhibitors).
Conclusions
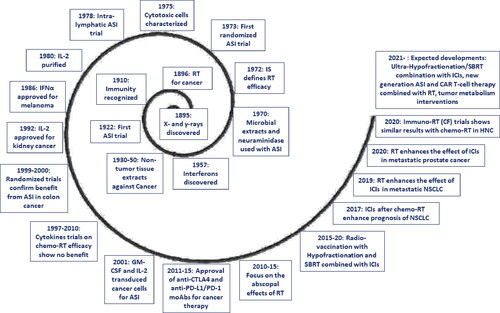
The ability of the human body, through its immune system, to recognize and eliminate cancer cells and to eradicate irradiated tumors has been well known since the first decade of the 20th century. The question raised at that era regarding the therapeutic interventions demanded to restore the body’s immune rejection mechanisms in patients with cancer remains largely unanswered. summarizes the key-stone events that curve the long path of immuno-radiotherapy. Vaccination with irradiated cancer cells, the so-called Active Specific Immunotherapy, proved effective. The positive published clinical data are strongly supported by experimental studies showing the radio-vaccination effects of radiotherapy. Cytokines have undoubtedly a role in the activation of cytotoxic T-cells and macrophages that contribute to the rejection of irradiated tumors, although clinical trials conducted in the ‘90s failed to reveal their potential. More recently, we realized that immune checkpoint molecules expressed by cancer cells or regulatory immune cells are the main obstacle that compromised the efficacy of radio-vaccination and its combination with cytokines. In the new era of immune checkpoint inhibitors, it is anticipated that relevant agents will unmask the potential of active specific immunotherapy policies and the benefit from old and novel awaited cytokines. The most appropriate way to combine old, new, and forthcoming immunotherapy agents with radiation remains elusive. The design of clinical research protocols demands profound knowledge of the biology of immune response and the radio-vaccination potency of different radiotherapy schedules. Knowledge of the history and experience obtained after a century of clinical attempts and the multitude of immuno-vaccination and other procedures devised will certainly contribute to the better design of the future of the most promising immuno-radiotherapy field of research. The passion expressed by the hundreds of researchers that built the foundations of our current status allowing us to hopefully gaze into the future should stand out as exemplary to inspire our efforts to cure cancer.
Figure 3. Key-stone events in the development of immuno-radiotherapy, during the long 125 years since the discovery of ionizing radiation (RT = radiotherapy; IS = immune system; ASI = active specific immunotherapy; moAB = monoclonal antibody; ICI = immune checkpoint inhibitors; NSCLC = non-small-cell lung cancer; CF = conventional fractionation; CAR-T = chimeric antigen receptor Tcell; SBRT = stereotactic body radiotherapy).
Conflicts of interest
There are no conflicts of interest to report
References
- Obituary: EH, Grubbe MD, FACP. Br Med J. 1960; 2:609
- Baird BJ, Sung CK, Beadle BM, Divi V. Treatment of early-stage laryngeal cancer: a comparison of treatment options. Oral Oncol. 2018; 87:8–16. doi:10.1016/j.oraloncology.2018.09.012.
- Kamran SC, D’Amico AV. Radiation therapy for prostate cancer. Hematol Oncol Clin North Am. 2020;34(1):45–69.
- Chang SS, Bochner BH, Chou R, et al. Treatment of non-metastatic muscle-invasive bladder cancer: AUA/ASCO/ASTRO/SUO guideline. J Urol. 2017;198(3):552–559. doi:10.1016/j.juro.2017.04.086.
- Tandberg DJ, Tong BC, Ackerson BG, Kelsey CR. Surgery versus stereotactic body radiation therapy for stage I non-small cell lung cancer: A comprehensive review. Cancer. 2018;124(4):667–678. doi:10.1002/cncr.31196.
- Brown S, Banfill K, Aznar MC, et al. The evolving role of radiotherapy in non-small cell lung cancer. Br J Radiol. 2019;92(1104):20190524.
- Alterio D, Marvaso G, Ferrari A, et al. Modern radiotherapy for head and neck cancer. Semin Oncol. 2019;46(3):233–245.
- Vordermark D. Radiotherapy of cervical cancer. Oncol Res Treat. 2016;39(9):516–520. doi:10.1159/000448902.
- Willers H, Allen A, Grosshans D, et al. Toward A variable RBE for proton beam therapy. Radiother Oncol. 2018;128(1):68–75. doi:10.1016/j.radonc.2018.05.019.
- Koukourakis MI, Mitrakas AG, Giatromanolaki A. Therapeutic interactions of autophagy with radiation and temozolomide in glioblastoma: evidence and issues to resolve. Br J Cancer. 2016;114(5):485–496. doi:10.1038/bjc.2016.19.
- Milanovic M, Fan DNY, Belenki D, et al. Senescence-associated reprogramming promotes cancer stemness. Nature. 2018;553(7686):96–100. doi:10.1038/nature25167.
- Garon EB, Hellmann MD, Rizvi NA, et al. Five-Year overall survival for patients with advanced non‒small-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. J Clin Oncol. 2019;37(28):2518–2527.
- Bashford EF, Murray JA, Cramer W. The natural and induced resistance of mice to the growth of cancer. Proc R Soc Lond. B. 1907; 79:164–187.
- Giatromanolaki A, Sivridis E, Koukourakis MI. The pathology of tumor stromatogenesis. Cancer Biol Ther. 2007;6(5):639–645.
- Mikulska ZB, Smith C, Alexander P. Evidence for an immunological reaction of the host directed against its own actively growing primary tumor. J Natl Cancer Inst. 1966;36(1):29–35.
- Berczi I, Strausbauch P, Sehon AH. Rejection of tumor cells in vitro. Science. 1973;180(4092):1289–1291. doi:10.1126/science.180.4092.1289.
- Jurin M, Suit HD. In vivo and in vitro studies of the influence of the immune status of C3Hf-Bu mice on the effectiveness of local irradiation of a methylcholanthrene-induced fibrosarcoma. Cancer Res. 1972;32(10):2201–2211.
- Shiku H, Kisielow P, Bean MA, et al. Expression of T-cell differentiation antigens on effector cells in cell-mediated cytotoxicity in vitro. Evidence for functional heterogeneity related to the surface phenotype of T cells. J Exp Med. 1975;141(1):227–241. doi:10.1084/jem.141.1.227.
- Kiessling R, Klein E, Wigzell H. Natural" killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution, according to genotype. Eur J Immunol. 1975;5(2):112–117.
- Ojo E. Positive correlation between the levels of natural killer cells and the in vivo resistance to syngeneic tumor transplants as influenced by various routes of administration of Corynebacterium parvum bacteria. Cell Immunol. 1979;45(1):182–187. doi:10.1016/0008-8749(79)90374-5.
- Hassan ZM, Rees RC, Potter CW. Corynebacterium parvum stimulation of adherent and non-adherent cytotoxic cells in mice. Br J Cancer. 1981;44(4):532–538. doi:10.1038/bjc.1981.222.
- Evans R, Alexander P. Mechanism of immunologically specific killing of tumour cells by macrophages. Nature. 1972;236(5343):168–170. doi:10.1038/236168a0.
- Keller R. Modulation of cell proliferation by macrophages: a possible function apart from cytotoxic tumour rejection. Br J Cancer. 1974;30(5):401–415. doi:10.1038/bjc.1974.214.
- Schirrmacher V, Landolfo S, Zawatzky R, Kirchner H. Immunogenetic studies on the resistance of mice to highly metastatic DBA/2 tumor cell variants. II. Influence of minor histocompatibility antigens on tumor resistance, gamma-interferon induction, and cytotoxic response. Invasion Metastasis. 1981;1(3):175–194.
- Isaacs A, Lindenmann J. Virus interference. I. The Interferon. Proc R Soc Lond B Biol Sci. 1957;147(927):258–267. [Database] doi:10.1098/rspb.1957.0048.
- Chany C. An inhibiting factor of intracellular multiplication of viruses called Interferon, originating from cancer cells. The phenomenon of viral autoinhibition. C R Hebd Seances Acad Sci. 1960; 250:3903–3905.
- Lee SH, Ozere RL, Van Rooyen CE. Interferon production by human leucocytes in vitro. Reduced levels in lymphatic leukemia. Proc Soc Exp Biol Med. 1966;122(1):32–39.
- Gresser I, Bourali C, Lévy JP, et al. Increased survival in mice inoculated with tumor cells and treated with interferon preparations. Proc Natl Acad Sci U S A. 1969;63(1):51–57. doi:10.1073/pnas.63.1.51.
- Gresser I, Bourali C. Antitumor effects of interferon preparations in mice. J Natl Cancer Inst. 1970;45(2):365–376.
- Gutterman JU, Blumenschein GR, Alexanian R, et al. interferon-induced tumor regression in human metastatic breast cancer, multiple myeloma, and malignant lymphoma. Ann Intern Med. 1980;93(3):399–406.
- Horning SJ, Levine JF, Miller RA, et al. Clinical and immunologic effects of recombinant leukocyte A interferon in eight patients with advanced cancer. JAMA. 1982;247(12):1718–1722. doi:10.1001/jama.1982.03320370032025.
- Golub SH, D’Amore P, Rainey M. Systemic administration of human leukocyte interferon to melanoma patients. II. Cellular events associated with changes in natural killer cytotoxicity. J Natl Cancer Inst. 1982; 68:711–717.
- Mochizuki DY, Watson J, Gillis S. Biochemical separation of interleukin 2. J Immunol Methods. 1980;39(3):185–201.
- Mills GB, Paetkau V. Generation of cytotoxic lymphocytes to a syngeneic tumor by using co-stimulator (Interleukin 2). J Immunol. 1980;125(5):1897–1903.
- Thayer A. Interleukin-2 wins FDA market clearance. Chem Eng News Archive. 1992;70(19):5. doi:10.1021/cen-v070n019.p005.
- Eggermont AM, Suciu S, Santinami M, et al. EORTC Melanoma Group. Adjuvant therapy with pegylated interferon alfa-2b versus observation alone in resected stage III melanoma: final results of EORTC 18991, a randomized phase III trial. Lancet. 2008;372(9633):117–126. ; doi:10.1016/S0140-6736(08)61033-8.
- Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–1355. doi:10.1126/science.aar4060.
- MacDowell EC, Taylor MJ, Potter JS. The dependence of protection against a transplantable mouse leukemia upon the genetic constitution of the immunizing tissue. Proc Natl Acad Sci U S A. 1935;21(8):507–508. doi:10.1073/pnas.21.8.507.
- Sturm E. Induced resistance to a transplantable lymphatic leukemia in rats. Cancer Res. 1941; 1:627–628.
- Murphy JB, Sturm A. The effect of adrenal grafting on transplanted lymphatic leukemia. Cancer Res. 1950;10(3):191–193.
- Sturm E, Murphy JB. The effect of adrenalectomy on the susceptibility of rats to a transplantable leukemia. Cancer Res. 1944; 4:384–388.
- Symes MO, Mitchell JP, Eckert H, et al. Transfer of adoptive immunity by intra-arterial injection of tumor-immune pig lymph node cells: treatment of recurrent urinary bladder carcinoma after radical radiotherapy. Urology. 1978;12(4):398–401. doi:10.1016/0090-4295(78)90288-1.
- Clowes GHA, Baeslack FW. Further evidence of immunity against cancer in mice after spontaneous recovery. Med News. 1905; 87:968–971.
- Besredka A, Gross L. De l’immunisation contre le sarcome de la souris par vole intracutane. Ann. Inst. Pasteur. 1935; 55:491–500.
- Gross L. Intradermal Immunization of C3H Mice against a Sarcoma That Originated in an Animal of the Same Line. Cancer Res. 1943; 3:326–333.
- Foley EJ. Antigenic properties of methylcholanthrene-induced tumors in mice of the strain of origin. Cancer Res. 1953;13(12):835–837.
- Lewis MR, Aptekman MB. Atrophy of tumors caused by strangulation and accompanied by development of tumor immunity in rats. Cancer. 1952; 6:411–413.
- Stjernswärd J, Vánky F, Klein E. Lymphocyte stimulation by autochthonous human solid tumours. Br J Cancer Suppl. 1973; 1:72–76.
- Avatkins E, Ogata Y, Anderson LL. Activation of Host lymphocytes cultured with cancer cells, treated with neuramidase. Nature (New Biol.). 1971; 231:83.
- Vánky F, Stjernswärd J, Klein G, Nilsonne U. Serum-mediated Inhibition of lymphocyte stimulation by autochthonous human tumors. J Natl Cancer Inst. 1971;47(1):95–103.
- Simmons RL, Rios A, Lundgren G, Ray PK. Immunotherapy of methylcholanthrene fibrosarcoma using neuraminidase. Fed Proc. 1971; 30:246.
- Weiss L, Mlavhew E, Ulrieh K. The effect of neuraminidase on the fate of transfused lymphocytes. Lab Invest. 1966; 15:1304.
- Simmons RL, Rios A, Ray PK. Immunogenicitv and antigenicity of lymphoid cells treated with neoiraminidase. Nature (New Biol.). 1971;231(23):179–181. doi:10.1038/newbio231179a0.
- Juillard GJ, Boyer PJ, Snow HD. Intralymphatic infusion of autochthonous tumor cells in canine lymphoma. Int J Radiat Oncol Biol Phys. 1976;1(5-6):497–503.
- Kellock TH, Chambers H, Russ S. An attempt to produce immunity to malignant disease in man. Lancet. 1922;199(5136):217–219. doi:10.1016/S0140-6736(01)25621-9.
- Anderson MJ, Kelly F, Wood SE, et al. Evaluation of Leukocyte function six years after tumour autograft in human mammary cancer. Br J Cancer. 1973; 28 (Suppl. I):83.
- Anderson JM, Kelly F, Gettinby G, Wood SE. Prolonged survival after immunotherapy (irradiated cancer autografts) or mammary cancers, assessed by a measure of therapeutic deficiency. Cancer. 1977;40(1):30–35. doi:10.1002/1097-0142(197707)40:1<30::AID-CNCR2820400107>3.0.CO;2-S.
- Laucius JF, Bodurtha AJ, Mastrangelo JM, Bellet RE. A Phase II study of autologous irradiated tumor cells plus BCG in patients with metastatic malignant melanoma. Cancer. 1977;40(5):2091–2093. doi:10.1002/1097-0142(197711)40:5<2091::AID-CNCR2820400517>3.0.CO;2-H.
- Hedley DW, McElwain TJ, Currie GA. Specific active immunotherapy does not prolong survival in surgically treated patients with stage IIB malignant melanoma and may promote early recurrence. Br J Cancer. 1978;37(4):491–496. doi:10.1038/bjc.1978.76.
- Hudson CN, McHardy JE, Curling OM, et al. Active specific immunotherapy for ovarian cancer. Lancet. 1976;2(7991):877–879. doi:10.1016/S0140-6736(76)90539-0.
- Juillard GJ, Boyer PJ, Yamashiro CH. A phase I study of active specific intra-lymphatic immunotherapy (ASILI). Cancer. 1978;41(6):2215–2225. doi:10.1002/1097-0142(197806)41:6<2215::AID-CNCR2820410622>3.0.CO;2-X.
- Stack BH, McSwan N, Stirling JM, et al. Cell-mediated immunity in operable bronchial carcinoma: the effect of injecting irradiated autologous tumour cells and BCG. Thorax. 1979;34(1):68–73. doi:10.1136/thx.34.1.68.
- McCune CS, Patterson WB, Henshaw EC. Active specific immunotherapy with tumor cells and Corynebacterium parvum: a phase I study. Cancer. 1979;43(5):1619–1623. doi:10.1002/1097-0142(197905)43:5<1619::AID-CNCR2820430509>3.0.CO;2-O.
- McCune CS, Schapira DV, Henshaw EC. Specific immunotherapy of advanced renal carcinoma: evidence for the polyclonality of metastases. Cancer. 1981;47(8):1984–1987. doi:10.1002/1097-0142(19810415)47:8<1984::AID-CNCR2820470814>3.0.CO;2-J.
- Souter RG, Gill PG, Gunning AJ, Morris PJ. Failure of specific active immunotherapy in lung cancer. Br J Cancer. 1981;44(4):496–501. doi:10.1038/bjc.1981.217.
- Wiseman C, Jessup JM, Smith TL, et al. Inflammatory breast cancer treated with surgery, chemotherapy and allogeneic tumor cell/BCG immunotherapy. Cancer. 1982;49(6):1266–1271. doi:10.1002/1097-0142(19820315)49:6<1266::AID-CNCR2820490631>3.0.CO;2-6.
- Mahaley MS, Jr, Bigner DD, Dudka LF, et al. Immunobiology of primary intracranial tumors. Part 7: Active immunization of patients with anaplastic human glioma cells: a pilot study. J Neurosurg. 1983;59(2):201–207.
- Jessup JM, McBride CM, Ames FC, et al. Active specific immunotherapy of Dukes B2 and C colorectal carcinoma: comparison of two doses of the vaccine. Cancer Immunol Immunother. 1986; 21:233–239.
- Gray BN, Walker C, Andrewartha L, et al. Controlled clinical trial of adjuvant immunotherapy with BCG and neuraminidase-treated autologous tumour cells in large bowel cancer. J Surg Oncol. 1989;40(1):34–37. doi:10.1002/jso.2930400109.
- Adler A, Gillon G, Lurie H, et al. Active specific immunotherapy of renal cell carcinoma patients: a prospective randomized study of hormono-immuno-versus hormonotherapy. Preliminary report of immunological and clinical aspects. J Biol Response Mod. 1987;6(6):610–624.
- Berd D, Mastrangelo MJ. Active immunotherapy of human melanoma exploiting the immunopotentiating effects of cyclophosphamide. Cancer Invest. 1988;6(3):337–349. doi:10.3109/07357908809080657.
- Wiseman CL, Rao VS, Kennedy PS, et al. Clinical responses with active specific intralymphatic immunotherapy for cancer–a phase I-II trial. West J Med. 1989;151(3):283–288.
- Berd D, Maguire HC, Jr, McCue P, Mastrangelo MJ. Treatment of metastatic melanoma with an autologous tumor-cell vaccine: clinical and immunologic results in 64 patients. J Clin Oncol. 1990;8(11):1858–1867. doi:10.1200/JCO.1990.8.11.1858.
- Schlag P, Manasterski M, Gerneth T, et al. Active specific immunotherapy with Newcastle-disease-virus-modified autologous tumor cells following resection of liver metastases in colorectal cancer. First evaluation of clinical response of a phase II-trial. Cancer Immunol Immunother. 1992;35(5):325–330. doi:10.1007/BF01741145.
- Dillman RO, DeLeon C, Beutel LD, et al. Short-term autologous tumor cell lines for the active specific immunotherapy of patients with metastatic melanoma. Crit Rev Oncol Hematol. 2001;39(1-2):115–123. doi:10.1016/s1040-8428(01)00110-x.
- Lotem M, Peretz T, Drize O, et al. Autologous cell vaccine as a post operative adjuvant treatment for high-risk melanoma patients (AJCC stages III and IV). The new American Joint Committee on Cancer. Br J Cancer. 2002;86(10):1534–1539. doi:10.1038/sj.bjc.6600251.
- Galligioni E, Quaia M, Merlo A, et al. Adjuvant immunotherapy treatment of renal carcinoma patients with autologous tumor cells and bacillus Calmette-Guèrin: five-year results of a prospective randomized study. Cancer. 1996; 77:2560–2566. doi:10.1002/(SICI)1097-0142(19960615)77:12<2560::AID-CNCR20>3.0.CO;2-P.
- Stack BH, McSwan N, Stirling JM, et al. Autologous x-irradiated tumour cells and percutaneous BCG in operable lung cancer. Thorax. 1982;37(8):588–593. doi:10.1136/thx.37.8.588.
- Vermorken JB, Claessen AM, van Tinteren H, et al. Active specific immunotherapy for stage II and stage III human colon cancer: a randomized trial. Lancet. 1999;353(9150):345–350. doi:10.1016/S0140-6736(98)07186-4.
- Harris JE, Ryan L, Hoover HC, Jr, et al. Adjuvant active specific immunotherapy for stage II and III colon cancer with an autologous tumor cell vaccine: eastern cooperative oncology group study E5283. JCO. 2000;18(1):148–157. doi:10.1200/JCO.2000.18.1.148.
- Bekesi JG, Roboz JP, Holland JF. Therapeutic effectiveness of neuraminidase-treated tumor cells as an immunogen in man and experimental animals with leukemia. Ann N Y Acad Sci. 1976;277(00):313–331. doi:10.1111/j.1749-6632.1976.tb41710.x.
- Powles R, Toy JL. The effect of immunotherapy on survival of patients with acute myelogenous leukaemia after relapse. Haematologia (Budap). 1976;10(1):5–9.
- Ohno R, Nakamura H, Kodera Y, et al. Randomized controlled study of chemoimmunotherapy of acute myelogenous leukemia (AML) in adults with Nocardia rubra cell-wall skeleton and irradiated allogeneic AML cells. Cancer. 1986;57(8):1483–1488. doi:10.1002/1097-0142(19860415)57:8<1483::AID-CNCR2820570808>3.0.CO;2-7.
- Hayat M, Jehn U, Willemze R, et al. A randomized comparison of maintenance treatment with androgens, immunotherapy, and chemotherapy in adult acute myelogenous leukemia. A leukemia-lymphoma group trial of the EORTC. Cancer. 1986;58(3):617–623. doi:10.1002/1097-0142(19860801)58:3<617::AID-CNCR2820580304>3.0.CO;2-1.
- Urbanitz D, Büchner T, Pielken H, et al. Neuraminidase-treated allogeneic blasts for maintenance in acute myelogenous leukemia: results of a prospective randomized trial. Haematol Blood Transfus. 1987; 30:64–68. doi:10.1007/978-3-642-71213-5_11.
- Kirchner HH, Atzpodien APJ. Adjuvant treatment of locally advanced renal cancer with autologous virus-modified tumor vaccines. World J Urol. 1995; 13:171–173.
- Pomer S, Schirrmacher V, Thiele R, et al. Tumor response and 4 year survival data of patients with advanced renal cell carcinoma treated with autologous tumor vaccine and subcutaneous R-IL-2 and IFN-alpha(2b). Int J Oncol. 1995; 6:947–954.
- Dillman RO, Depriest C, McClure SE. High-dose IL2 in metastatic melanoma: better survival in patients immunized with antigens from autologous tumor cell lines. Cancer Biother Radiopharm. 2014;29(2):53–57. doi:10.1089/cbr.2013.1565.
- Mordoh J, Pampena MB, Aris M, et al. Phase II study of adjuvant immunotherapy with the CSF-470 vaccine plus bacillus Calmette-Guerin plus recombinant human granulocyte macrophage-colony stimulating factor vs medium-dose interferon alpha 2B in stages IIB, IIC, and III cutaneous melanoma patients: a single institution. Front Immunol. 2017; 8:625.
- Kusumoto M, Umeda S, Ikubo A, et al. Phase 1 clinical trial of irradiated autologous melanoma cells adenovirally transduced with human GM-CSF gene. Cancer Immunol Immunother. 2001;50(7):373–381. doi:10.1007/s002620100213.
- Tani K, Azuma M, Nakazaki Y, et al. Phase I study of autologous tumor vaccines transduced with the GM-CSF gene in four patients with stage IV renal cell cancer in Japan: clinical and immunological findings. Mol Ther. 2004;10(4):799–816.
- Buchner A, Pohla H, Willimsky G, et al. Phase 1 trial of allogeneic gene-modified tumor cell vaccine RCC-26/CD80/IL-2 in patients with metastatic renal cell carcinoma. Hum Gene Ther. 2010;21(3):285–297.
- Lutz E, Yeo CJ, Lillemoe KD, Biedrzycki B, et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann Surg. 2011;253(2):328–335.
- Kugler A, Seseke F, Thelen P, et al. Autologous and allogenic hybrid cell vaccine in patients with metastatic renal cell carcinoma. Br J Urol. 1998;82(4):487–493.
- Krause SW, Neumann C, Soruri A, et al. The treatment of patients with disseminated malignant melanoma by vaccination with autologous cell hybrids of tumor cells and dendritic cells. J Immunother. 2002;25(5):421–428.
- Homma S, Kikuchi T, Ishiji N, et al. Cancer immunotherapy by fusions of dendritic and tumour cells and rh-IL-12. Eur J Clin Invest. 2005;35(4):279–286.
- Wei Y, Sticca RP, Holmes LM, et al. Dendritoma vaccination combined with low dose interleukin-2 in metastatic melanoma patients induced immunological and clinical responses. Int J Oncol. 2006;28(3):585–593.
- Koukourakis MI, Giatromanolaki A. Tumor microenvironment, immune response and post-radiotherapy tumor clearance. Clin Transl Oncol. 2020;22(12):2196–2205.
- Heaton KM, Ju G, Grimm EA. Human interleukin 2 analogs that preferentially bind the intermediate-affinity interleukin 2 receptor lead to reduced secondary cytokine secretion: implications for the use of these interleukin 2 analogs in cancer immunotherapy. Cancer Res. 1993;53(11):2597–2602.
- Koukourakis MI, Giatromanolaki A. Lymphopenia and intratumoral lymphocytic balance in the era of cancer immuno-radiotherapy. Crit Rev Oncol Hematol. 2021; 159(103226):103226.
- Interferon approved for marketing by FDA. Chem Eng News. 1986; 64:7.
- Crowther D, Scarffe JH, Howell A, et al. Growth factor-assisted chemotherapy–the Manchester experience. Ciba Found Symp. 1990; 148:201–210.
- Heufler C, Koch F, Schuler G. Granulocyte/macrophage colony-stimulating factor and interleukin 1 mediate the maturation of murine epidermal Langerhans cells into potent immunostimulatory dendritic cells. J Exp Med. 1988;167(2):700–705.
- Borden EC. Interferons α and β in cancer: therapeutic opportunities from new insights. Nat Rev Drug Discov. 2019;18(3):219–234.
- Torrisi J, Berg C, Harter K, et al. Phase I combined modality clinical trial of alpha-2-interferon and radiotherapy. Int J Radiat Oncol Biol Phys. 1986;12(8):1453–1456.
- Färkkilä M, Jääskeläinen J, Kallio M, et al. Randomised, controlled study of intratumoral recombinant gamma-interferon treatment in newly diagnosed glioblastoma. Br J Cancer. 1994;70(1):138–141. doi:10.1038/bjc.1994.263.
- Buckner JC, Schomberg PJ, McGinnis WL, et al. A phase III study of radiation therapy plus carmustine with or without recombinant interferon-alpha in the treatment of patients with newly diagnosed high-grade glioma. Cancer. 2001;92(2):420–433. doi:10.1002/1097-0142(20010715)92:2<420::AID-CNCR1338>3.0.CO;2-3.
- Tummarello D, Mari D, Graziano F, et al. A randomized, controlled phase III study of cyclophosphamide, doxorubicin, and vincristine with etoposide (CAV-E) or teniposide (CAV-T), followed by recombinant interferon-alpha maintenance therapy or observation, in small cell lung carcinoma patients with complete responses. Cancer. 1997;80(12):2222–2229. doi:10.1002/(SICI)1097-0142(19971215)80:12<2222::AID-CNCR2>3.0.CO;2-W.
- van Zandwijk N, Groen HJ, Postmus PE, et al. Role of recombinant interferon-gamma maintenance in responding patients with small cell lung cancer. A randomised phase III study of the EORTC lung cancer cooperative group. Eur J Cancer. 1997;33(11):1759–1766. doi:10.1016/s0959-8049(97)00174-3.
- Mantz CA, Vokes EE, Stenson K, et al. Induction chemotherapy followed by concomitant chemoradiotherapy in the treatment of locoregionally advanced oropharyngeal cancer. Cancer J. 2001;7(2):140–148.
- Gennatas C, Dardoufas C, Mouratidou D, et al. Surgical adjuvant therapy of rectal carcinoma: a controlled evaluation of leucovorin, 5-fluorouracil and radiation therapy with or without interferon-alpha2b. Ann Oncol. 2003;14(3):378–382. doi:10.1093/annonc/mdg105.
- Schmidt J, Abel U, Debus J, et al. Open-label, multicenter, randomized phase III trial of adjuvant chemoradiation plus Interferon Alfa-2b versus fluorouracil and folinic acid for patients with resected pancreatic adenocarcinoma. J Clin Oncol. 2012;30(33):4077–4083.
- Basu P, Jenson AB, Majhi T, et al. Phase 2 randomized controlled trial of radiation therapy plus concurrent interferon-alpha and retinoic acid versus cisplatin for stage III cervical carcinoma. Int J Radiat Oncol Biol Phys. 2016;94(1):102–110.
- Kimura H, Yamaguchi Y. A phase III randomized study of interleukin-2 lymphokine-activated killer cell immunotherapy combined with chemotherapy or radiotherapy after curative or noncurative resection of primary lung carcinoma. Cancer. 1997;80(1):42–49. doi:10.1002/(SICI)1097-0142(19970701)80:1<42::AID-CNCR6>3.0.CO;2-H.
- De Stefani A, Forni G, Ragona R, et al. Improved survival with perilymphatic interleukin 2 in patients with resectable squamous cell carcinoma of the oral cavity and oropharynx. Cancer. 2002;95(1):90–97. doi:10.1002/cncr.10654.
- Thompson JA, Fisher RI, Leblanc M, et al. Total body irradiation, etoposide, cyclophosphamide, and autologous peripheral blood stem-cell transplantation followed by randomization to therapy with interleukin-2 versus observation for patients with non-Hodgkin lymphoma: results of a phase 3 randomized trial by the Southwest Oncology Group (SWOG 9438). Blood. 2008;111(8):4048–4054. ). doi:10.1182/blood-2007-09-111708.
- Curti B, Crittenden M, Seung SK, et al. Randomized phase II study of stereotactic body radiotherapy and interleukin-2 versus interleukin-2 in patients with metastatic melanoma. J Immunother Cancer. 2020;8(1):e000773.
- Bunn PA, Jr, Crowley J, Kelly K, et al. Chemoradiotherapy with or without granulocyte-macrophage colony-stimulating factor in the treatment of limited-stage small-cell lung cancer: a prospective phase III randomized study of the Southwest Oncology Group. JCO. 1995;13(7):1632–1641. doi:10.1200/JCO.1995.13.7.1632.
- Deng L, Liang H, Xu M, et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41(5):843–852. doi:10.1016/j.immuni.2014.10.019.
- Herrera FG, Bourhis J, Coukos G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA Cancer J Clin. 2017;67(1):65–85.
- Dagoglu N, Karaman S, Caglar HB, Oral EN. Abscopal effect of radiotherapy in the immunotherapy era: systematic review of reported cases. Cureus. 2019;11(2):e4103.
- Grimaldi AM, Simeone E, Giannarelli D, et al. Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology. 2014; 3:e28780. doi:10.4161/onci.28780.
- Koukourakis IM, Giakzidis AG, Kouroupi M, et al. Cutaneous squamous-cell carcinoma of the head-neck area refractory to chemo-radiotherapy: benefit from anti-PD-1 immunotherapy. BJR Case Rep. 2021; 6:20200170.
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919–1929. ; doi:10.1056/NEJMoa1709937.
- Wu YL, Tsuboi M, He J, et al. Herbst RS; ADAURA Investigators. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N Engl J Med. 2020;383(18):1711–1723. doi:10.1056/NEJMoa2027071.
- Fizazi K, Drake CG, Beer TM, et al. Final analysis of the ipilimumab versus placebo following radiotherapy phase iii trial in postdocetaxel metastatic castration-resistant prostate cancer identifies an excess of long-term survivors. Eur Urol. 2020;78(6):822–830. ;. doi:10.1016/j.eururo.2020.07.032.
- Theelen WSME, Peulen HMU, Lalezari F, et al. Effect of Pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol. 2019;5(9):1276–1282. doi:10.1001/jamaoncol.2019.1478.
- McBride S, Sherman E, Tsai CJ, et al. Randomized phase II trial of nivolumab with stereotactic body radiotherapy versus nivolumab alone in metastatic head and neck squamous cell carcinoma. J Clin Oncol. 2021;39(1):30–37.
- Cohen EE, Ferris RL, Psyrri A, et al. 910O-Primary results of the phase III JAVELIN head & neck 100 trial: Avelumab plus chemoradiotherapy (CRT) followed by avelumab maintenance vs CRT in patients with locally 1280 advanced squamous cell carcinoma of the head and neck (LA SCCHN). Ann Oncol. 2020; 31:S658. doi:10.1016/j.annonc.2020.08.1025.
- Bourhis J, Sire C, Tao Y, et al. LBA38 Pembrolizumab versus cetuximab, concomitant with 1283 radiotherapy (RT) in locally advanced head and neck squamous cell carcinoma (LA-HNSCC): Results of the GORTEC 2015-01 ‘PembroRad’ randomized trial. Ann Oncol. 2020; 31:S1168. doi:10.1016/j.annonc.2020.08.2268.
- Giatromanolaki A, Koukourakis IM, Chatzipantelis P, et al. Rectal cancer induces a regulatory lymphocytic phenotype in the tumor-draining lymph nodes to promote cancer cell installation. Immunol Res. 2020;68(6):363–372.
- Minn I, Rowe SP, Pomper MG. Enhancing CAR T-cell therapy through cellular imaging and radiotherapy. Lancet Oncol. 2019;20(8):e443-51–e451. doi:10.1016/S1470-2045(19)30461-9.
