ABSTRACT
In this paper Genetic Programming (GP) method was used to predict the removal of hexavalent chromium as one of main pollutant of wastewater using nanotube carbon as the adsorbent. One set of experimental data was chosen for this aim. The considered parameters as input of the network were adsorbent dosage, initial solution pH, initial concentration of Cr(VI), contact time and temperature and the output parameter of the network was final concentration of Cr(VI). GP applied for two groups of data, namely, kinetic and equilibrium and two correlations presented for these groups. The determined correlations using the GP had excellent precision. The correlations were used to determine appropriate model for both kinetic and equilibrium of adsorption. The results showed that the kinetic and equilibrium of adsorption fitted on the pseudo-second-order and Langmuir isotherm models, respectively. Activation energy and enthalpy of adsorption were determined using the models.
Introduction
Chromium cations in different valences are heavy metal pollutants of industrial wastewater. Chromium cation exists in two forms, hexavalent and trivalent. Hexavalent chromium Cr(VI) is more toxic and pollutant which can remain in food chain of human (Mohanty et al. Citation2005). It may cause cancer (Kaufman, Dinicola, and McIntosh Citation1970) and epigastric pain, nausea, vomiting, severe diarrhea and hemorrhage (Browning Citation1969). Unfortunately this cation can be found in very industries such as metal fishing, leather tanning, electroplating, nuclear power plant, textile (Kowalski Citation1994). It is necessary to remove the Cr(VI) from wastewater before disposal. The tolerance limit for Cr(VI) for discharge into inland surface water is 0.1 mg/Land in potable water is 0.05 mg/L (Kobya Citation2004), but concentration of Cr(VI) in wastewater industries is in a range of 0.5–270 mg/L.
Adsorption has many advantages in comparison of other methods to remove heavy metal ions such as chemical precipitation (Philipot, Chaffange, and Sibony Citation1984), chemical oxidation or reduction, ion exchange (Jorgensen Citation1979), electrochemical treatment (Huang and Wu Citation1975) and membrane. The disadvantages of these mentioned methods are incomplete metal removal, requirements for equipment and monitoring systems, high reagent and energy requirements (Aksu, Ånen, and Demircan Citation2002; Baran et al. Citation2007). The main disadvantages of adsorption method are its requirement to special adsorbent in special cases. Today nanotube carbon as a main nanostructure material shows exclusive adsorption capacity to remove most of heavy metals.
Basically, mathematical modeling and simulation help researchers to speedup the prediction of system behavior precisely. Soft computing is a series of novel and practical methods to simulate the complex systems such as adsorption process. In recent years, soft-computing methods became one of the most useful tools that could implement on many systems. The advantages of these methods are their power in prediction, optimization and fast calculation. Genetic Programming (GP) is one of the most powerful methods of soft computing that is based on biological evolution concept. GP is Genetic Algorithm (GA) where each individual is a computer program. It is a machine learning method uses to optimize a population of computer programs according to a fitness landscape determined by a program’s ability to perform a given computational task (Kinnear Citation1994).
In previous studies, Saroj Baral, Das and Rath (Citation2006) found that sawdust is an effective adsorbent to remove Cr(VI). They also presented the thermodynamic parameters and studied the effect of pH on the removal process. Esmaeili, Ghasemi and Rustaiyan (Citation2010) studied removal of toxic Cr(VI) ions from wastewater using two different activated carbon marine algae Gracilaria (red algae) and Sargassum sp. (brown algae). They showed that activated carbon prepared from algae can be used for removal of chromium, also they studied the kinetic and equilibrium of adsorption process. Gholipour, Hashemipour Rafsanjani and Soltani Goharrizi (Citation2011) studied the Cr(VI) removal from aqueous solution via adsorption on granular activated carbon. By determining the thermodynamic parameters, they suggested that the adsorption process is spontaneous and endothermic. Also they used Artificial Neural Networks (ANNs) to predict the Cr(VI) removal and their results showed the accuracy of ANNs for predicting this process. Shirzad Siboni et al. (Citation2011) studied the kinetic and equilibrium of the removal of Cr(VI) from aqueous solutions using modified holly sawdust. They showed that pseudo-second-order model and Langmuir isotherm model can describe kinetic and equilibrium of this removal, respectively. Gholipour and Hashemipour (Citation2012) evaluated multiwall carbon nanotubes (MWCNTs) performance in adsorption and desorption of Cr(VI). Their results showed that MWCNTs are effective adsorbents and they estimated adsorption rate constant (K), Gibbs free energy (), enthalpy (
) and entropy (
) for Cr(VI) adsorption. Dula, Siraj and Addisu Kitte (Citation2014) presented chemically activated carbon prepared from locally available waste of bamboo for removing Cr(VI). Behind studying the kinetic and equilibrium of the adsorption process, they studied adsorption efficiency and capacity of hexavalent chromium.
In this study, the adsorption behavior of Cr(VI) with Carbon Nanotube (CNT) is simulated using GP. For training GP network, a set of experimental data related to the adsorption process is used. The aim of using GP is generation of many data to predict the kinetic and equilibrium models and calculate the model’s parameters precisely.
Methodology
Genetic Programming
One set of experimental data was chosen for removal of hexavalent Cr(VI) by adsorption (Gholipour and Hashemipour Citation2012). Adsorbent dosage, initial solution pH, initial concentration of Cr(VI), contact time and temperature were considered as the input parameters of the GP network effecting final concentration of hexavalent chromium which is the output of GP network. These data divided into two groups, namely, kinetic and equilibrium data. Two correlations presented for each of two groups using GP. Seventy percent, 15% and 15% of each groups of data used for training, testing and validating, respectively. Some of the parameters needed to know for GP are given in .
Table 1. Parameters used in GP.
Relative to the population size, tournament sizes tend to be small. A tournament size of 1 would be equivalent to selecting individuals random, and a tournament size equal to the population size would be equivalent to selecting the best individual at any given point. Tree depth and Max gene depth are used to generate the initial population.
Kinetic of adsorption
The kinetic of adsorption describes the rate of adsorption process and it is a function of adsorbate concentration and temperature. Two kinetic models were considered to describe rate of adsorption, namely, pseudo-first-order model and pseudo-second-order model.
The pseudo-first-order rate expression is as follows (Ho and McKay Citation1999):
where qt (mg/L) is the adsorbate temperature, qe (mg/L) is adsorbate concentration at equilibrium, is the rate constant. The integrated form of Equation (1) becomes as follows (Costa Citation2003):
With linear regression of experimental data based on Equation (2), the rate constant (k1) can be calculated using the slope of the regression.
The pseudo–second-order model describes in Equation (3)
In this equation is the rate constant. This equation can be integrated and presented in form of Equation (4) (Esmaeili et al. Citation2008):
which can be written in the form of
If the experimental data plot as versus
, the parameters
and
can be calculated from the slope and intercept of the regression.
Equilibrium isotherm
The most common model used for modeling of adsorption equilibrium is Langmuir isotherm. The isotherm relation is as follows (Langmuir Citation1916):
where qe (mg/L) is the adsorbate concentration at the equilibrium, qmax (mg/L) is the maximum adsorption capacity, Ce (mg/L) is the concentration in liquid phase, named as affinity constant and is constantly related to the affinity of the binding sites (Gonul and Zumriye Citation2002).
Another common model of equilibrium is Freundlich isotherm (Citation1906) that uses for adsorption of liquids and expresses as follows:
where qe (mg) is the adsorbate concentration at the equilibrium, Kf is the equilibrium constant and presents the order of adsorption rate.
Results and discussion
Kinetic results
The following correlation presented for describing the final concentration of Cr(VI) as a function of adsorption conditions using GP. This correlation is used to generate kinetic data of adsorption.
where is final concentration of hexavalent chromium,
is temperature, t (h) is contact time, dad (gr) is adsorbent dosage and
is initial concentration of hexavalent chromium. For showing the precision of this correlation, square correlation coefficient (R2) was determined. Calculated R2 for this correlation was 0.99. By using this high precision correlation, it is tried to generate new data of
versus
at different temperatures to study the kinetic of the removal process. The generated data were applied to two mentioned models for rate of this process and comparison between precision of these models determined and presented in at initial concentration 20 and 40 mg/L.
Table 2. Comparison between precision of pseudo-first-order and pseudo-second-order equation at pH 2 and adsorbent dosage 0.01 g.
As one can see in , the pseudo-second-order equation was better than pseudo-first-order equation to predict the kinetic data in both initial concentrations. Therefore, the kinetic parameters of pseudo-second order are calculated from the regression and presented in for both initial concentrations.
Table 3. Constant rate of the pseudo-second-order kinetic at pH 2 and adsorbent dosage 0.01 g.
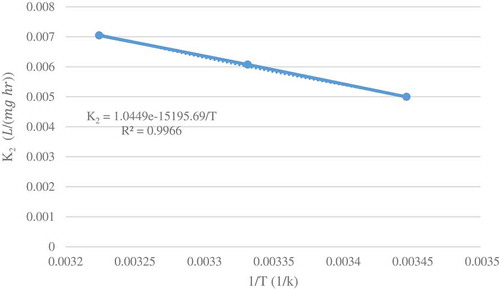
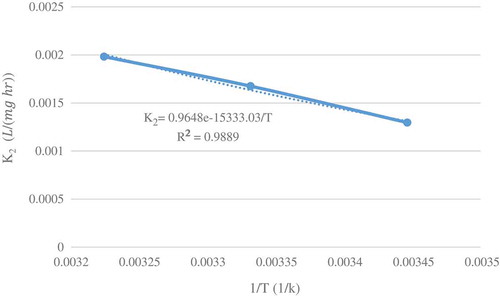
Arrhenius functionality of the rate constant was applied for data of and plotted for initial concentration 20 and 40 mg/L in and , respectively. The determined square correlation coefficients (R2 = 0.9966 for initial concentration 20 mg/L and R2 = 0.9889 for initial concentration 40 mg/L) showed the precision of data and good fitting of the functionality. According to this equation, and
determined as 15166.26 J/mol and 1.00485 (L/(mg h)).
Equilibrium results
The following correlation was presented for adsorption equilibrium concentration of Cr(VI) using GP:
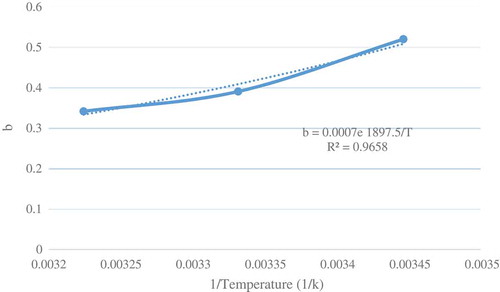
where Ce (mg/L) is equilibrium concentration of Cr(VI), T°C is temperature, dad (g) is adsorbent dosage and C0 (mg/L) is initial concentration of Cr(VI). Calculated R2 for this correlation was 0.99 that shows a high precision. After generating data, it is tried to find the best model to predict equilibrium state. The regression calculation for Langmuir and Freundlich isotherms was done and the comparison of the square correlation coefficient between these two models reported in the . As can be seen from this table, Longmuir isotherm is more accurate than Froehlich. Therefore, Van’t Hoff equation applied for data of plotted in . According to this equation, and
determined as 18614.48 J/mol and 0.0007 (L/(mg h)).
Table 4. Comparison of the square correlation coefficients between Langmuir and Freundlich isotherm models.
Table 5. Constants of the Langmuir equation at the initial concentration of 20 mg/L.
As one can see from , Freundlich model also applied to the generated data but the results were not reliable.
Conclusion
In this study, the simulation of adsorption process as an important process for removal of heavy metal (Cr(VI)) from the industrial wastewater was investigated. Detail description of both kinetic and equilibrium of adsorption were considered. A set of experimental data was used at different operating conditions to generate a correlation for describing the process. Using GP, two different correlations for each of kinetic and equilibrium states determined and these correlations were used to simulate the process and generate many data for predicting kinetic and equilibrium parameters such as activation energy and adsorption enthalpy and their pre-exponential factors. Square correlation coefficient between correlations results and experimental data showed precision of GP. The analysis of these data indicated that the pseudo-second-order model can better predict the kinetic behavior of the process than the pseudo-first-order model. In addition, the Langmuir isotherm is designated as the better model to describe equilibrium of the adsorption process than Freundlich isotherm. Finally, the activation energy and the enthalpy of the adsorption are calculated as 15166.26 and 1897.5 J/mol, respectively.
References
- Aksu, Z., F. G. Ånen, and Z. Demircan. 2002. Biosorption of Chromium (VI) ions by Mowital B30H resin immobilized activated sludge in a packed bed: Comparison with granular activated carbon. Processing Biochemical 38 (2):175–86. doi:10.1016/S0032-9592(02)00053-5.
- Baran, A., E. Bıçak, Ô. Hamarat-Baysal, and S. Önal. 2007. Comparative studies on the adsorption of Cr (VI) ions on to various sorbents. Bioresource Technology 98 (3):661–65. doi:10.1016/j.biortech.2006.02.020.
- Browning, E. 1969. Chromium in toxicity of industrial metals, 2nd ed. London: Butterworths Co.
- Costa, M. 2003. Potential hazards of hexavalent chromate in our drinking water. Toxicology and Applied Pharmacology 188:1–5. doi:10.1016/S0041-008X(03)00011-5.
- Dula, T., K. Siraj, and S. Addisu Kitte. 2014. Adsorption of hexavalent chromium from aqueous solution using chemically a carbon prepared from locally available waste of bamboo (Oxytenanthera abyssinica), Hindawi Publishing Corporation ISRN Environmental Chemistry Volume 2014. Article ID 438245, 9. doi: 10.1155/2014/438245.
- Esmaeili, A., P. Beirami, A. Rustaiyan, F. Rafiei, S. Ghasemi, and F. Assadian. 2008. Evaluation of the marine alga Gracilaria corticata for the biosorption of Cu (II) from wastewater in a packed column. Journal of Marine Environmental Engineering 9:65–73.
- Esmaeili, A., S. Ghasemi, and A. Rustaiyan. 2010. removal of hexavalent chromium using activated carbon derived from marine algae Gracilaria and Sargassum SP. Journal of Marine Science and Technology 18 (4):587–92.
- Freundlich, H. 1906. Uber die adsorption in losungen. Zeitschrift Fur Physikalische Chemie 57:385–470.
- Gholipour, M., and H. Hashemipour. 2012. Evaluation of multi-walled carbon nanotubes performance in adsorption and desorption of hexavalent chromium. Chemical Industry & Chemical Engineering Quarterly 18 (4):509−523. doi:10.2298/CICEQ111104025G.
- Gholipour, M., H. Hashemipour Rafsanjani, and A. Soltani Goharrizi. 2011. Optimization and modeling of hexavalent chromium removal from aqueous solution via adsorption on multiwalled carbon nanotubes. Research Journal of Applied Sciences, Engineering and Technology 3 (9):880–86.
- Gonul, D., and A. Zumriye. 2002. Removal of chromium (VI) from saline wastewater by Dunaliella species. Process Biochemistry 38:751–62. doi:10.1016/S0032-9592(02)00204-2.
- Ho, Y. S., and G. McKay. 1999. Pseudo-second order model for sorption processes. Process Biochemistry 34:451–65. doi:10.1016/S0032-9592(98)00112-5.
- Huang, C. P., and M. M. Wu. 1975. Journal - Water Pollution Control Federation 47:2437–46.
- Jorgensen, S. E. 1979. Industrial Wastewater Management 7:81–92.
- Kaufman, D. B., W. Dinicola, and R. McIntosh. 1970. Acute potassium dichromate poisoning in man. American Journal of Diseases of Children 119 (4):374–76. doi:10.1001/archpedi.1970.02100050376021.
- Kinnear, E., Jr. 1994. Advances in genetic programming. Cambridge, MA: Massachusetts institute of technology.
- Kobya, M. 2004. Removal of Cr (VI) from aqueous solutions by adsorption onto hazelnut shell activated carbon, kinetic and equilibrium studies. Bioresource Technology 91 (3):317–21. doi:10.1016/j.biortech.2003.07.001.
- Kowalski, Z. 1994. Treatment of chromic tannery wastes. Journal of Hazardous Materials 37 (1):137–44. doi:10.1016/0304-3894(94)85042-9.
- Langmuir, I. 1916. The constitution and fundamental properties of solids and liquids. Journal of the American Chemical Society 38:2221–95. doi:10.1021/ja02268a002.
- Mohanty, K., M. Jha, B. C. Meikap, and M. N. Biswas. 2005. Removal of chromium (VI) from dilute aqueous solutions by activated carbon developed from Terminalia Arjuna nuts activate with zinc chloride with zinc chloride. Chemical Engineering Science 60 (11):3049–59. doi:10.1016/j.ces.2004.12.049.
- Philipot, J. M., F. Chaffange, and J. Sibony. 1984. Water Sciences Technological 17:1121–32.
- Saroj Baral, S., S. N. Das, and P. Rath. 2006. Hexavalent chromium removal from aqueous solution by adsorption on treated sawdust. Biochemical Engineering Journal 31:216–22. doi:10.1016/j.bej.2006.08.003.
- Shirzad Siboni, M., M. R. Samarghandi, S. Azizian, W. G. Kim, and S. M. Lee. 2011. The removal of hexavalent chromium from aqueous solutions using modified holly sawdust: Equilibrium and kinetics studies. Environmental Engineering Research June, 16 (2):55–60. doi:10.4491/eer.2011.16.2.55.