?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
For successful prognosis of cardiovascular diseases (CVDs), an early and quick diagnosis is essential. Heart disease and strokes are the predominant causes and account for more than 80% of CVD deaths, whilst one-third of these deaths occurs prematurely in people under 70 years of age. For CVD diagnosis, patients need to show an elevated level of biomarkers in the blood sample associated with severe pain in the chest, and diagnostic electrocardiogram (ECG). The majority of CVD patients making CVD diagnosis difficult for physicians show a surprisingly normal ECG pattern. Artificial intelligence techniques can radically improve and optimize CVD outcomes. AI has the potential to provide novel tools and techniques to collect and interpret data and make faster and more accurate decisions reducing hospitalization cost, thereby increasing the quality of life. AI has also improved medical knowledge by unlocking clinically relevant information from the voluminous and complex data received from various resources. This paper reviews the present biosensors and describes various AI techniques, which can effectively be used for early and accurate detection of CVD, thereby improving cardiac care.
Introduction
Cardiovascular diseases (CVDs) are mainly caused due to the formation of a plaque in the artery walls that narrows the arteries, giving rise to a serious condition called atherosclerosis. This narrowing of arteries obstructs blood flow and makes the free flow of blood difficult, leading to a heart attack or stroke. CVDs are one of the leading causes of death in the world and a major obstacle for sustainable human growth and wellbeing (GBD Citation2017Causes of Death Collaborators 2018). A study reported that the occurrence of the majority of CVDs was observed in developing countries having low to middle income (Keates et al. Citation2017). It is reported that CVD deaths are less in high-income countries and comparatively more in low- and middle-income countries (Jagannathan et al. Citation2019). However, in the last few decades, the absolute burden of CVDs has been seen more in developing countries and is expected to increase further as the population ages (Jagannathan et al. Citation2019; Keates et al. Citation2017). To assess the CVD associated risk factors, several health examination surveys were conducted in some of the countries with low and middle income (Joseph et al. Citation2017; Kwan et al. Citation2016).
Bio Sensors for Risk Assessment and Early Detection of CVDs
CVD treatment-related cost and other health-care expenditures are escalating, and there is a crucial requirement for early detection followed by proper treatment of asymptomatic cardiovascular diseases (Daniel Duprez Citation2006). There is a dire need to understand the factors responsible for the initiation, related symptoms, and early and accurate detection of CVD, thereby reducing the associated high risk of unexpected death and saving huge hospitalization costs and time.
Current Status of CVD Detection Method
Current CVD detection methods rely significantly on laboratory tests and take several hours or sometimes days to produce the results. According to WHO criteria for the diagnosis of CVD, patients normally show some characteristic conditions such as severe pain in the chest, changes in diagnostic ECG, and elevated levels of the biomarker in blood sample (Yusuf et al. Citation1984).
Current CVD diagnostic techniques involve blood tests to assess relevant biomarker levels. The biomarker is measured on bio-samples such as blood, urine, or tissue tests. Other diagnosis techniques include data recording obtained from a person’s blood pressure, ECG, etc. Imaging tests like an echocardiogram or CT scan are also included.
There are different imaging techniques such as electrocardiograms, computed tomography, and angiography, which are also used for CVD detection. Although these clinical methods provide vital information about cardiovascular health, they consume lengthy time durations. They also require sample preparation and depend on the skill and experience of the physician to interpret the results correctly, which delays the treatment. This long-time schedule in diagnosis sometimes results in the deterioration of the condition of the patient.
Biomarker in the Detection of CVD
The vascular wall discharge molecules into the blood, which gives necessary information about the relevant pathological processes occurring. The concentrations of the released molecules give potential information about different pathological processes and can act as biomarkers.
An ideal cardiac biomarker has some characteristic features such as strong clinical sensitivity, very specific in marking the target molecules, quick release in the blood leading to fast diagnosis, maintaining high elevation level for longer duration in the blood, and able to assay quantitatively.
A wide range of potential biomarkers requires simultaneous analysis for the correct diagnosis of CVD (Anderson Citation2005; McDonnell et al. Citation2009; Vasan Citation2006). A research study reported a set of 177 plasma markers for the detection of cardiovascular diseases (Anderson Citation2005).
The quantification of biomarkers is required to detect CVD quickly and accurately to plan a proper strategy for the cardiac care of the patient. A quick and reliable detection of these biomarkers helps physicians to distinguish between diseases that show similar symptoms. As the clinically important sensing ranges of some of the essential biomarkers have very low concentrations, they need highly sensitive assay methods.
Biosensor for Revolution in CVD Diagnosis
Biosensors have revolutionized CVD diagnosis and prognosis. A bio-sensing device is fabricated to detect and measure specific target molecules. The underlying principle used in the design of biosensor is to immobilize a biological receptor material on an appropriate transducer’s surface, which further transforms this biochemical signal into measurable electronic signals, which can be optical, piezoelectric, electrochemical, magnetic, or acoustic signal (Daniels and Pourmand Citation2007; Krishnamoorthy et al. Citation2008; Kwon et al. Citation2011; Monson et al. Citation2009).
A variety of sensors are fabricated for CVD detection, which is very accurate, quick in detection, easy to carry, and without causing any damage to the environment. The nanotechnology industry is rapidly growing and creating highly efficient diagnostic tools involving multidisciplinary research involving nanofluids, microelectronics, and analytical chemistry (Altintas, Fakanya, and Tothill Citation2014).
Various sensor platforms are based on the optical and mechanical properties of the biosensing materials. Electrochemical-based detection techniques are preferred due to their high sensitivity, reduced cost, and faster detection, and electrochemical impedance spectroscopy (EIS) has exhibited excellent results in low concentration detection of many clinically important biomarkers used for diagnosis of CVDs. The application of biomarkers, which can be recognized in blood has attracted the interest of many researchers recently in this area (Honikel et al. Citation2018).
Different Types of Cardiac Biosensors
Existing cardiac-specific biomarkers detect CVDs using material properties such as optical, acoustic, electrochemical, etc., which require a crucial time duration ranging from hours to a few days for final results of the tests carried out in the laboratory. Optical biosensors are very sensitive and efficient biosensors. These sensors detect biomarkers by observing the variation in frequency, phase, amplitude, and state of polarization of the input light responding to the biorecognition process. Optical biosensors make use of material properties for detection of CVD such as calorimetry, fluorescence, luminescence, fiber optics etc. (Fan et al. Citation2008).
The optical characteristics of chromogenic reporters are used in ELISA-based biomarkers. Colorimetric, fluorogenic, or luminous signals are produced by these biomarkers. ELISA has been widely utilized to detect cardiac markers (Cho et al. Citation2009; Darain et al. Citation2009; Leung et al. Citation2005; Park, Cropek, and Banta Citation2010). Research is going on to further miniaturize the sensor platform without compromising on its detection ability.
Surface plasmon resonance (SPR) is an effective and widely used optical transduction sensing technique used in most commercial optical biosensors. These biosensors work on special electromagnetic waves – surface plasmon and identify variations in the refractive index (RI) on the metal surface (Homola et al. Citation2006; Park, Cropek, and Banta Citation2010).
In electrochemical biosensors, an immobilized detection probe holds together the target molecules selectively. The detection of target molecules is based on observing the variations in currents or voltages at a local surface. The potentiometric, amperometric, and impedimetric transducers used in these biosensors convert vital chemical information into a measurable amperometric signal. These biosensors are low cost and easy to miniaturize (Tweedie et al. Citation2006).
A group of researchers used micro and nano polystyrene polymer structures to design electrochemical biosensors. The study concluded that reducing the surface texture to nano-size improved the sensitivity of detection to CRP biomarkers (Kunduru et al. Citation2010).
Biological cells, pathogens, toxin antibodies, toxins, proteins, etc., can be categorized by connecting them to microbeads, which are superparamagnetic in nature. The size of these particles is in the range from a few nanometers to micrometers. The structure of magnetic beads comprises tiny magnetite crystallites encapsulated in ceramic or plastic spheres. Biological species like DNA or antibodies are coated on these beads, which selectively bind to the target analyte (Arakaki et al. Citation2004). This principle is used in making magnetic biosensors.
Limitations of Biosensor Technology in the Detection of CVDs
The number of patients suffering from cardiovascular diseases is growing at an alarming rate in the world. Precious lives can be saved if CVDs are diagnosed at an early stage of their progression. This will enable physicians to provide proper treatment and faster recovery of patients (Schernthaner et al. Citation2020).
It is critical to fabricate efficient and straightforward CVD diagnostic devices, which can quickly detect multiple cardiac biomarkers in biological fluids in a meager volume. Accurate detection using biomarkers is complicated as most of the cardiac biomarkers also mark common inflammation (Aydin et al. Citation2019; Dhingra and Vasan Citation2017). Hence, it becomes challenging to differentiate cardiovascular risks. A patient may have an inflammatory process and no cardiovascular disease at all, and still, biomarkers may report false positives (Aydin et al. Citation2019; Dhingra and Vasan Citation2017; Ghantous et al. Citation2020).
Therefore, there is an urgent need to explore new techniques and tools to make fast and accurate detections of cardiovascular risks, helping physicians in making correct clinical decisions. It has become mandatory to identify new and non-inflammatory biomarkers for CVD, develop biorecognition elements, explore biomarkers, which can directly detect CVD in biological fluids and develop miniature, portable electronic transducers (Ghantous et al. Citation2020).
It is a big challenge to have CVD diagnostic tools with high sensitivity, specificity, low-cost, fast and accurate biosensors. Artificial Intelligence provides excellent tools for the discovery of new biosensing materials for early and accurate detection of cardiovascular diseases (Vashistha et al. Citation2018).
Computational techniques clubbed with artificial intelligence (AI) are transforming our understanding of vital information from available medical data. These techniques interpret the data in the form of structured, optimized, and meaningful information, which is readily available to physicians for a timely and accurate diagnosis (Bohr and Memarzadeh Citation2020; Vashistha et al. Citation2018).
Rapidly evolving technologies based on Artificial Intelligence are noninvasively illuminating the fundamental processes contributing to cardiovascular diseases (Krittanawong et al. Citation2021). New insights into cardiovascular diseases and strokes have important therapeutic implications for an expanding population of patients. Digitized medical data combined with Information and Telecommunication techniques is revolutionizing the diagnosis and treatment of complex cardiovascular abnormalities (Bohr and Memarzadeh Citation2020; Vashistha et al. Citation2018).
Medical practitioners need to learn and follow rapidly evolving AI tools and techniques and apply them in interpreting the health-related data received from different sources in making their clinical decisions. AI applications in cardiac care open a range of possibilities to provide new personalized healthcare services (Barrett et al. Citation2019). Personalized new contacts between patients and medical practitioners are being established with mHealth and telemedicine to move healthcare from passive to pervasive activity. Physicians and scientists are integrating AI into for faster, more accurate and effective diagnosis and treatment (Barrett et al. Citation2019; Bohr and Memarzadeh Citation2020; Krittanawong et al. Citation2021).
Application of Artificial Intelligence in CVD Detection
AI technologies are being used in cardiac precision medicine, clinical assessment, cardiac imaging analysis, and automated robotic systems in cardiology. This brings faster and more accurate results in detecting CVDs (Haleem et al. Citation2021).
Image techniques have offered innovative tools to interpret data and arrive at a correct clinical assessment effectively.
Machine learning and deep learning techniques in AI not only assist medical practitioners with faster and more accurate clinical decisions but also significantly improve medical knowledge by handling huge volume and complex health data, and revealing clinically significant information about CVDs (Jiang et al. Citation2017).
The application of Information and Communication Technology is becoming central to creating a personalized and efficient healthcare service. It not only helps aged and chronically ill cardiovascular patients to receive adequate medical care in the comfort of their homes but also significantly reduces hospitalization costs and improves quality of life (Romiti et al. Citation2020). Early and accurate diagnosis and prognosis assessment is crucial to enhance CVD outcomes. The focus, however, rests on Imaging when AI is used in cardiovascular medicine.
Machine Learning and Deep Learning are Artificial Intelligence techniques, which can be effectively applied for early and accurate diagnosis, prediction of CVD outcomes and prognosis assessment of CVD.
A considerable volume of quantitative, qualitative, and transactional datasets are generated from electronic health records and other resources, which require AI techniques to be effectively and meaningfully interpreted (Murdoch and Detsky Citation2013). Some of the AI techniques used in cardiovascular medicine and digital healthcare applications are described below:
Telemedicine and Mobile Health (mHealth)
Telemedicine and mobile health (mHealth) are gaining popularity and playing a significant role in the deterrence of cardiovascular diseases and overall, up-gradation of cardiac care services. Computers, smartphones, mobile health apps, and patient monitoring services all fall under the umbrella of mHealth. Artificial Intelligence (AI) is very useful for remote follow-up of patients, medication reminders, and real-time medical counseling and early warnings of symptoms. This vital information generated can be used to access health-care information and general improvements in health-care services (Burke et al. Citation2015). mHealth supports individuals to maintain their health under physicians monitoring and allows them to continue their daily activities. From a clinicians perspective, AI can prove to be extremely effective in collecting voice medical data and connecting electronic medical records, thereby significantly reducing the work of clinicians (Johnson et al. Citation2018; Seetharam, Kagiyama, and Sengupta Citation2019). Mobile devices have shown great promise in the prevention of CVDs, with self-monitoring apps and their utilization such as diet monitoring, physical activity, blood pressure (BP), and sleep monitoring apps. These applications help patients maintain a healthy exercise routine, proper weight, controlled blood glucose, and normal BP and lipid profiles (Azar et al. Citation2013; Johnson et al. Citation2018; Neve et al. Citation2010; Rivera et al. Citation2016; Walsh, Topol, and Steinhubl Citation2014). Apple reported a study (Perez et al. Citation2019) to assess if the Apple heart Study App used for the analysis of pulse rate data collected from the Apple Watch could identify irregular heart rhythms.
Internet of Things (Iot)
The Internet of Things (IoT) comprises a network of physical devices, buildings, vehicles, and other objects integrated with electronic components, sensors, software, and network connections to collect and exchange the data. In cardiovascular diseases where timely medical help is highly essential to save patients’ lives, IoT can prove to be a boon. IoT can effectively send the data received from the patient to remote physicians who can access it and can monitor patients’ physical status in real-time (Kulkarni and Vijaykumar Citation2016; Perl et al. Citation2019; Stehlik, Schmalfuss, and Bozkurt et al. Citation2020).
In cardiovascular medicine, collected data related to blood pressure, ECG, and SpO2 will help the physician to diagnose and forecast the necessary treatment. IoT techniques, along with real-time analytical algorithms, can be instrumental in sending pre-warning to patients about possible cardiac attacks (Kulkarni and Vijaykumar Citation2016; Stehlik, Schmalfuss, and Bozkurt et al. Citation2020).
Wearable sensors combined with Machine Learning analytics can not only improve clinical outcomes but also significantly reduce hospitalizations in heart failure cases (Kulkarni and Vijaykumar Citation2016; Perl et al. Citation2019; Stehlik, Schmalfuss, and Bozkurt et al. Citation2020).
Machine Learning
Machine learning (ML) is a branch of artificial intelligence that offers a set of new algorithms and tools for the construction of inferential and predictive models, driven by data. Deep learning (DL) is a type of machine learning approach, which utilizes neural networks similar to the human brain. Neural networks are characterized by automated algorithms that can extract meaningful patterns from huge amounts of data such as images, audio, or text. It imitates human brain complexity by creating hierarchical representations from data with numerous degrees of abstraction (Mitchell Citation1997; Müller and Guido Citation2016).
Physicians diagnose the disease according to their training, knowledge, experience, and clinical results. Machine learning could prove to be extremely beneficial in expanding and improving their medical knowledge and understanding. ML models have been used by physicians for the accurate prediction and diagnosis of diseases based on clinical factors and symptoms and to treat patients satisfactorily. Echocardiographic data and clinical factors combined with AI tools can diagnose, classify, estimate the severity, and predict the adverse events in CVDs (Al’Aref et al. Citation2019; Cikes et al. Citation2019; Horiuchi et al. Citation2018; Lancaster et al. Citation2019). Ouyang et al. successfully designed an innovative video-based deep learning algorithm: EchoNet-Dynamic (Ouyang et al. Citation2020). This model can evaluate cardiac functions with an accuracy better than human experts from echocardiogram videos alone.
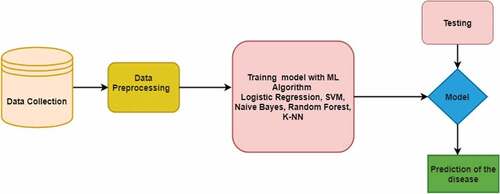
depicts the stages involved in building an ML model for disease prediction. In the first step, relevant clinical data is collected. The data is cleaned and is split into two sets – train and test. The training data is used to train the model using ML algorithms such as SVM, LR, K-NN, etc. The test data is used to check the accuracy of the model and evaluate its performance. The next step would be to select a different model entirely or to introduce extra variables to improve the model’s performance.
Machine Learning Algorithms
ML algorithms can be categorized into three types:
Supervised learning: Used to create a predictive model, which predicts the outcome based on historical data. The learning algorithm receives labeled data as input and produces the desired output. Regression and classification tasks belong to supervised learning. For example, predict rainfall based on previous years’ rainfall data (Regression task). Images of fish labeled “fish” will help the algorithm derive the rules to identify the picture of fish (Classification task) (Oladipupo Citation2010; Sarker Citation2021)
Unsupervised learning: Here, the data is unlabeled, and the learning algorithm extracts hidden patterns, associations from the input data. Some common examples of unsupervised learning include clustering, principal component analysis, association/sequence mining. For example, in the data collected from an e-commerce website, ML algorithm discovers products that are bought together frequently (Oladipupo Citation2010; Sarker Citation2021).
Semi-supervised machine learning: Semi-supervised learning is a technique that combines the benefits of both supervised and unsupervised methods. It is suitable in applications where a large amount of unlabeled data is available and it is hard or expensive to acquire labeled data. Text classification, fraud detection, and machine translation are a few of the most common examples of applications where semi-supervised learning is used (Nan Wang et al. Citation2020; Oladipupo Citation2010).
Reinforcement learning: This entails learning through interaction with a dynamic environment that gives feedback in the form of rewards and punishments. For example, actions of self-driving cars like driving speed, route selection are based on the feedback received from the environment. The model learns continuously, and the best solution is decided based on the maximum reward (Nan Wang et al. Citation2020).
The following section gives an overview of some of the commonly used ML algorithms
Decision Tree Induction
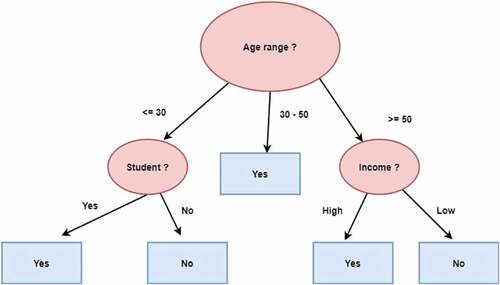
A decision tree is a supervised machine learning technique; it can be used to solve both regression, and classification problem – yet, is mostly used for classification. The algorithm splits the data set into smaller subsets recursively while creating a tree representation of the data, in which each leaf node corresponds to a class label, and the internal nodes are decision nodes, which represent the attributes. At each node, the algorithm selects the attribute that best partitions the training examples.
As an example, the decision tree shown in predicts whether a person is likely to purchase a computer. The tree is built using training data i.e., tuples with known class labels. The attributes used for branching are age if a person is a student and his credit rating. The tuple’s attribute values are tested against the decision tree for a given new tuple. A path that indicates the class prediction for that tuple is traced from the root to a leaf node (Han and Kamber Citation2011; Oladipupo Citation2010).
Basic algorithm
The tree is built in a top-down recursive manner.
Initially, all the training instances are at the root of the tree.
For attribute values, categorical values are preferred. If the values are continuous, they are discretized before being used in the model.
Training examples are recursively partitioned into subsets depending on the attributes selected. Based on a statistical calculation (e.g., Information benefit, Gain Ratio, or Gini Index), the branching attributes are chosen.
The partitioning is repeated till
All the instances for a given node have the same class
No more input features remain or too few examples left to make an informative split.
Apply the model to test data and evaluate the accuracy of the model.
Support Vector Machines
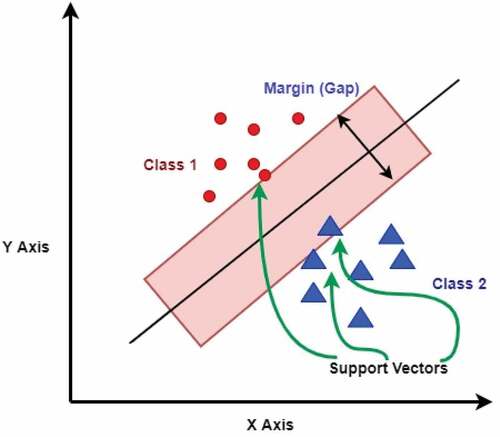
SVMs are a popular type of supervised machine learning algorithms used mostly for classification, although they may also be used for regression work. In the SVM algorithm, feature values are plotted as coordinate values in the n-dimensional space. The algorithm then finds a hyperplane that separates the dataset into classes. The hyperplane represents a decision boundary. The core idea of SVM is to find a maximum marginal hyperplane (MMH) in an iterative manner that best divides the dataset into classes with minimum error (Han and Kamber Citation2011; Joachims Citation1998).
Suppose that for a given dataset, we have to distinguish green triangles from red stars. We need to find a line that separates the data into two classes, creating a decision boundary. It is possible that there can be many lines that can do this.
The algorithm tries to find support vectors that are points that lie closest to both classes (). The next step is to compute the distance between the dividing plane and the support vectors. This distance is known as margin. The model tries to find a decision boundary for which the margin is maximum, i.e., the distance between the classes is as wide as possible, and this hyperplane is the ideal one (Joachims Citation1998). When the data is in two dimensions, the hyperplane is of one dimension. In general, for an n-dimensional data space, the algorithm creates an n-1 dimensional hyperplane.
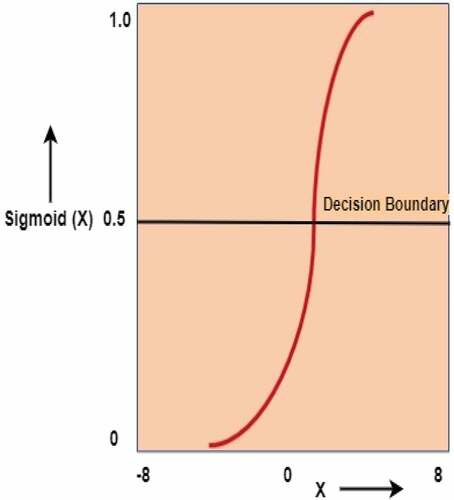
Logistic Regression
A logistic regression model fits the input data into an “S” shaped logistic curve (). The Sigmoid logistic function is used as its output to return a probability value to two or discrete groups, which can be mapped. The sigmoid function maps real values into probability values between 0 and 1 () (Ainapure et al. Citation2021; Müller and Guido Citation2016).
The equation for the Sigmoid function is this:
f(x) = output between 0 and 1 (probability prediction)
x = input to the function
e = exponential constant, a value of approximately 2.71828.
A threshold value p is picked above which it is categorized as a positive class, and below which is categorized as a negative class.
p ≥ 0.5, class = + and p < 0.5, class = -
We may choose the highest expected class for logistic regression in multiple classes.
Naïve Bayes
The Naive Bayes classifier is a probabilistic machine-learning model. It predicts the class membership probabilities based on Bayes’ Theorem as given below (Müller and Guido Citation2016; Sarker Citation2021; Han and Kamber Citation2011):
Given evidence i.e., sample data X, the posterior probability of a hypothesis H, P (H|X), follows the Bayes theorem:
The probability of occurrence of H can be found, given that X has occurred. Here, X is the evidence, and H is the hypothesis. The assumption made is that the predictors/features are independent.
P (H|X): The probability that the hypothesis holds given the observed sample X
P (H): Initial (prior) probability
P(X): Probability that sample data is observed
P (X|H) (posteriori probability), the probability of observing the sample X, given that the hypothesis holds.
Consider a training set of instances and their associated class labels, let vector X represent the parameters/features. Each instance is represented by a feature vector X = (x1, x2, …, xn). Suppose there are m classes C1, C2, …, Cm. We need to find the class Ci with maximum probability, i.e., P(Ci|X)
Applying Bayes’ theorem,
needs to be maximized, as P(X) is constant for all classes. The naïve Bayesian classifier predicts that a tuple X belongs to the class Ci if and only if the probability P (Ci|X) is the highest among
P (Ck | X), 1 ≤ k ≤ m; j < > i, for all the m classes (Han and Kamber Citation2011).
Artificial Neural Networks (ANN)
ANN algorithms are inspired by the functioning of biological neural networks; ANN is an attempt to create a network of artificial neurons working in parallel to mimic certain cognitive powers of humans (Mitchell Citation1997). Classical AI techniques are very good at puzzle and problem solving, playing games like grandmaster-level chess, Othello and complex mathematical computations. However, they perform poorly at mimicking human perceptions like recognizing an object, hearing, etc. This is where the strength of ANN lies. Deep learning models based on ANN have led to cutting edge innovations in computer vision, speech recognition, and medical science (Patel and Goyal Citation2008).
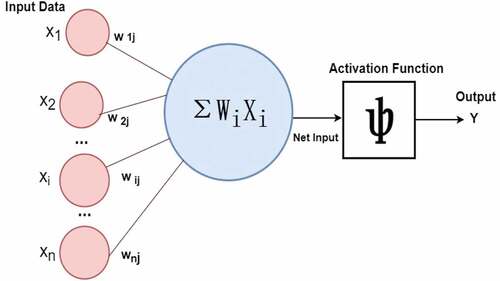
Artificial Neural Networks (ANNs) are multi-layer n/w comprised of a large number of computing units called neurons organized in interconnected layers (e.g., as in ), ANN has (Han and Kamber Citation2011)
“An input layer that accepts the independent variables or inputs of the model”
“One or many hidden layers”
“An output layer that generates predictions”
Figure 5. A multilayer feed-forward neural network [Han and Kamber Citation2011].
![Figure 5. A multilayer feed-forward neural network [Han and Kamber Citation2011].](/cms/asset/0489c838-3b66-48db-9c93-e03aeccd238a/uaai_a_2031816_f0005_oc.jpg)
Each node in a layer is connected to every other node in the next layer. A deep neural network has a large number of hidden layers. The ANN uses non-linear activation functions such as sigmoid, TanH, ReLU (Rectified Linear Unit), and Softmax (Han and Kamber Citation2011; Müller and Guido Citation2016).
As shown in , each arrow represents a connection between two neurons in adjacent layers and the flow of information. Each neuron is given a numeric weight. The weights, together with the activation function, define each neuron’s output. The ith neuron in the jth layer has a weight denoted by wi,j. The network is trained by feeding a large number of input examples (samples) into the neurons in the input layer and applying the training algorithm. Each neuron multiplies the weight with the input and generates an output, which is transferred to the next layer. The training algorithm iteratively adjusts the network’s weights so that it can minimize prediction error and produce desired output for a particular input on completing the training (Oladipupo Citation2010).
Neural networks are trained using a process called backpropagation (Zurada Citation1992), which is ideal for simple Pattern Recognition and Mapping Tasks
Steps in the training algorithm (Mitchell Citation1997; Zuranda 1992) :
Initialize the weights with small random values
The input layer is fed with a training data set
Forward pass: use provided weights and inputs for performance prediction, applying the activation function. Outputs are then passed forward to neurons in the next layer.
Calculate the difference between the real and forecasted performance. The main objective of the training is to reduce the prediction, that is, between the expected and the actual performance.
If there is an error, the algorithm adjusts the weights values.
Backpropagation, is the technique used to adjust the weights using gradient descent function. It calculates the gradient of the error function with respect to the neural network’s weights. The calculation proceeds backwards through the network.
Backward Pass: using the derived formulas, new weights are calculated.
Repeat the forward pass with the new weights
Iterate the process of backward and forward pass until the error is close or equal to zero.
Clustering
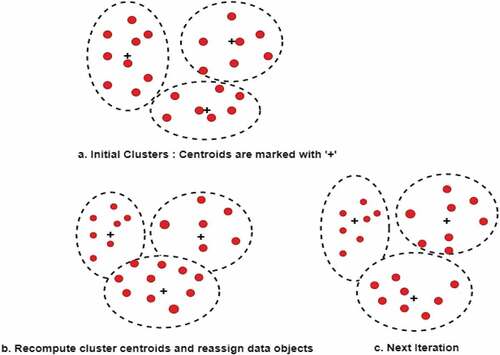
Clustering is an unsupervised machine learning technique. It is used when the class labels of the data objects are not known or not present, unlike in a classification task. Clustering is the process of assigning data objects to a number of groups or clusters, in such a way that the objects within a cluster have high intra-cluster similarity and minimum inter-cluster similarity. K-means clustering is the most widely used clustering algorithm where ‘k’ indicates the number of clusters into which we wish to partition the data objects. Initially, ‘k’ random data points are chosen as cluster centroids and the data objects are assigned to the cluster with the closest centroid. In the next step, for each cluster, the cluster centroid is recomputed, and each data point is reassigned to the closest cluster. The distance between the data object and the centroid is calculated based on Euclidian or Manhattan distance measure. The process is repeated until there is no change in the cluster assignment to objects (Han and Kamber Citation2011; Müller and Guido Citation2016). The clustering process with value of k = 3 is illustrated in .
The ML algorithms particularly SVM, ANN, and K-means discussed in the previous section, have exhibited very good, i.e.,, accuracy ~90% for the prediction of various cardio vascular diseases such as coronary artery disease, heart failure, stroke, cardiac arrhythmias, and COVID disease (Ainapure et al. Citation2021; Krittanawong et al. Citation2020). The important features considered for training the model are age, sex, cholesterol, fasting blood sugar, resting blood pressure, BMI etc. (Ainapure et al. Citation2021; Bharti et al. Citation2021; Krittanawong et al. Citation2020; Nikam et al. Citation2020).
Magnetic Resonance Imaging (CMR)
CMR imaging is emerging as a strong option for assessing several cardiovascular pathological entities. It is a very effective tool in for noninvasive measurement of left and right ventricular volumes and ejection fraction (Demirkiran et al. Citation2019).
In cardiac MRI, ventricular segmentation is an important application for implementing ML models. It not only quantifies the volumetry but also increases the efficiency and reproducibility of clinical assessments (Avendi, Kheradvar, and Jafarkhani Citation2017; Tan et al. Citation2018; Winther et al. Citation2018). It helps in detailed tissue characterization essential to diagnose, manage and treat cardiovascular diseases (Reinstadler, Thiele, and Eitel Citation2015). It has the ability to determine myocardial and vascular injuries precisely. A research study used deep learning algorithms for the segmentation of the right ventricular chamber (Avendi, Kheradvar, and Jafarkhani Citation2017). Another important application of ML has been observed in recognition of subacute or chronic myocardial scar (Baessler et al. Citation2018).
Echocardiography
Echocardiography plays a very crucial role in the diagnosis and treatment of CVDs and for correct quantitative evaluation of cardiovascular functions and structures.
AI has been successfully applied to automated measurement features including left ventricular EF, chamber dimensions, wall thickness etc. (Alsharqi et al. Citation2018; Cheema et al. Citation2019; Davis et al. Citation2018).
AI tools, specifically Machine Learning, offer new possibilities of better accuracy in interpreting images in clinical echocardiography practice. ML models reveal different features in an image; identify a range of disease patterns by considering each pixel in the image and their connections (Alsharqi et al. Citation2018). These models interpret the unused data generated by multidimensional imaging techniques like 3D echocardiography and speckle tracking thereby significantly reducing the analysis time and improving reproducibility (Alsharqi et al. Citation2018; Cheema et al. Citation2019; Davis et al. Citation2018).
Cardiac Computed Tomography
Computed tomography (CT) is a noninvasive imaging technique, which is gaining popularity in the diagnosis and monitoring of coronary artery disease (CAD). CT provides excellent visualization of the coronary arteries with high spatial resolution (Levin et al. Citation2019). ML models effectively use image analysis for quick and accurate diagnosis and cardiac risk assessment.
Key Limitations of Machine Learning in Cardiac Care
Machine learning (ML) techniques have shown a lot of promise in fast and accurate detection of CVDs. But there are some limitations in its use such as:
A large dataset is required for training depending on the model used to forecast accuracy using machine learning (Ohu et al. Citation2020).
Manual labeling is required for most of the dataset, so that labeled data is used by the ML model as a reference for training, testing, and validation (Ohu et al. Citation2020).
Large amounts of data can strengthen the power of ML, but it becomes increasingly difficult to assess their quality (Siontis et al. Citation2020).
Labeling large amounts of data manually can be expensive and time-consuming (Ohu et al. Citation2020).
ML techniques are at risk of overfitting the training data and underfitting the new data.
One of the key issues with deep-learning ML approaches is that they are a black box due to the agnostic nature of how a collection of input data is examined to produce an output (Siontis et al. Citation2020).
Another issue is that such black box models may prevent patients and doctors from engaging in meaningful shared decision-making because it is unknown what drives the model’s advice (Siontis et al. Citation2020).
Conclusions and Perspectives
n spite of significant development in the diagnosis and risk assessment techniques of CVDs, it still remains one of the leading causes of morbidity and mortality around the world. Medical records indicate that CVDs begin with the progression of risk factors, which turn into the development of subclinical atherosclerosis and finally end in overt CVD. Biomarkers are used to diagnose the disease, identify high-risk individuals, and enable physicians to develop effective CVD treatment strategies. Although clinical assessment results form the basis for patient management, they have their own constraints in terms of time, accuracy, portability etc.
With the fast spread of several diseases affecting heart and blood vessels, cases of CVDs are also on the rise, making diagnosis and prognosis both very complex and difficult. As a result, the need for new biomarkers will continue to grow. The expanding research in the discovery of new biomarkers commands a systematic organization and fast and accurate interpretation of data.
Accelerating the discovery of new biomarkers requires multidisciplinary research. It has become essential to radically shift from traditional methods of material discovery to AI-enabled modern techniques. The discovery of new biomarker materials follows a process involving conception, synthesis, and characterization of the material. These steps are carried out sequentially, and only limited materials can be tested at a time. Computational techniques combined with AI are transforming our understanding of material properties and providing a better interpretation of data generated through different material characterization techniques. The faster discovery of materials enables the researchers to develop new biomarkers for accurate and quick detection of diseases.
Technological advancements in the field of artificial intelligence (AI) have opened new areas and tools for the creation of novel modeling and predictive techniques for clinical applications in CVDs and have begun to infuse and reform cardiovascular medicine.
AI tools can significantly change the way cardiology is practiced today. Cardiac Imaging, Machine Learning, and Cognitive computing can provide the opportunity to develop efficient and accurate tools for the interpretation of data and make correct clinical decisions.
Rapidly emerging AI techniques are noninvasively revealing the fundamental processes underlying cardiovascular diseases. Digitalization of health-care practices is remarkably proving to be an effective diagnostic tool for rectifying complex heart-related abnormalities.
Multimodality images, electronic health records, and mHealth devices safely store underutilized data for each patient. AI is capable of improving the status quo, and learn from these voluminous data, generating new knowledge and understanding, and enabling physicians to apply them in distinct situations. With each heartbeat containing significant information, cardiovascular medicine is one such area, which can combine with AI to march toward personalized and precise cardio care.
The design and development of biosensors enabled by AI can be called as the next-generation biosensors and are a promising solution to the current problems in the detection and treatment of CVD. Moreover, AI also shows great potential in designing and crafting efficient wearable medical devices for real-time checking of heart rate, rhythm, and thoracic fluid. However, there are currently several challenges to adopting AI in clinical practice, with consistent efforts in this direction, AI can provide a new dimension to more personalized and precise cardiac healthcare services in the coming few years. The general understanding of AI techniques by physicians and clinicians will bring a drastic change in cardiovascular diagnosis and prognosis.
Disclosure Statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Ainapure, B. S., R. N. Pise, A. A. Wagh, and J. Tejnani. 2021. Prognosis of COVID- 19 Patients with machine learning techniques. Annals of the Romanian Society for Cell Biology 25 (6):20183–1770.
- Al’Aref, S. J., K. Anchouche, G. Singh, P. J. Slomka, K. K. Kolli, A. Kumar, M. Pandey, G. Maliakal, A. R. van Rosendael, A. N. Beecy, et al. 2019. Clinical applications of machine learning in cardiovascular disease and its relevance to cardiac imaging. European Heart Journal 40(24):1975–86. View at: Publisher Site | Google Scholar doi:10.1093/eurheartj/ehy404.
- Alsharqi, M., W. J. Woodward, J. A. Mumith, D. C. Markham, R. Upton, and P. Leeson. 2018. Artificial intelligence and echocardiography. Echo Research and Practice 5:R115–R125. View at: Publisher Site | Google Scholar doi:10.1530/ERP-18-0056.
- Altintas, Z., W. M. Fakanya, and I. E. Tothill. October 2014. Cardiovascular disease detection using bio-sensing techniques. Talanta 128(1):177–86. doi: 10.1016/j.talanta.2014.04.060.
- Anderson, L. 2005. Candidate-based proteomics in the search for biomarkers of cardiovascular disease. Journal of Physiology 563:23. doi:10.1113/jphysiol.2004.080473.
- Arakaki, S., T. Hideshima, D. Nakagawa, T. Niwa, T. Tanaka, T. Matsunaga, and T. Osaka. 2004. Detection of biomolecular interaction between biotin and streptavidin on a self-assembled monolayer using magnetic nanoparticles. Biotechnology and Bioengineering 88:543–46. doi:10.1002/bit.20262.
- Avendi, M. R., A. Kheradvar, and H. Jafarkhani. 2017. Automatic segmentation of the right ventricle from cardiac MRI using a learning-based approach. Magnetic Resonance in Medicine 78 (6):2439–48. View at: Publisher Site | Google Scholar. doi:10.1002/mrm.26631.
- Aydin, S., K. Ugur, S. Aydin, İ. Sahin, and M. Yardim. 2019. Biomarkers in acute myocardial infarction: Current perspectives. Vascular Health and Risk Management 15:1. doi:10.2147/VHRM.S166157.
- Azar, K. M. J., L. I. Lesser, B. Y. Laing, J. Stephens, M. S. Aurora, L. E. Burke, and L. P. Palaniappan . 2013. Mobile applications for weight management. American Journal of Preventive Medicine 45(5):583–89. doi:10.1016/j.amepre.2013.07.005.
- Baessler, B., M. Mannil, S. Oebel, D. Maintz, H. Alkadhi, and R. Manka. 2018. Subacute and chronic left ventricular myocardial scar: Accuracy of texture analysis on nonenhanced cine MR images. Radiology 286 (1):103–12. View at: Publisher Site | Google Scholar. doi:10.1148/radiol.2017170213.
- Barrett, M., J. Boyne, J. Brandts, H. P. Brunner-La Rocca, L. De Maesschalck, K. De Wit, B. Zippel-Schultz, C. Eurlings, D. Fitzsimons, and O. Golubnitschaja. 2019. Artificial intelligence supported patient self-care in chronic heart failure: A paradigm shift from reactive to predictive, preventive and personalised care. Epma Journal 10 (4):445–64. doi:10.1007/s13167-019-00188-9.
- Bharti, R., A. Khamparia, M. Shabaz, G. Dhiman, S. Pande, and P. Singh. 2021. Prediction of heart disease using a combination of machine learning and deep learning. Computational Intelligence and Neuroscience. 11. Article ID 8387680 doi:10.1155/2021/8387680.
- Bohr, A., and K. Memarzadeh. 2020. The rise of artificial intelligence in healthcare applications. In Artificial Intelligence in healthcare, 25–60. Academic Press. doi: 10.1016/B978-0-12-818438-7.00002-2.
- Burke, L. E., J. Ma, K. M. J. Azar, G. G. Bennett, E. D. Peterson, Y. Zheng, W. Riley, J. Stephens, S. H. Shah, B. Suffoletto, et al. 2015. Current science on consumer use of mobile health for cardiovascular disease prevention. Circulation 132(12):1157–213. View at: Publisher Site | Google Scholar doi:10.1161/CIR.0000000000000232.
- Cheema, B. S., C. Hsieh, D. Adams, A. Narang, and J. Thomas. 2019. Automated guidance and image capture of echocardiographic views using a deep learning-derived technology. Circulation 140: ([poster]). pp. A15694-A15694.
- Cho, I. H., E. H. Paek, Y. K. Kim, J. H. Kim, and S. H. Paek. 2009. Chemiluminometric enzymelinked immunosorbent assays (ELISA)-on-a-chip biosensor based on cross-flow chromatography. Analytica Chimica Acta 632:247–55. doi:10.1016/j.aca.2008.11.019.
- Cikes, M., S. Sanchez-Martinez, B. Claggett, N. Duchateau, G. Piella, C. Butakoff, A. C. Pouleur, D. Knappe, T. Biering-Sørensen, V. Kutyifa, et al. 2019. Machine learning-based phenogrouping in heart failure to identify responders to cardiac resynchronization therapy. European Journal of Heart Failure 21(1):74–85. View at: Publisher Site | Google Scholar doi:10.1002/ejhf.1333.
- Daniel Duprez, F. E. S. C. January 2006. Early detection of cardiovascular disease - the future of cardiology? E-Journal of Cardiology Practice 4:19–26.
- Daniels, J. S., and N. Pourmand. 2007. Label-free impedance biosensors: Opportunities and challenges. Electroanalysis 19:1239–57. doi:10.1002/elan.200603855.
- Darain, F., P. Yager, K. L. Gan, and S. C. Tjin. 2009. On-chip detection of myoglobin based on fluorescence. Biosensors & Bioelectronics 24:1744–50. doi:10.1016/j.bios.2008.09.004.
- Davis, A., K. Billick, K. Horton, M. Jankowski, J. E. Peg Knoll, A. P. Marshall, R. Palma, and D. B. Adams. 2018. Three-dimensional echocardiographic quantification of the left-heart chambers using an automated adaptive analytics algorithm: Multicentre validation study. European Heart Journal Cardiovascular Imaging 19:47–58.
- Demirkiran, A., H. Everaars, R. P. Amier, C. Beijnink, M. J. Bom, M. J. W. Götte, R. B. van Loon, J. L. Selder, A. C. van Rossum, R. Nijveldt, et al. 2019. Cardiovascular magnetic resonance techniques for tissue characterisation after acute myocardial injury. European Heart Journal Cardiovascular Imaging 20(7):723–34. 10.1093/ehjci/jez094 [PubMed] [CrossRef] [Google Scholar] F1000 Recommendation doi: 10.1093/ehjci/jez094.
- Dhingra, R., and R. S. Vasan. 2017. Biomarkers in cardiovascular disease: Statistical assessment and section on key novel heart failure biomarkers. Trends in Cardiovascular Medicine 27 (2):123–33. doi:10.1016/j.tcm.2016.07.005.
- Fan, X., I. M. White, S. I. Shopova, H. Zhu, J. D. Suter, and Y. Sun. 2008. Sensitive optical biosensors for unlabeled targets: A review. Analytica Chimica Acta 620:8–26. doi:10.1016/j.aca.2008.05.022.
- GBD 2017 Causes of Death Collaborators. 2018. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: A systematic analysis for the global burden of disease study. Lancet 392:1736–88.
- Ghantous, C. M., L. Kamareddine, R. Farhat, F. A. Zouein, S. Mondello, F. Kobeissy, and A. Zeidan. 2020. Advances in cardiovascular biomarker discovery. Biomedicines 8 (12):552.
- Haleem, A., M. Javaid, R. P. Singh, and R. Suman. 2021. Applications of Artificial Intelligence (AI) for cardiology during COVID-19 pandemic. Sustainable Operations and Computers 2:71–78. doi:10.1016/j.susoc.2021.04.003.
- Han, J., and M. Kamber. 2011. Data Mining: Concepts and Techniques. 3rd ed. Burlington: Morgan Kaufmann.
- Homola, J, Piliarik, M. 2006. Surface Plasmon Resonance (SPR) Sensors. In: Homola J. (eds) Surface Plasmon Resonance Based Sensors. Springer Series on Chemical Sensors and Biosensors, vol 4. Springer, Berlin, Heidelberg. https://doi.org/10.1007/5346_014
- Honikel, M. M., C. E. Lin, D. Probst, and J. T. La Belle. 2018. Facilitating earlier diagnosis of cardiovascular disease through point-of-care biosensors: A review. Critical Reviews in Biomedical Engineering 46 (1):53–82. doi:10.1615/CritRevBiomedEng.2018025818.
- Horiuchi, Y., S. Tanimoto, A. H. M. M. Latif, K. Y. Urayama, J. Aoki, K. Yahagi, T. Okuno, Y. Sato, T. Tanaka, K. Koseki, et al. 2018. Identifying novel phenotypes of acute heart failure using cluster analysis of clinical variables. International Journal of Cardiology 262:57–63. View at: Publisher Site | Google Scholar doi:10.1016/j.ijcard.2018.03.098.
- Introduction to machine learning with python. A. C. Müller, and S. Guido, Sebastopol, CA, USA: O’Reilly Media, Inc. October 2016.
- Jagannathan, R., S. A. Patel, M. K. Ali, and K. V. Narayan. 2019. Global updates on cardiovascular disease mortality trends and attribution of traditional risk factors. Current Diabetes Reports 19 (7):1–12. doi:10.1007/s11892-019-1161-2.
- Jiang, F., Y. Jiang, H. Zhi, Y. Dong, H. Li, S. Ma, Y. Wang, Q. Dong, H. Shen, and Y. Wang. 2017. Artificial intelligence in healthcare: Past, present and future. Stroke and Vascular Neurology 2:4. doi:10.1136/svn-2017-000101.
- Joachims, T. 1998. Making large-scale SVM learning practical. Adv. Kernel Methods - Support Vector Learn, MIT Press.
- Johnson, K. W., S. J. Torres, B. S. Glicksberg, K. Shameer, R. Miotto, M. Ali, E. Ashley, and J. T. Dudley . 2018. Artificial intelligence in cardiology. Journal of the American College of Cardiology 71::2668–79. doi:10.1016/j.jacc.2018.03.521.
- Joseph, P., D. Leong, M. McKee, S. S. Anand, J. D. Schwalm, K. Teo, S. Yusuf, and S. Yusuf. 2017. Reducing the global burden of cardiovascular disease, part 1: The epidemiology and risk factors. Circulation Research 121 (6):677–94. doi:10.1161/CIRCRESAHA.117.308903.
- Keates, A. K., A. O. Mocumbi, M. Ntsekhe, K. Sliwa, and S. Stewart. 2017. Cardiovascular disease in Africa: Epidemiological profile and challenges. Nature Reviews. Cardiology 14 (5):273–93. doi:10.1038/nrcardio.2017.19.
- Krishnamoorthy, S., A. A. Iliadis, T. Bei, and G. P. Chrousos. 2008. An interleukin-6 ZnO/SiO(2)/Si surface acoustic wave biosensor. Biosensors & Bioelectronics 24:313–18. doi:10.1016/j.bios.2008.04.011.
- Krittanawong, C., A. J. Rogers, K. W. Johnson, Z. Wang, M. P. Turakhia, J. L. Halperin, and S. M. Narayan. 2021. Integration of novel monitoring devices with machine learning technology for scalable cardiovascular management. Nature Reviews. Cardiology 18 (2):75–91. doi:10.1038/s41569-020-00445-9.
- Krittanawong, C., H. U. H. Virk, S. Bangalore, Z. Wang, K. W. Johnson, R. Pinotti, H. Zhang, S. Kaplin, B. Narasimhan, T. Kitai, et al. 2020. Machine learning prediction in cardiovascular diseases: A meta-analysis. Scientific Reports 10:16057. doi:10.1038/s41598-020-72685-1.
- Kulkarni, A., and S. Vijaykumar. 2016. Application of internet of things in artificial heart pacemakers and its impact on security. International Journal of Current Trends in Engineering & Research (IJCTER) 2 (5):604–10. View at: Google Scholar.
- Kunduru, V., M. Bothara, J. Grosch, S. Sengupta, P. K. Patra, and S. Prasad. 2010. Nanostructured surfaces for enhanced protein detection toward clinical diagnostics. Nanomedicine: Nanotechnology, Biology and Medicine 6 (5):642–50. doi:10.1016/j.nano.2010.03.002.
- Kwan, G. F., B. M. Mayosi, A. O. Mocumbi, J. J. Miranda, M. Ezzati, Y. Jain, G. Robles, E. J. Benjamin, S. V. Subramanian, and G. Bukhman. 2016. Endemic cardiovascular diseases of the poorest billion. Circulation 133 (24):2561–75. PubMed] [Google Scholar. doi:10.1161/CIRCULATIONAHA.116.008731.
- Kwon, Y. C., M. G. Kim, E. M. Kim, Y. B. Shin, S. K. Lee, S. D. Lee, M. J. Cho, and H. S. Ro. 2011. Development of a surface plasmon resonance-based immunosensor for the rapid detection of cardiac troponin I. Biotechnology Letters 33:921–27. doi:10.1007/s10529-010-0509-0.
- Lancaster, M. C., A. M. Salem Omar, S. Narula, H. Kulkarni, J. Narula, and P. P. Sengupta. 2019. Phenotypic clustering of left ventricular diastolic function parameters: Patterns and prognostic relevance. JACC. Cardiovascular Imaging 12 (7):1149–61. View at: Publisher Site | Google Scholar. doi:10.1016/j.jcmg.2018.02.005.
- Leung, W. M., C. P. Chan, M. F. Leung, R. Renneberg, K. Lehmann, I. Renneberg, M. Lehmann, A. Hempel, and J. F. C. Glatz. 2005. Novel digital-style rapid test simultaneously detecting heart attack and predicting cardiovascular disease risk. Analytical Letters 38:423–39. doi:10.1081/AL-200045139.
- Levin, D. C., L. Parker, E. J. Halpern, and V. M. Rao. 2019. Coronary CT angiography: Reversal of earlier utilisation trends. Journal of the American College of Radiology: JACR 16:147–55. [PubMed] [CrossRef] [Google Scholar] doi:10.1016/j.jacr.2018.07.022.
- McDonnell, B., S. Hearty, P. Leonard, and R. O’Kennedy. 2009. Cardiac biomarkers and the case for point-of-care testing. Clinical Biochemistry 42:549–61. doi:10.1016/j.clinbiochem.2009.01.019.
- Mitchell, T. 1997. Machine Learning. New York: McGraw Hill.
- Monson, C. F., L. N. Driscoll, E. Bennion, C. J. Miller, and M. Majda. 2009. Antibody–antigen exchange equilibria in a field of an external force: Design of reagentless biosensors. Analytical Chemistry 81:7510–14. doi:10.1021/ac9010759.
- Murdoch, T. B., and A. S. Detsky. 2013. The inevitable application of big data to health care. Journal of the American Medical Association 309 (13):1351–52. View at: Publisher Site | Google Scholar. doi:10.1001/jama.2013.393.
- Nan Wang, H., N. Liu, -Y.-Y. Zhang, D.-W. Feng, F. Huang, D.-S. Li, and Y.-M. Zhang . 2020. Reinforcement learning: A survey. Frontiers of Information Technology and Electronic Engineering 21(12):1726–44. doi:10.1631/FITEE.1900533.
- Neve, M., P. J. Morgan, P. R. Jones, and C. E. Collins. 2010. Effectiveness of web-based interventions in achieving weight loss and weight loss maintenance in overweight and obese adults: A systematic review with meta-analysis. Obesity Reviews 11 (4):306–21. View at: Publisher Site | Google Scholar. doi:10.1111/j.1467-789X.2009.00646.x.
- Nikam, A., S. Bhandari, A. Mhaske, and S. Mantri. 2020. Cardiovascular disease prediction using machine learning models. IEEE Pune Section International Conference (PuneCon), Pune, India. doi:10.1109/PuneCon50868.2020.9362367.
- Ohu, I., P. K. Benny, S. Rodrigues, and J. N. Carlson. 2020. Applications of machine learning in acute care research. Journal of the American College of Emergency Physicians Open 1 (5):766–72. doi:10.1002/emp2.12156.
- Ouyang, D., B. He, A. Ghorbani, N. Yuan, J. Ebinger, C. P. Langlotz, P. A. Heidenreich, R. A. Harrington, D. H. Liang, E. A. Ashley, et al. 2020. Video-based AI for beat-to-beat assessment of cardiac function. Nature 580(7802):252–56. doi:10.1038/s41586-020-2145-8.
- Park, J. P., D. M. Cropek, and S. Banta. 2010. High affinity peptides for the recognition of the heart disease biomarker troponin I identified using phage display. Biotechnology and Bioengineering 105:678–86. doi:10.1002/bit.22597.
- Patel, J., and R. Goyal. 2008. Applications of artificial neural networks in medical science. Current Clinical Pharmacology 2 (3):217–26. doi:10.2174/157488407781668811.
- Perez, M. V., K. W. Mahaffey, H. Hedlin, J. S. Rumsfeld, A. Garcia, T. Ferris, V. Balasubramanian, A. M. Russo, A. Rajmane, L. Cheung, et al. 2019. Large-scale assessment of a smartwatch to identify atrial fibrillation. New England Journal of Medicine 381(20):1909–17. View at: Publisher Site | Google Scholar doi:10.1056/NEJMoa1901183.
- Perl, L., E. Soifer, J. Bartunek, D. Erdheim, F. Köhler, W. T. Abraham, and D. Meerkin . 2019. A novel wireless left atrial pressure monitoring system for patients with heart failure, first ex-vivo and animal experience. Journal of Cardiovascular Translational Research 12(4):290–98. doi:10.1007/s12265-018-9856-3.
- Reinstadler, S. J., H. Thiele, and I. Eitel. 2015. Risk stratification by cardiac magnetic resonance imaging after ST-elevation myocardial infarction. Current Opinion in Cardiology 30 (6):681–89. 10.1097/HCO.0000000000000227 [PubMed] [CrossRef] [Google Scholar].
- Rivera, J., A. McPherson, J. Hamilton, C. Birken, M. Coons, S. Iyer, A. Agarwal, C. Lalloo, and J. Stinson . 2016. Mobile apps for weight management: A scoping review. JMIR mHealth and uHealth 4(3):e87. doi:10.2196/mhealth.5115.
- Romiti, S., M. Vinciguerra, W. Saade, I. A. Cortajarena, and E. Greco. 2020. Artificial intelligence (AI) and cardiovascular diseases: An unexpected Alliance. Cardiology Research and Practice 8. Article ID 4972346 doi:10.1155/2020/4972346.
- Sarker, I. H. 2021. Machine learning: algorithms, real-world applications and research directions. SN Computer Science 2 (160). doi: 10.1007/s42979-021-00592-x.
- Schernthaner, G., N. Shehadeh, A. S. Ametov, A. V. Bazarova, F. Ebrahimi, P. Fasching, Ž. Visockienė, P. Kempler, I. Konrāde, and N. M. Lalić. 2020. Worldwide inertia to the use of cardiorenal protective glucose-lowering drugs (SGLT2i and GLP-1 RA) in high-risk patients with type 2 diabetes. Cardiovascular Diabetology 19 (1):1–17. doi:10.1186/s12933-020-01154-w.
- Seetharam, K., N. Kagiyama, and P. P. Sengupta. 2019. Application of mobile health, telemedicine and artificial intelligence to echocardiography. Echo Research and Practice 6 (2):R41–R52. doi:10.1530/ERP-18-0081.
- Siontis, K. C., X. Yao, J. P. Pirruccello, A. A. Philippakis, and P. A. Noseworthy. 2020. How will machine learning inform the clinical care of atrial fibrillation? Circulation Research 127 (1):155–69. doi:10.1161/CIRCRESAHA.120.316401.
- Stehlik, J., C. Schmalfuss, B. Bozkurt, Nativi-Nicolau, J., Wohlfahrt, P., Wegerich, S., Rose, K., Ray, R., Schofield, R., Deswal, A., et al. 2020. Continuous wearable monitoring analytics predict heart failure hospitalisation: The LINK-HF multicenter study. Circulation. Heart Failure 13(3): e006513.
- Taiwo Oladipupo, Ayodele. 2010. Types of Machine Learning Algorithms, New Advances in Machine Learning, Yagang Zhang (Ed.), ISBN: 978-953-307-034-6, InTech. http://www.intechopen.com/books/new-advances-in-machine-learning/types-of-machine-learning-algorithms
- Tan, L. K., R. A. McLaughlin, E. Lim, Y. F. Abdul Aziz, and Y. M. Liew. 2018. Fully automated segmentation of the left ventricle in cine cardiac MRI using neural network regression. Journal of Magnetic Resonance Imaging 48 (1):140–52. View at: Publisher Site | Google Scholar. doi:10.1002/jmri.25932.
- Tweedie, M., R. Subramanian, P. Lemoine, I. Craig, E. T. McAdams, J. A. McLaughlin, B. Maccraith, and N. Kent. 2006. Fabrication of impedimetric sensors for label-free point-of-care immunoassay cardiac marker systems, with passive microfluidic delivery. Conference Proceedings – IEEE Engineering in Medicine and Biology Society, 1 4610–14.
- Vasan, R. 2006. Biomarkers of cardiovascular disease: Molecular basis and practical considerations. Circulation 113:2335. doi:10.1161/CIRCULATIONAHA.104.482570.
- Vashistha, R., A. K. Dangi, A. Kumar, D. Chhabra, and P. Shukla. 2018. Futuristic biosensors for cardiac health care: An artificial intelligence approach. 3 Biotechnology 8 (8):1–11. doi:10.1007/s13205-018-1368-y.
- Walsh, J. A., E. J. Topol, and S. R. Steinhubl. 2014. Novel wireless devices for cardiac monitoring. Circulation 130 (7):573–81. View at: Publisher Site | Google Scholar. doi:10.1161/CIRCULATIONAHA.114.009024.
- Winther, H. B., C. Hundt, B. Schmidt, C. Czerner, J. Bauersachs, F. Wacker, and J. Vogel-Claussen . 2018. ν-net: Deep Learning for Generalized Biventricular Mass and Function Parameters Using Multicenter Cardiac MRI Data. JACC. Cardiovascular Imaging 11(7):1036–38. doi:10.1016/j.jcmg.2017.11.013.
- Yusuf, S., M. Pearson, H. Sterry, S. Parish, D. Ramsdale, P. Rossi, and P. Sleight. 1984. The entry ECG in the early diagnosis and prognostic stratification of patients with suspected acute myocardial infarction. European Heart Journal 5:690–96. doi:10.1093/oxfordjournals.eurheartj.a061728.
- Zurada, J. M. 1992. Introduction to artificial neural systems. West, St. Paul, MN, USA