?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In this research, artificial neural network (ANN) model having three layers was developed for precise estimation of Cr(III) sorption rate varying from 17% to 99% by commercial resins as a result of obtaining 38 experimental data. ANN was trained by using the data of sorption process obtained at different pH (2–7) values with Amberjet 1200H and Diaion CR11 amount (0.01–0.1 g) dosage, initial metal concentration (4.6–31.7 ppm), contact time (5–240 min), and a temperature of 25°C. A feed-forward back propagation network type with one hidden layer, different algorithm (transcg, trainlm, traingdm, traincgp, and trainrp), different transfer function (logsig, tansig, and purelin) for hidden layer and purelin transfer function for output layer were used, respectively. Each model trained for cross-validation was compared with the data that were not used. The trainlm algorithm and purelin transfer functions with five neurons were well fitted to training data and cross-validation. After the best suitable coefficient of determination and mean squared error values were found in the current network, optimal result was searched by changing the number of neurons range from 1 to 20 in the current network hidden layer.
Introduction
An artificial neural network (ANN) is a mathematical model inspired by the human brain and nervous system. It is very different from a conventional computer program. ANN learns as human brain be given to any of examples of the types of data/pattern. Then, the ANN can produce a new result by applying to unknown data based on this learning.
The structure of the neural network has three components as follows: neurons, connections, and learning algorithm. This structure consists of three layers, namely input, hidden, and output. The raw data of experimental sources or external world were received by the input layer. Then, the data were presented to the user by the output layer. Between the input and output layers, the hidden layers are present where the data are processed. The number of the nerve cells in the hidden layer is significant both for the performance of the network and its length (Kaya, Kayci, and Uyar Citation2015). Although ANN analysis has been revealed approximately 60 years ago, ANN applications have been developed and used to various analysis and modeling problems in the last three decades (Kalogirou et al. Citation2015). Successfully used areas of the ANNs are engineering, medicine, mathematics, economics, neurology, psychology, meteorology, prediction of mineral exploration sites, pattern, recognition of sound and speech, military target identification, weather and market trends forecasting, robotic control, electrical and thermal load prediction, and so forth (Kalogirou Citation2001). In order to predict adsorption process and to model complicated functional relationships, ANNs can be used as most powerful tools (Sha and Edwards Citation2007). Various applications of ANN have also been achieved for the prediction of adsorption/sorption process mainly considered in the present work (Amani-Ghadim and Seyed Dorraji Citation2015; Ghaedi et al. Citation2015a; Ghaedi, Ghaedi, and Karami Citation2015; Ghaedi et al. Citation2015b; Ghanbari and Vaferi Citation2015; Kuvendziev et al. Citation2015; Maghsoudi et al. Citation2015; Shojaeimehr et al. Citation2014; Singha, Bar, and Das Citation2014; Solsvik, Chao, and Jakobsen Citation2015; Turan, Mesci, and Ozgonenel Citation2011).
In this study, the ANN model was created by considering 38 different sorption experiment parameters and the percentages of removal efficiency (r.e) for Amberjet 1200H and Diaion CR11 dependent on these parameters. Initially, the model was trained by using 30 batch study data set, then compared with other 8 batch study data (for validating data) that were unknown input in the network to test the model.
On the other hand, heavy metal contamination resulted in aqueous wastewater due to industrial activities is an important problem because they are non-biodegradable, highly toxic, and have a tendency to accumulate in the environment. Among these heavy metals, chromium with its trivalent and hexavalent forms requires particular attention since its disposal into aqueous medium may result in significant health risks. Therefore, heavy metal removal especially chromium ions from aqueous medium is a serious challenge and processes required to remove them need to be developed. Among many traditional treatment methods such as reduction, chemical precipitation, and membrane separation as well as adsorption and photocatalytic reduction, ion-exchange is one of the most preferred methods with its effectiveness and high sorption performance.
The aim of the present study is to evaluate the r.e of Amberjet 1200H and Diaion CR11 for sorption behavior in aqueous solution by using ANN analysis. For this purpose, 38 experimental data were benefited in the developed models.
Statement of Problem
Adsorbent
Two commercially available resins were used throughout the experiments. Amberjet 1200H which is a strong acid cation exchanger with sulfonate functional group on styrene-divinylbenzene copolymer and was purchased from Rohm & Haas Co. (USA) whereas Diaion CR11 having iminodiacetic acid as chelating functional group on a cross-linked polystyrene matrix was obtained from Sigma-Aldrich (Germany). The chemicals used throughout the experiments were of analytical grade. In order to prepare the solutions required in the experiments, double-distilled water was used.
Adsorbate
The batch study experiments were carried out in order to evaluate the performances of commercial resins to remove trivalent chromium from aqueous solutions. The concentrated solution of Cr(III) was prepared by dissolving the salt of Cr(NO3)3 · 9H2O purchased from Merck (Germany) company. Adjustment of solution pH was performed by using NaOH and HCl (Merck) solutions.
Sorption Studies
The standard metal solutions with different concentrations of Cr(III) were prepared by diluting the stock solution of Cr(III). For the experiments, the ion exchange resins were weighed in 25 mL Cr(III) solution at a constant stirring rate and at 25°C ± 1°C using a magnetic stirrer. After filtration with Whatman filter paper, the equilibrium Cr(III) concentration in the filtrate was analyzed with AAS (Analytical JenaContrAA 300) at 357.8 nm wavelength.
In order to investigate the effects of parameters, batch sorption experiments were carried out at 25°C ± 1°C for varying time intervals (5–240 min), by changing the resin amount from 0.01 to 0.1 g, by performing experiments at different pH values between 2 and 7 as well as by varying the solution concentration between 4.6 and 31.7 ppm. The solution pH was adjusted with 0.01 M NaOH and HCl solutions.
Artificial Neural Network
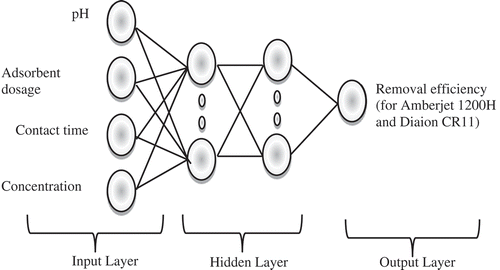
In this presented study, we developed a three-layer neural network model and MATLAB 7 platform with ANN tools was used to predict the performance of adsorption process. To develop the ANN model, 38 experimental data were used. A feed-forward back propagation (FFBP) algorithm was considered in the model. The model consists of input (contact time, adsorbent dosage, pH, and initial concentration), hidden, and output (r.e) layers as shown in .
The model has a single hidden layer configuration with five neurons initially. While “tansig”, ‘‘logsig”, and ‘‘purelin” were used as transfer functions at hidden layer, “purelin” function was tested as the output layer transfer function. In , the transfer functions used were given. The number of neurons in the hidden layer is significant for the sensitivity of ANN training. Too many or too little, the number of neurons may result in the over fitting (Kumar and Porkodi Citation2009). During the network design, finding the optimum number of neuron in the hidden layer is an important issue (Dutta et al. Citation2010). So in this study, when we find out the most suitable ANNs model with the algorithm and transfer function in the hidden layer, we changed the neurons number range from 1 to 20 in the hidden layer for optimization.
Table 1. Transfer functions used in algorithms.
In order to avoid numerical overflows and increase prediction accuracy, all the experimental data for ANN models were normalized between 0 and 1 (Sola and Sevilla Citation1997). The most commonly applied is the normalization expression as follows:
where Xi is original data, Xmin and Xmax are minimum and maximum experimental data, respectively.
The performances of the developed ANN model in the present study were evaluated through two different statistical criteria that were previously used in numerous studies. These criteria are mean squared error (MSE – Equation (2)) which measures the network’s performance and coefficient of determination (R2 – Equation (3)). The formula for the calculation of MSE and R2 are
where Yprd,i is the estimated data of ANN model, Yexp,i is the batch study data, Ym is the average of the batch study data, and n is the number of data.
Suggested Method and Results
Among heavy metals in the environment, chromium with its two oxidation states, namely, Cr(III) and Cr(VI), is known with its toxicity and hazardous effects on human health. Despite the fact that Cr(VI) is more toxic than Cr(III), the second one has also potential risks in terms of environment and health especially in the presence of an oxidizing agent since these two oxidation states of chromium are convertible with each other. For this reason, an experimental study was carried out to remove Cr(III) ions from aqueous solution by using commercially available resins. Thirty-eight experimental data sets were used to develop the ANN model with FFBP algorithm for predicting the r.e of Amberjet 1200H and Diaion CR11 for their sorption behavior in aqueous solution. The ranges of input and output data set parameters and their related statistic are indicated in .
Table 2. The ranges of data set and their statistic.
Optimization of ANNs
One of the main points in designing an ANN is the selection of appropriate parameters. For an optimized model, the selected network type, training, and transfer functions might not be appropriate. The suitable training algorithm at different layers, the number of neurons, the number of hidden layers, determination of the transfer and training functions are highly responsive parameters in the design of ANNs. The training results of ANN model should be compared with the validation data (simulated data) that were not shown to the network for cross-validation purposes. If the training results do not agree with the validation results, it would not be possible to develop a suitable model for r.e. Several parameters were used with different correlations in order to find the most suitable network model. Models were designed to generate the minimum MSE and maximum coefficient of determination (R2) values with five neurons in hidden layers, and tansig, logsig, and purelin transfer functions. When Matlab software training is completed, performances of training, validation, and test results are determined via specificity coefficient. In this study, specificity coefficients of test results given in tables are the ones which belong to training and validation specificity coefficients above 0.75. Some algorithms of training functions were used as trainscg, trainlm, traingdm, traincgp, and trainrp. For the confirmation of designed models, network training data and the other set of experimental data (validation data) were compared. These comparisons have been determined as good suitable ANN architecture.
The developed models have been modified to optimize by changing the number of neurons in the hidden layer. Thus, the number of neurons has also been optimized in the hidden layer.
R.e for Amberjet 1200H Modeling with ANNs
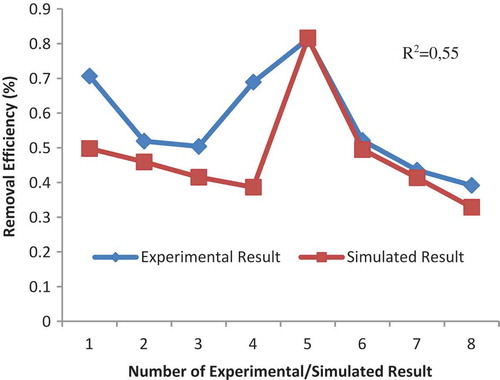
Some algorithms of training and transfer functions in the hidden layer have been tested. However, purelin transfer function has been maintained constant in the output layer. The test data R2, simulated data R2, and MSE values have been found out. Finally, the developed ANNs model’s all data are given in . shows that among the algorithms used, the training function that used trainlm and transfer function that used purelin provided the most satisfactory results for r.e. The lowest MSE = 0.04289 and highest R2test = 0.98 for training result and R2simulated = 0.55 for simulated result are found for r.e.
Table 3. Results of training and transfer functions used for removal efficiency for Amberjet 1200H.
The most suitable ANN model of simulated result and experimental result for the performance of adsorption process is given in .
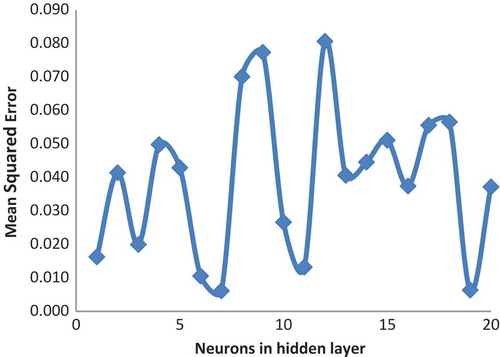
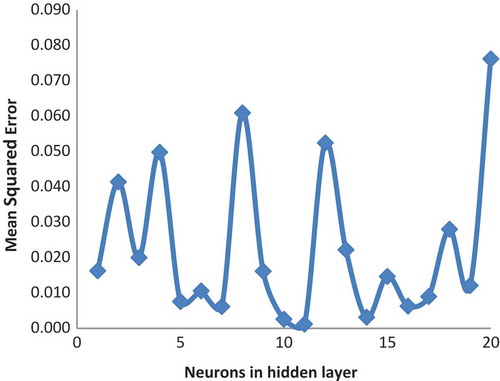
On developing the most suitable ANN model (training function as a traingdm and transfer function as a purelin), in order to model optimization, the neuron number was changed range from 1 to 20. Optimal neuron number 7, R2test = 0.99, and MSE = 0.006162 were found. When we look at the value of the performance, it is understood that the new model is an optimized one. The data belong to the developed optimal ANN model, which are presented in . Also, illustrates the relation between MSE and the number of neurons at a hidden layer for the r.e of Amberjet 1200H.
Table 4. Comparison of 20 neurons in the hidden layer for Amberjet 1200H.
R.e for Diaion CR11 Modeling with ANNs
The similar analysis for Amberjet 1200H resin was also carried out for Diaion CR11 resin and the obtained tables and figures are as follows:
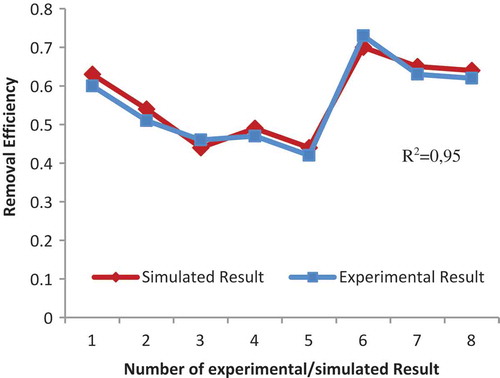
The different training and transfer functions that were tested for Diaion CR11 resin are given in . The table shows that the traincgp training function and logsig transfer function give the most suitable results for r.e. shows the comparison between experimental and simulated data of the neural network model. Also, illustrates that the relation between MSE and the number of neuron at hidden layer for the r.e of Diaion CR11.
Table 5. Results of training and transfer functions used for Diaion CR11.
In this analysis, simulated R2 was found as 0.95 and MSE was found 0.0075858 for simulated data.
The similar optimization for Amberjet 1200H resin was also performed for Diaion CR11 resin by changing the number of neurons ranges from 1 to 20 in the hidden layer. also shows the values of optimized model. Optimal neuron number 7, R2test = 0.98, and MSE = 0.006684 were found for Diaion CR11 resin.
Table 6. Comparison of 20 neurons in the hidden layer for Dıaıon CR11.
ANN modeling was used to investigate the experimental data for the r.e of Cr(III) outside the laboratory. Although many studies have been performed for the evaluation of sorption processes via ANN, this study is the first one which estimates the behavior for the removal process of Cr(III) ions by commercial resins, namely Diaion CR11 and Amberjet 1200H. By this modeling, it is resulted that Amberjet 1200H can predict r.e with 55% accuracy whereas Diaion CR11 can predict with 95% accuracy. Therefore, using Diaion CR11 for removal of Cr(III) is much more effective than the other resin and it will be possible to predict the r.e under different experimental conditions.
Conclusion
Thirty-eight different sorption experiments were performed for two different commercial resins. The ANN models were applied to these sorption systems. The first step, ANN architecture was created with FFBP network type, different training and transfer functions, and five neurons in the hidden layer. The performance of models was measured using MSE and R2. As a result, the network models were developed to be the best results with the lowest MSE and highest R2. In the first model with Amberjet 1200H resin, R2test = 0,98, R2simulated = 0.55 and MSE = 0.04289 were found while in the model with Diaion CR11 resin, R2test = 0,97, R2simulated = 0.95 and MSE = 0.0075858 were found. However, in order to determine optimum models, the current ANN architecture neuron numbers were changed with 20 neurons, respectively, in the hidden layer. Consequently, ANN model for Diaion CR11 resin was very good suitable results with seven neurons, R2test = 0.98, R2simulated = 0.97 and MSE = 0.006884 than Amberjet 1200H resins with R2test = 0.99, R2simulated = 0.85 and MSE = 0.006162. Herein, it can be observed that both test and simulated data agree with each other. Consequently, the r.e of Cr(III) was dependent on the initial metal concentration, amount of resin, pH, and contact time. The performance of Diaion CR11 resin for the removal of Cr(III) solution was successfully predicted by applying optimum architecture given as a three-layered neural network with seven neurons in the hidden layer, by using traincgp as training function, logsig as transfer function and using the back-propagation algorithm.
In this study, it can be concluded that ANN model, developed especially for Diaion CR11 resin, is a strong tool to calculate the prediction of removal efficiency more rapidly and accurately. Moreover, results obtained from the developed ANN model indicated that it is also possible to determine removal percentages very close to, even same, the experimental results without performing high-cost experiments.
References
- Amani-Ghadim, A. R., and M. S. Seyed Dorraji. 2015. Modeling of photocatalyatic process on synthesized ZnO nanoparticles: Kinetic model development and artificial neural networks. Applied Catalysis B: Environmental 163:539–46. doi:10.1016/j.apcatb.2014.08.020.
- Dutta, S., S. A. Parsons, C. Bhattacharjee, S. Bandhyopadhyay, and S. Datta. 2010. Development of an artificial neural network model for adsorption and photocatalysis of reactive dye on TiO2 surface. Expert Systems with Applications 37 (12):8634–38. doi:10.1016/j.eswa.2010.06.090.
- Ghaedi, A. M., M. Ghaedi, and P. Karami. 2015. Comparison of ultrasonic with stirrer performance for removal of sunset yellow (SY) by activated carbon prepared from wood of orange tree: Artificial neural network modeling. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 138:789–99. doi:10.1016/j.saa.2014.11.019.
- Ghaedi, M., A. Daneshfar, A. Ahmadi, and M. S. Momeni. 2015a. Artificial neural network-genetic algorithm based optimization for the adsorption of phenol red (PR) onto gold and titanium dioxide nanoparticles loaded on activated carbon. Journal of Industrial and Engineering Chemistry 21:587–98. doi:10.1016/j.jiec.2014.03.024.
- Ghaedi, M., N. Zeinali, M. Maghsoudi, and M. K. Purkait. 2015b. Artificial neural network (ANN) method for modeling of sunset yellow dye adsorption using nickel sulfide nanoparticle loaded on activated carbon: Kinetic and isotherm study. Journal of Dispersion Science and Technology 36 (9):1339–48. doi:10.1080/01932691.2014.964359.
- Ghanbari, S., and B. Vaferi. 2015. Experimental and theoretical investigation of water removal from DMAZ liquid fuel by an adsorption process. Acta Astronautica 112:19–28. doi:10.1016/j.actaastro.2015.03.011.
- Kalogirou, S. A. 2001. Artificial neural networks in renewable energy systems applications: A review. Renewable and Sustainable Energy Reviews 5 (4):373–401. doi:10.1016/S1364-0321(01)00006-5.
- Kalogirou, S. A., G. A. Florides, P. D. Pouloupatis, P. Christodoulides, and J. Joseph-Stylianou. 2015. Artificial neural networks for the generation of a conductivity map of the ground. Renewable Energy 77:400–07. doi:10.1016/j.renene.2014.12.033.
- Kaya, Y., L. Kayci, and M. Uyar. 2015. Automatic identification of butterfly species based on local binary patterns and artificial neural network. Applied Soft Computing 28:132–37. doi:10.1016/j.asoc.2014.11.046.
- Kumar, K. V., and K. Porkodi. 2009. Modelling the solid–Liquid adsorption processes using artificial neural networks trained by pseudo second order kinetics. Chemical Engineering Journal 148 (1):20–25. doi:10.1016/j.cej.2008.07.026.
- Kuvendziev, S., M. Marinkovski, K. Lisichkov, and P. Paunović. 2015. Artificial neural network modeling of Cd (II) ions adsorption on nano-porous inorganic sorbents. In Nanoscience advances in CBRN agents detection, information and energy security, 469–76. Dordrecht: Springer.
- Maghsoudi, M., M. Ghaedi, A. Zinali, A. M. Ghaedi, and M. H. Habibi. 2015. Artificial neural network (ANN) method for modeling of sunset yellow dye adsorption using zinc oxide nanorods loaded on activated carbon: Kinetic and isotherm study. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 134:1–9. doi:10.1016/j.saa.2014.06.106.
- Sha, W., and K. L. Edwards. 2007. The use of artificial neural networks in materials science based research. Materials & Design 28 (6):1747–52. doi:10.1016/j.matdes.2007.02.009.
- Shojaeimehr, T., F. Rahimpour, M. A. Khadivi, and M. Sadeghi. 2014. A modeling study by response surface methodology (RSM) and artificial neural network (ANN) on Cu 2+ adsorption optimization using light expended clay aggregate (LECA). Journal of Industrial and Engineering Chemistry 20 (3):870–80. doi:10.1016/j.jiec.2013.06.017.
- Singha, B., N. Bar, and S. K. Das. 2014. The use of artificial neural networks (ANN) for modeling of adsorption of Cr (VI) ions. Desalination and Water Treatment 52 (1–3):415–25. doi:10.1080/19443994.2013.813682.
- Sola, J., and J. Sevilla. 1997. Importance of input data normalization for the application of neural networks to complex industrial problems. IEEE Transactions on Nuclear Science 44 (3):1464–68. doi:10.1109/23.589532.
- Solsvik, J., Z. Chao, and H. A. Jakobsen. 2015. Modeling and simulation of bubbling fluidized bed reactors using a dynamic one-dimensional two-fluid model: The sorption-enhanced steam–Methane reforming process. Advances in Engineering Software 80:156–73. doi:10.1016/j.advengsoft.2014.09.011.
- Turan, N. G., B. Mesci, and O. Ozgonenel. 2011. The use of artificial neural networks (ANN) for modeling of adsorption of Cu (II) from industrial leachate by pumice. Chemical Engineering Journal 171 (3):1091–97. doi:10.1016/j.cej.2011.05.005.