Inflammation is the body’s natural response to injury or biotic and abiotic challenges to the host. Controlled inflammation is required in order to maintain tissue homeostasis and physiological processes under various intrinsic and extrinsic perturbations. However, uncontrolled inflammation leads to various degrees of immune-pathologies such as susceptibility to infection or autoimmune diseases. Various biotic and abiotic factors induce inflammation, including, pathogenic invasion, physical injury, stress, obesity, and chemical or radiation insult to the host. At the cellular or molecular level, inflammation is induced by complex signaling through innate immune sensors expressed on immune and non-immune cells, and through specialized adaptive immune cells. Inflammatory mediators can be amines (e.g. histamine), lipid derivatives (e.g. prostaglandins), the peptide, bradykinin or proteins (small fragments of complements and inflammatory cytokines). These inflammatory mediators can cause cell damage, can result in a cytokine storm, and can lead to damage to the vital organs. This special issue of International Reviews of Immunology focuses on inducers of inflammation and how inflammation shapes immunity and its dysregulation results in the development of diseases and disorders ().
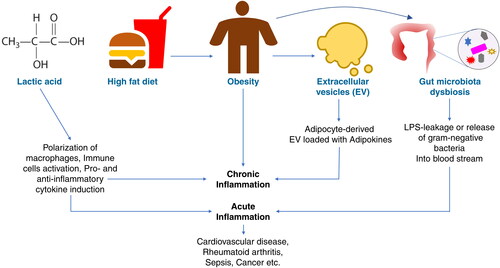
In mammals, lactate is a metabolic by-product of anaerobic respiration, a glycolytic pathway that ensures quick energy replenishment in the form of adenosine triphosphate (ATP) for the cells and prevention of muscle fatigue. Lactate acts as a circulating fuel in the blood that goes to the liver and is converted into pyruvate by the enzyme lactate dehydrogenase. Pyruvate is then converted into glucose via a metabolic pathway known as gluconeogenesis in the liver. Notably, lactate production increases when demand for ATP increases. In the past three decades, lactate has also been proved to be a very important signaling molecule that regulates various signaling pathways including inflammation-associated immune pathways. In this special issue, Zhou et al. [Citation1] and Luo et al. [Citation2] shed light on how endogenous lactate regulates inflammation in various immunological events, such as via macrophage polarization, T-cell immune dysfunction and its link with infectious and noninfectious diseases such as tumors. These two articles will be of interest to a broad readership in the field of immunology, as well as researchers investigating metaflammation and immunometabolic disorders and those in associated fields ().
Extracellular vesicles (EVs) are cell-derived membranous structures generated from broad cell-types including hematopoetic and non-hematopoetic cells. They range in size from the smallest exosomes and medium-sized micro-vesicles to apoptotic bodies and large-sized macrolets. Extracellular vesicles carry several biomolecules such as proteins (enzymes, receptors, cytokines etc.), lipids, genetic materials (miRNA, RNA, DNA) in them. Hence, by virtue of carrying a variety of biomolecules, EVs qualify to participate in several cellular processes, namely, signaling pathways that regulate cell growth, metabolism, immunity and inflammation, and thereby help in intercellular communication. Depending on what biomolecules they carry, EVs either induce or suppress physiological processes. In this special issue, Kumar et al. [Citation3] discuss how EVs secreted from adipose tissue initiate inflammation at target cells by carrying several adipokines (pro-inflammatory cytokines) in obese people. Obesity is the manifestation of an unwanted accumulation of fat (lipids) and a slow metabolic rate. Obesity predisposes individuals to many diseases such as cardiovascular diseases, diabetes mellitus, hypertension, atherosclerosis etc. Obesity and inflammation have a mutual relationship, i.e. chronic inflammation causes obesity and obesity promotes inflammation. The article by Kumar et al. [Citation3] elaborates the role of EVs in obesity-induced inflammation which gives an insight into the therapeutic and diagnostic potential of EVs in obesity. This article will be helpful to cellular immunologists, and especially researchers working at the junction of cellular trafficking, signaling and immunology ().
Gut microbiota constitutes an important part of the body and have the potential to regulate the overall health of the host. Gut microbiota comprises a variety of microorganisms such as bacteria, fungi, protozoa and archaea. The cellular components or metabolites derived from these microbiota have the capacity to induce or suppress systemic inflammation upon leakage into the circulation. One such cellular component is lipopolysaccharide (LPS) which is present on the cell membrane of gram-negative bacteria. Lipopolysaccharide is known to induce inflammation through activation of host-cell surface receptors (toll-like receptors) or cytoplasmic (Casp11) receptors. By activating these receptors, LPS causes the production and secretion of pro-inflammatory molecules which could cause systemic inflammation, sepsis and even the death of the host. Hence, the leakage of LPS or gram-negative bacteria through the gut into the blood circulation could lead to activation of immune cells and thereby inflammation. However, the intestinal barrier prevents such leakage. Several factors such as diet, host genetics, age, antibiotic drugs and inflammation regulate gut permeability and the gut microbiota ratio, and thereby gut dysbiosis. In this special issue, Du et al. [Citation4] explain how a change in the gut microbiota ratio due to a high-fat diet can lead to a change in the amount of LPS that leaks through the gut to cause different degrees of inflammation and inflammation-associated obesity. This article will be interesting to researchers or clinicians working on personalized medicine, particularly those looking at the metagenomes of gut microbiota and their links with physiology and the immune system ().
Funding
The author(s) reported there is no funding associated with the work featured in this article.
References
- Zhou HC, Yan XY, Yu WW, et al. Lactic acid in macrophage polarization: the significant role in inflammation and cancer. Int Rev Immunol. 2022;41(1):4–18. doi:https://doi.org/10.1080/08830185.2021.1955876.
- Luo Y, Li L, Chen X, et al. Effects of lactate in immunosuppression and inflammation: progress and prospects. Int Rev Immunol. 2022;41(1):19–29. doi:https://doi.org/10.1080/08830185.2021.1974856.
- Kumar V, Kiran S, Kumar S, et al. Extracellular vesicles in obesity and its associated inflammation. Int Rev Immunol. 2022;41(1):30–44. doi:https://doi.org/10.1080/08830185.2021.1964497.
- Du, et al. Lipopolysaccharides derived from gram-negative bacterial pool of human gut microbiota promote inflammation and obesity development. Int Rev Immunol. 2022;41(1):45–56. doi:https://doi.org/10.1080/08830185.2021.1996573.