ABSTRACT
The burden of cardiovascular disease (CVD) is still rising after decades of growth. CVD is the leading cause of death in the Kingdom of Saudi Arabia (KSA), accounting for more than 45% of all CVD-related deaths. The development of cardiac rehabilitation (CR) programs is significantly aided by artificial intelligence (AI) approaches, including machine learning and deep learning models. However, the KSA has a limited supply of CR, and AI methods are unavailable. This review aims to assess contemporary research on AI approaches’ application, potential, and efficacy in CR as a call to action for harnessing it in the KSA. Using the keywords artificial intelligence, AI, machine learning, deep learning models, cardiac rehabilitation, and Saudi Arabia, electronic databases of PubMed, CINHAL, Web of Science, PEDro, and SCOPUS were searched to find relevant articles. Evidence from the literature supports the idea that using AI techniques in CR can improve the ability to effectively diagnose more patients in areas without doctors in Saudi Arabia. Using AI in CR has constrained CR resources, which will lessen the need for outsourcing and enhance healthcare. Patients can receive an accurate diagnosis online thanks to machine learning algorithms and the expanding capabilities of AI.
Introduction
Cardiovascular diseases (CVDs) are the most significant cause of death worldwide and a major contributor to disability (Roth et al. Citation2020). The prevalence of total CVD doubled to 523 million in 2019 from 271 million in 1990. The CVD deaths steadily increased from 12.1 million to approximately 18.6 million in the same period. Years with disability doubled from 17.7 million to 34.4 million over that period. Moreover, the CVD burden continues its decades-long rise for almost all countries outside high-income countries (Roth et al. Citation2020). The age-standardized CVD rate has risen alarmingly, whereas it was declining in some high-income countries, particularly in the Kingdom of Saudi Arabia (KSA) (Roth et al. Citation2020).
KSA is the largest country in the Arabian Peninsula with an area of 2,150,000 km2 (Bindawas and Vennu Citation2016), a population of more than 35 million (GASAT Citation2022), and a rapidly adapting increasingly urbanized lifestyle in the country (Soofi and Youssef Citation2015). Parallel to the rapid urbanization and developing economies in KSA, more than 45% of all deaths account for CVDs (Aljefree and Ahmed Citation2015), which is incredibly high among women (Alshaikh et al. Citation2016). In addition, the world’s biggest killer of ischemic heart disease (IHD) is contributing to a mortality of 7–14 for both sexes per 100,000 populations per year in the country (Huang et al. Citation2021). There is a call to action for secondary prevention in KSA.
Cardiac rehabilitation (CR) is an effective secondary prevention strategy for CVDs related mortality and morbidity cost-effectively (Balady et al. Citation2007; McMahon, Ades, and Thompson Citation2017; Wong et al. Citation2012). Shreds of evidence from randomized controlled trials and meta-analyses suggest that CR is cost-effective (Edwards et al. Citation2017; Shields et al. Citation2018), especially with exercise decreasing morbidity and mortality compared to usual care. This significantly reduces risk factors, improves clinically meaningful health-related quality of life (McGregor et al. Citation2020), and promotes a healthy lifestyle in patients with cardiovascular disease (Anderson and Taylor Citation2014; Edwards et al. Citation2017; Xia et al. Citation2018). However, home-based training appears more cost-effective, with higher satisfaction than center-based training for patients with low-to-moderate cardiac risk (Bravo-Escobar et al. Citation2017; Edwards et al. Citation2017). CR for low-risk patients can deliver equivalent benefits, if not more than medical therapies (Naci and Ioannidis Citation2015). The results of the recent meta-analysis indicated that technology-assisted cardiac rehabilitation is a potential alternative to center-based CR to remove CR barriers during the prolonged pandemic (Chong et al. Citation2021). In addition, a recent systematic review suggested that harnessing artificial intelligence (AI) in CR holds excellent potential for early detection of cardiac events, allowing for home-based monitoring and improved clinician decision-making (Sotirakos et al. Citation2022).
AI techniques, such as machine learning (ML), deep learning (DL), and cognitive computing, are on their way to having an impact on practically every aspect of human conditions, and cardiology is no exception (Mathur et al. Citation2020). AI techniques can radically change how cardiology practices, especially imaging, interpret data and make clinical decisions (Romiti et al. Citation2020). AI techniques can also improve medical knowledge due to the increase in the volume and complexity of the data, unlocking clinically relevant information (Murdoch and Detsky Citation2013). Likewise, AI techniques are becoming pivotal to creating a pervasive healthcare service through which patients with CVDs can receive CR at their homes, reducing hospitalizations and improving their quality of life (Sotirakos et al. Citation2022). Thus, harnessing AI techniques in CR can play a critical role in the early detection and diagnosis of CVDs, along with outcome prediction and prognosis evaluation (Sotirakos et al. Citation2022). Moreover, AI techniques are required to interpret massive datasets, such as quantitative, qualitative, and transactional data (Murdoch and Detsky Citation2013). As a result, physicians can make better clinical decisions by detecting clinically relevant information that can be found in the massive amount of data (Jiang et al. Citation2017; Visco et al. Citation2021).
Despite these benefits, a recent review of the literature suggested that tremendous gaps exist in how many patients can access CR worldwide (Turk-Adawi, Sarrafzadegan, and Grace Citation2014). According to that review, CR services are available in less than 40% of countries worldwide. More centrally, the characteristics of CR programs, the components delivered, their capacity, and the degree of patient access are known to be insufficient, including in Latin America and the Caribbean (LAC) countries (≥1 CR program in 24 LAC countries) (Chacin-Suarez et al. Citation2021). Given this uncertainty, the previous global survey ascertained CR availability, volumes and their drivers, and density (Turk-Adawi et al. Citation2019). According to that survey, CR is available in 54.7% (111 out of 203) countries globally (Turk-Adawi et al. Citation2019). The survey also indicated the capacity of 5,753 programs serving 1,655,083 patients/year is grossly insufficient because of an estimated 20,279,651 incident IHD cases globally per year. The average of 1,677 programs in 12 Eastern Mediterranean countries served a median of 120 (Q25–Q75 = 93–227) patients per year each. In KSA, only 1 CR program helps a median of 90 patients per year each, as shown in that global survey (Turk-Adawi et al. Citation2019).
A recent review indicated four established CR programs in KSA (Rashed et al. Citation2020). According to that review, the Physical Therapy Department in the King Faisal Specialist Hospital and Research Centre in Riyadh implemented the first CR program. Another CR service for cardiac patients was implemented at Prince Sultan Cardiac Center in Al-Ahasa. However, there is no specific information about the characteristics and duration of the program. In addition, King Khalid University Hospital (KKUH) and King Abdul-Aziz University Hospital (KAUH) have CR facilities. Still, there are several obstacles regarding patient safety standards, limited capacity, and staff qualifications (Rashed et al. Citation2020). Given the enormous burden of CVDs (Aljefree and Ahmed Citation2015; Huang et al. Citation2021) and the vastly insufficient availability of CR in KSA (Turk-Adawi et al. Citation2019), there have been no comprehensive studies that have explored AI techniques in CR delivery (Sotirakos et al. Citation2022). Thus, this article aims to review the recent literature on the scope, future, and effectiveness of AI techniques in CR as a call to action for suggesting harness of it in KSA.
Method of Literature Collection
It is customary to make the paper selection criteria for a literature review clear to ensure representativeness and confidence in the information source. The method for choosing the papers is explained as follows:
The three main components of the search scope are AI, healthcare, and CR, covering the spectrum, future, and efficacy of applying AI in CR. The collection scope is based on the primary content of this work. An incomplete reverse snowball search is conducted on the article’s reference list identified by an early keyword search to uncover more pertinent papers that the keyword might not have acquired.
The categories dictate the keywords for each part. For instance, “machine learning” OR “deep learning” AND “healthcare” are applied after fusing the keywords as AI can be divided into ML and DL according to the type of extracted characteristics. The terms “artificial intelligence,” “machine learning,” “deep learning,” and “cardiac rehabilitation” are combined to create the keywords for CR. The CR in the KSA uses the same searching procedure.
The literature that was primarily found comes from journals. The most significant pertinent works have been considered, including research articles, randomized studies, systematic reviews, and meta-analyses published in English. Most of the reviewed articles compiled in this report came from the electronic databases of PubMed, Web of Science, CINHAL Plus, and PEDro. In addition, external web archives like ResearchGate, SCOPUS, and Google Scholar are utilized.
The title and abstract of the papers that were discovered during the search were used to identify and categorize them. I studied the full texts of the relevant articles to ensure they met the criteria for high relevance and high-quality material, and I then considered them if I thought they were appropriate. As a result, collecting existing articles could serve as a good foundation for this paper’s review effort.
Cardiac Rehabilitation
The World Health Organization (WHO) is defined CR as “The sum of activity and interventions required to ensure the best possible physical, mental, and social conditions so that patients with chronic or post-acute CVDs may, by their efforts, preserve or resume their proper place in society and lead an active life” (WHO Citation1993). CR, also known as cardiac rehab, is a customized inpatient and outpatient program comprising exercises and education (Kalix Citation1988). CR programs are patient-centered and delivered by a multidisciplinary team (such as a cardiologist, nurse, physiotherapist, dietician, and psychologist) to improve patients’ quality of life, psychosocial well-being, and functional capacity (Francis et al. Citation2019). They are cost-effective (Shields et al. Citation2018). CR is integral in providing comprehensive care to patients suffering from CVDs. Therefore, it plays a critical role in the secondary prevention of coronary heart disease (Balady et al. Citation2007; McMahon, Ades, and Thompson Citation2017). CR contains exercise programs, emotional support, and education regarding lifestyle changes to reduce CVDs risks, and lifestyle changes include eating heart-healthy food, maintaining a normal weight, and quitting smoking (Balady et al. Citation2007; Leon et al. Citation2005). All cardiac rehabilitation/secondary prevention programs, according to the American Heart Association (AHA) and the American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR), should include specific core components aimed at reducing cardiovascular risk, fostering healthy behaviors, and compliance with these behaviors, reducing disability and encouraging patients with cardiovascular disease to live an active lifestyle (Suaya et al. Citation2007).
Phases in Cardiac Rehabilitation
The CR consists of three phases (Suaya et al. Citation2007). Phase I is an inpatient program that occurs when the patient is hemodynamically stable in a safe, supervised setting within 48 hours of a cardiovascular event (T. J. Wang et al. Citation2020). In this phase, the patient performs passive and active movements to maintain muscle tone and reduce sedentary risks and unfavorable complications.
Phase II is an outpatient program that recommends patients begin within 2–7 days following a percutaneous intervention, 4–6 weeks after cardiac surgery, or the intervening 6–8 weeks after discharge from the hospital (Shajrawi et al. Citation2020; Zhang et al. Citation2018). Patients must generally obtain a physician’s referral to participate in this program because of fears of overexertion or a recurrence of heart issues (Shajrawi et al. Citation2020; Supervia et al. Citation2019). Typically, participation in this program begins with an intake evaluation of cardiac risk factors, such as lipid measures, blood pressure, body composition, depression/anxiety, and tobacco use (Grace et al. Citation2016). In addition, an exercise stress test is performed to determine if exercise is safe to allow for the customized exercise program (Supervia et al. Citation2019). Risk factors are addressed, and patients’ goals are established to achieve their targets through a cardiac-trained Registered Nurse, Physiotherapist, or exercise physiologist. The patient’s heart rate and blood pressure may be monitored during exercise to check the activity intensity (Supervia et al. Citation2019). Overall, the duration of CR varies from program to program and can range from six weeks to several years. Usually, the better well-established median of 24 sessions is offered worldwide (Chaves et al. Citation2020; Santiago de Araujo Pio et al. Citation2017).
Phase III is a long-term maintenance program available to interested patients after finishing phase II of CR (Chowdhury et al. Citation2021). In this program, benefits are optimized with long-term adherence. However, this program is cost-effective as patients generally have to pay out-of-pocket. CR programs are recommended by the American Heart Association/American College of Cardiology (Smith et al. Citation2011) and the European Society of Cardiology (Piepoli et al. Citation2016), among other associations (Guha et al. Citation2017) based on their role in proving CVDs outcomes (Taylor, Dalal, and McDonagh Citation2022).
However, CR is significantly under-used globally with widely varying rates because of causes by the following multi-level factors (Grace, Kotseva, and Whooley Citation2021; Santiago de Araujo Pio et al. Citation2020). The health system level (lack of available programs) (Turk-Adawi et al. Citation2019), the provider level (low referral rates by physicians) (Ghisi et al. Citation2013), and the patient-level factors, such as transportation, distance, cost, competing responsibilities, lack of awareness and other health conditions are responsible (Shanmugasegaram et al. Citation2012). Other factors, such as women (Samayoa et al. Citation2014), ethnocultural minorities (Midence et al. Citation2014), older patients (Grace et al. Citation2009), those of lower socioeconomic status with comorbidities, and those living in rural areas (Leung et al. Citation2010) are less likely to access CR even the fact that these patients often need it most (Ruano-Ravina et al. Citation2016). To mitigate these barriers to CR use (Santiago de Araujo Pio et al. Citation2019), it is crucial for treating cardiac patients using the phone (Falter et al. Citation2019; Parak et al. Citation2021) and other technologies to institute automatic, systematic, or electronic referrals to CR (Grace et al. Citation2011). Moving to a generally safe home-based CR (Thomas et al. Citation2019), which depends on remote monitoring and coaching tactics, has now been seen to be a viable option for boosting participation rates (Sotirakos et al. Citation2021). This was one of the most effective strategies used during the COVID-19 pandemic too wherein all these rehabilitation programs were seen to be carried out remotely because of not only the risk of infection but also the overburdened healthcare system. These issues have opened new doors for harnessing AI techniques (Mathur et al. Citation2020; Romiti et al. Citation2020) to penetrate the traditional CR programs and expand the spectrum of interventions that can be done by clinicians by overcoming the challenges faced by not only the patients but also the clinicians.
Artificial Intelligence in Healthcare
Artificial intelligence (AI) techniques are on their way to impacting practically every aspect of human conditions (Mathur et al. Citation2020). AI refers to the use of technology that can make decisions based on data (Duan, Edwards, and Dwivedi Citation2019). In clinical practice, AI unveils a new realm of possibilities (Khalsa et al. Citation2021). By evaluating patterns in patient data to predict outcomes and guide treatment, AI can be efficiently utilized in healthcare to provide constructive data to clinicians (Miller Citation1994). AI has spread its roots in almost every area of medicine (Patel et al. Citation2009; Schork Citation2019). The first application of AI occurred in 1976 when it was used to successfully identify severe abdominal pain via computer analysis (Gunn Citation1976). In the real world, medical practice is changing hand in hand with the development of new AI systems, and problems from different areas have been successfully solved using AI algorithms (Davenport and Kalakota Citation2019). In terms of illness discovery and targeted treatment, the application of AI approaches in setting up or creating precision medicine is critical (Schork Citation2019). Moreover, with technology, clinical personnel can deliver a very efficient healthcare service (Visco et al. Citation2021).
AI algorithms showed promising results in clinical (Bhinder et al. Citation2021; Blease et al. Citation2019; Cao et al. Citation2021; Hibler, Qi, and Rossi Citation2016; Tran et al. Citation2021); systems (Christopoulou et al. Citation2020; Liang et al. Citation2019; Pouke and Hakkila Citation2013), and industry settings (Pisarchik, Maksimenko, and Hramov Citation2019). For example, in clinical settings, AI algorithms are an initial triage tool for accurately diagnosing and risk-stratifying patients with coronary artery disease (Wang et al. Citation2021). The accuracy of machine learning models on clinician diagnostic ability has been compared directly in a few studies (Stewart et al. Citation2021). Patient mortality, medication effects, and adverse events following treatment for acute coronary syndrome have been predicted using other algorithms (Wang et al. Citation2021). The various settings of AI models potentially enabled earlier detection of cardiac events occurring outside the hospital (Sotirakos et al. Citation2022). The utility of AI in classifying heart sounds and diagnosing valvar disease is another growing area of research (Chen et al. Citation2021). However, the challenges of AI in limited data on social determinants of health as they pertain to cardiovascular disease (Zhao et al. Citation2021). Despite challenges, AI allows for a good experience by resourcing files to find the best treatment for patients in areas where healthcare is scarce (Guo and Li Citation2018). The ability of AI to adjust to a level of individualized care that is nearly non-existent in developing countries also allows the patient to have their treatment modified based on what works for them (Guo and Li Citation2018).
Artificial Intelligence in Cardiac Rehabilitation
Most CVDs are multi-system clinical syndromes with no single quantitative indicator representing the complexity of the data needed to determine disease status (De Cannière et al. Citation2020). As a result, to adequately portray the physiological condition of CVD patients, a multi-parametric approach is required. Sensory inputs by a system integrate measurements from a heart rate monitor, an inertial measurement unit (IMU) (reporting the treadmill inclination), a Laser Range Finder-LRF (to estimate gait parameters), and periodic results from the Borg scale, as well as a user interface and an autonomous humanoid social robot platform-NAO (pronounced as NOW) (SoftBank Robotics Europe, France). Such a system was designed by (Lara et al. Citation2017)to present the three primary metrics considered in CR.
First, gait spatiotemporal parameters measurements from an LRF are used to estimate the patient’s cadence, step length, and speed of the patient. This node estimates the parameters of the kinematics of lower limbs and performs filtering of the oscillatory components contained in the user movement intention. Second, regarding cardiopulmonary parameters, a heart rate monitor (Zephyr HxM BT) is located on the user’s chest and reports a wireless and continuous heart rate measurement using Bluetooth communication. Third, physical activity difficulty parameters are used to evaluate the biological activity difficulty: one being the inclination of the treadmill and the other the patient’s reported problem with the exercise. A tactile computer monitor (i.e., a tablet) uses a graphical user interface to measure the patient’s fatigue using the Borg scale. In a CR supported by a social robot, an NAO robot periodically asks the patient to report a value on the Borg scale entered on the tablet. Novel methods to help patients with cardiac problems outside of a hospital environment have been proposed using mathematical algorithms and mobile applications (Lara et al. Citation2017; Medina Quero et al. Citation2017).
AI for Quitting Tobacco Initiative
Lifestyle modifications play an essential role in CR, and quitting tobacco is crucial for many individuals (Balady et al. Citation2007). Amazon Web Services and Google Cloud have supported San Francisco and New Zealand-based Digital People company Soul Machines to create Florence. Florence is a robot that develops a tobacco quit plan that is outlined as follows (Kaplan et al. Citation2001):
The patient is required to set a quit date. They must set up a quit date as soon as possible. The reason is that giving oneself a short period to quit will keep one focused and motivated to achieve a goal.
The patient is advised to involve their friends, family, and coworkers since it is essential to share their goal of quitting with those they frequently interact.
The patient is required to anticipate challenges to the upcoming quit attempt. The first few weeks especially are critical and the hardest to overcome due to the potential nicotine withdrawal symptoms and the obstacles usually presented while breaking any habit.
The patient is asked to remove tobacco products from their environment to avoid distractions.
Mobilizations and Walking Monitors Using Smartwatches
The program in CR is embedded in a wrist-worn device with a heart rate sensor, which provides real-time physical activity monitoring during sessions safely and effectively. Moreover, wearable devices have been demonstrated to favor strategies for changes to healthy habits and the promotion of healthful physical activity (Case et al. Citation2015). For this, a linguistic approach based on fuzzy logic was proposed to model the cardiac rehabilitation protocol and the expert knowledge from the cardiac rehabilitation team. Fuzzy logic has not only provided successful results in developing intelligent systems from sensor data streams but has also been described as an effective modeling tool in CR (Peláez-Aguilera et al. Citation2019). The following steps can effectively achieve this:
It integrates a high-quality protocol based on clinical guidelines for monitoring a patient’s heart rate in a personalized way that will suit the patient’s needs, like diet specifications, exercise type, and duration, and titrating the dose of medications regularly.
A wearable wrist-worn device with a heart rate sensor provides real-time monitoring during sessions.
A wearable-mobile-cloud platform is being used to collect and synchronize data between patients and the CR team.
Decision Support Systems (DSS)
DSS are computational applications that improve the abilities of an individual or group of individuals to make decisions (Main et al. Citation2010). In recent years, its use in clinical practice is gaining acknowledgment of its benefits in terms of reducing diagnostic errors (Linder et al. Citation2009), increasing conformity with clinical guidelines (Roshanov et al. Citation2013), and potentially increasing healthcare quality (Roshanov et al. Citation2013). There are different types of DSS, but the most suitable for clinical DSS is the knowledge-driven, which uses AI and statistical inference in codified knowledge to assist in specialized problem solutions, suggesting or recommending actions. Rules, clinical standards, or probabilistic networks can all be used to represent knowledge in a knowledge-driven clinical DSS (Kong, Xu, and Yang Citation2008).
Virtual Cardiac Rehabilitation (VCR)
A home-based CR program with guided exercise usually takes place post-acute CR. In the last two years, telerehabilitation has been implemented worldwide, and VCR was implemented to overcome the challenges created by the COVID-19 pandemic. An application that offers home-based CR through videos and monitors has been adapted as a solution to address this situation (Pérez-Robledo et al. Citation2021).
Sensor Derived Biomarkers
A reasonable interpretation of physical fitness is required to track a patient’s progress during a CR program. Various wearable sensors that can track multiple physiological signals reflective of physical fitness are available today, and their implementation within a cardiac population has been investigated extensively (Gevaert et al. Citation2020). However, for clinical acceptance, the easy interpretability of the AI models is crucial (De Canniere et al. Citation2020). The sensors can visualize the relationship between sensor-derived biomarkers and functional capacity, which monitors the clinical progression of patients throughout the CR program. These findings pave the way for the use of AI approaches that can be interpreted by healthcare professionals and help translate CR to a home environment (De Canniere et al. Citation2020). Studies have shown that the combination of sensor-derived biomarkers, i.e., during a standardized 6-Minute Walk Test (6MWT), chronotropic reaction, and effort can be used as a surrogate for functional capacity in a CR (De Canniere et al. Citation2020).
Future of Artificial Intelligence in Cardiac Rehabilitation
Covid-19 has thrown light on people’s capacity in terms of what they can do while they are stuck at home. The whole world has learned to function virtually and effectively as a consequence. There is no single problem in this world that does not have a solution. This being said, the day is not far from when we incorporate AI in our daily lives and make CR more simple with algorithms, devices, and virtual applications that can safely and accurately monitor safety, adherence, and compliance and ensure a successful treatment (Barrett et al. Citation2019; Gensini et al. Citation2017). This would be a significant path-breaking step in medicine and will result in a reduction in mortality and morbidity due to CVDs which are the most common cause of death worldwide, specifically in KSA. The AI techniques in CR provide various tools to enhance and extend the effectiveness of CR teams and programs (Johnson et al. Citation2018). Thus, more comprehensive and interdisciplinary courses ought to come, and innovative teams will bring out solutions with creative and brainstorming sessions in the future.
Because AI-based technologies assist clinicians in daily medical activities, enhancing illness identification and treatment, personalized patient care via telemonitoring may soon improve clinical practice (Visco et al. Citation2021). Indeed, AI may obviate much of the tedium of modern-day clinical practice, such as interacting with Electronic Health Records and billing, which will soon be intelligently automated to a much greater extent (Steinhubl and Topol Citation2015). Although personnel with specialized training often perform machine learning, these methods will become increasingly easy and commoditized in the future.
The expert knowledge of pathophysiology and clinical presentation that clinicians acquire over their training and career will remain vital. Therefore, clinicians should lead in deciding where to apply and how to interpret these models (Konstam et al. Citation2017). AI will help deliver improved patient care because clinicians can analyze more data in greater depth than ever. Reinforcement learning algorithms will be used as companion clinicians’ aids, inconspicuously supporting clinicians while expediting clinical treatment (Konstam et al. Citation2017; Steinhubl and Topol Citation2015). Advances in unsupervised machine learning will enable far greater characterization of patients’ disorders and ultimately lead to better treatment selection and improved outcomes (Shameer et al. Citation2018). Future physicians will be able to monitor, analyze, and respond to new streams of biological data collected remotely and automatically because of the spread of AI (Shameer et al. Citation2018).
A recent network meta-analysis of patients with heart failure, stroke, hypertension, and diabetes was performed by comparing the machine learning and deep learning models for CVDs prediction (Baashar et al. Citation2022). A piece of evidence from the network meta-analysis indicated that the deep learning algorithms predicted well in heart failure with an area under the curve of 0.843 [CI = 0.840–0.845], while the machine learning algorithm achieved an average accuracy of 91.1% in predicting heart failure. These findings suggest that the prediction of and knowledge about heart failure is effective with deep learning models, but these models in the field of CVDs are unknown. A very recent systematic review evaluated the efficacy of AI in cardiac rehabilitation using the current body of research in the literature (Sotirakos et al. Citation2022). Evidence from this review showed that AI-driven CR programs hold immense potential for patients and clinicians alike. Mainly, good clinical outcomes were observed with the telehealth delivery of CR, as shown previously (Jin et al. Citation2019). The evidence also showed that harnessing AI tools in existing telehealth cardiac rehabilitation programs could provide a personalized and adaptive rehabilitation program that responds to individual clinical needs (Falter, Scherrenberg, and Dendale Citation2020). Findings indicated that AI in CR improved communication between patient and clinician by increasing patient-clinician interaction and making cardiac rehabilitation more accessible to those in geographically or socially disadvantaged areas or during a pandemic (Widmer et al. Citation2017). Additionally, the review suggested that clinicians may benefit from AI-based tools by using clinical decision-making support tools or accessing a patient’s smartwatch data (Diciolla et al. Citation2015). Such a system may serve helpful in the early detection, diagnosis, and treatment of cardiac arrhythmias or in tailoring a rehabilitation program of care (Omura et al. Citation2017).
To summarize, the research indicates that CR programs are patient-centered, affordable, and provided by a multidisciplinary team. It is essential for the secondary prevention of coronary heart disease. It can help with a specialized program that includes workouts and education to enhance patients’ functional ability, quality of life, and psychosocial well-being. However, evidence from the literature revealed that due to the multi-level variables discussed above, CR is severely underutilized internationally and in the KSA, with rates that vary greatly. The evidence implies that using AI technology to lessen these barriers to CR use is essential for treating cardiac patients (Santiago de Araujo Pio et al. Citation2019). Given how AI techniques practically affect every area of human conditions (Mathur et al. Citation2020), a request is that the KSA utilize AI methods for identifying trends in patient data to forecast outcomes and direct care, especially in CR. Using algorithms, gadgets, and virtual applications that can reliably and safely monitor safety, adherence, and compliance to ensure a successful treatment, according to lessons learned from the COVID-19 epidemic, is another way that integrating AI in CR may be made simpler (Barrett et al. Citation2019; Gensini et al. Citation2017).
Artificial Intelligence Techniques in the Kingdom of Saudi Arabia
The KSA has taken severe and broad steps to harness AI techniques in various fields, especially after announcing a vision for 2030, in which the transition to digitalization is one of its biggest goals. Therefore, it considers AI an important strategic option to achieve this goal and its ambition to hold a highly distinguished position among the world’s nations. Moreover, the recent paper showed KSA’s efforts in developing AI techniques in different fields, especially in the education and healthcare sectors (Al-Jehani et al. Citation2021). The report showed that KSA has contributed by AI techniques to facilitating the performance of indispensable daily work, such as holding E-Learning, performing remote work, and even serving people who need medical information about Coronavirus. In addition, the paper showed the use of robots, innovative pharmacies, and some applications, such as TABAOD and TAWAKKALNA. Similar to how they help in daily medical tasks, AI approaches in CR across the KSA improve sickness recognition and treatment while customizing patient care. The monotony of contemporary clinical practice, such as communicating with electronic health records and billing, may also be much reduced by AI in the future. These tasks will soon be intelligently automated to a far more considerable degree.
Recommendations
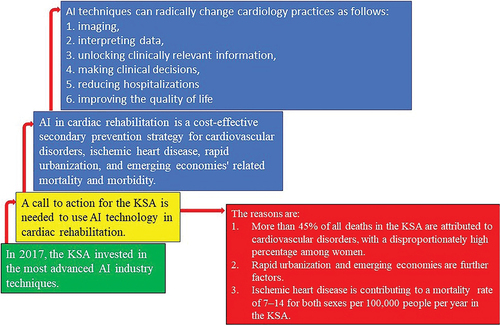
The flowchart of recommendations is shown in . In KSA, the most advanced AI industry techniques were invested in 2017 (Al-Jehani et al. Citation2021). However, this nation can consider a call to action for harnessing AI techniques in CR because more than 45% of all deaths account for CVDs (Aljefree and Ahmed Citation2015) parallel to the rapid urbanization and developing economies, exceptionally high among women (Alshaikh et al. Citation2016). In addition, the world’s biggest killer of IHD is contributing to a mortality of 7–14 for both sexes per 100,000 populations per year in this country (Huang et al. Citation2021). Thus, there has to be a call to action for secondary prevention in KSA using AI in CR, which is an effective secondary prevention strategy for CVDs related mortality and morbidity cost-effectively (Balady et al. Citation2007; McMahon, Ades, and Thompson Citation2017; Wong et al. Citation2012). Moreover, AI techniques can radically change the way cardiology practices (Romiti et al. Citation2020). Especially imaging, interpreting data, unlocking clinically relevant information (Murdoch and Detsky Citation2013), making clinical decisions, reducing hospitalizations, and improving quality of life (Sotirakos et al. Citation2022) aligned with the aspirations of Saudi Arabia’s vision 2030 (Sotirakos et al. Citation2022). Therefore, the capacity of AI to alter course is essential since it enables patients in the KSA to have their therapy updated based on what is most effective for them. Additionally, patients can receive an accurate diagnosis online thanks to sophisticated ML algorithms and the expanding capabilities of AI.
Conclusion
AI has roots in practically every area of medicine, including in CR. The algorithms used by AI have produced encouraging outcomes in clinical, system, and industry settings to give helpful information to physicians. The ability of various wearable sensors to measure multiple physiological signals indicative of physical fitness and their application within a cardiac population has been thoroughly studied. Artificial intelligence is predicted to simplify algorithms, hardware, and software in CR in the future so that it can accurately and safely monitor patient safety, adherence, and compliance. These results pave the path for applying AI techniques that support the translation of CR to the home environment and can be understood by healthcare practitioners. Therefore, in line with the KSA’s 2030 vision, this review issues a call to action for using AI approaches in CR. Because CVDs are the leading cause of death in KSA, using AI techniques in CR can increase the effectiveness of diagnosing more patients in KSA without doctors.
Acknowledgements
The author extends his appreciation for technical support to Dr. Vishal Vennu.
Disclosure Statement
No potential conflict of interest was reported by the author.
References
- Aljefree, N., and F. Ahmed. 2015. Prevalence of cardiovascular disease and associated risk factors among adult population in the gulf region: A systematic review. Advances in Public Health 2015:1–683. 2015. doi:10.1155/2015/235101.
- Al-Jehani, N. B., Z. A. Hawsawi, N. Radwan, and M. Farouk. 2021. Development of artificial intelligence techniques in Saudi Arabia: The impact on COVID-19 pandemic. literature review. Journal of Engineering Science and Technology 16(6):4530–47.
- Alshaikh, M. K., F. T. Filippidis, J. P. Baldove, A. Majeed, and S. Rawaf. 2016. Women in Saudi Arabia and the prevalence of cardiovascular risk factors: A systematic review. Journal of Environmental and Public Health 2016 2016:1–15. doi:10.1155/2016/7479357.
- Anderson, L., and R. S. Taylor. 2014. Cardiac rehabilitation for people with heart disease: An overview of cochrane systematic reviews. Cochrane Database System Review 2014 CD011273. doi:10.1002/14651858.CD011273.pub2.
- Baashar, Y., G. Alkawsi, H. Alhussian, L. F. Capretz, A. Alwadain, A. A. Alkahtani, and M. Almomani. 2022. Effectiveness of artificial intelligence models for cardiovascular disease prediction: Network meta-analysis. Computational intelligence and neuroscience 2022:5849995. doi:10.1155/2022/5849995.
- Balady, G. J., M. A. Williams, P. A. Ades, V. Bittner, P. Comoss, J. M. Foody, Barry Franklin, Bonnie Sanderson, and Douglas Southard . 2007. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: A scientific statement from the American heart association exercise, cardiac rehabilitation, and prevention committee, the council on clinical cardiology; the councils on cardiovascular nursing. epidemiology and prevention, and nutrition, physical activity, and metabolism; and the American association of cardiovascular and pulmonary rehabilitation. epidemiology and prevention, and nutrition, physical activity, and metabolism; and the American association of cardiovascular and pulmonary rehabilitation Circulation 115:2675–82. doi:10.1161/CIRCULATIONAHA.106.180945.
- Barrett, M., J. Boyne, J. Brandts, H. P. Brunner-La Rocca, L. De Maesschalck, K. De Wit, Lana Dixon, Eurlings, Casper Fitzsimons, Donna and Golubnitschaja, Olga . 2019. Artificial intelligence supported patient self-care in chronic heart failure: A paradigm shift from reactive to predictive, preventive and personalised care. The EPMA Journal. 10(4):445–64. doi:10.1007/s13167-019-00188-9.
- Bhinder, B., C. Gilvary, N. S. Madhukar, and O. Elemento. 2021. Artificial Intelligence in cancer research and precision medicine. Cancer Discovery 11 (4):900–15. doi:10.1158/2159-8290.CD-21-0090.
- Bindawas, S. M., and V. S. Vennu. 2016. Stroke rehabilitation. a call to action in Saudi Arabia. Neurosciences (Riyadh) 21 (4):297–305. doi:10.17712/nsj.2016.4.20160075.
- Blease, C., T. J. Kaptchuk, M. H. Bernstein, K. D. Mandl, J. D. Halamka, and C. M. DesRoches. 2019. Artificial intelligence and the future of primary care: exploratory qualitative study of UK general practitioners’ views. Journal of Medical Internet Research 21 (3):e12802. doi:10.2196/12802.
- Bravo-Escobar, R., A. Gonzalez-Represas, A. M. Gomez-Gonzalez, A. Montiel-Trujillo, R. Aguilar-Jimenez, R. Carrasco-Ruiz, and P. Salinas-Sanchez. 2017. Effectiveness and safety of a home-based cardiac rehabilitation programme of mixed surveillance in patients with ischemic heart disease at moderate cardiovascular risk: A randomised, controlled clinical trial. BMC cardiovascular disorders 17 (1):66. doi:10.1186/s12872-017-0499-0.
- Cao, J. S., Z. Y. Lu, M. Y. Chen, B. Zhang, S. Juengpanich, J. H. Hu, Li, Shi-Jie, Topatana, Win, Zhou, Xue-Yin, and Feng, Xu . 2021. Artificial intelligence in gastroenterology and hepatology: Status and challenges. World Journal of Gastroenterology: WJG. 27(16):1664–90. doi:10.3748/wjg.v27.i16.1664.
- Case, M. A., H. A. Burwick, K. G. Volpp, and M. S. Patel. 2015. Accuracy of smartphone applications and wearable devices for tracking physical activity data. JAMA 313 (6):625–26. doi:10.1001/jama.2014.17841.
- Chacin-Suarez, A., S. L. Grace, C. Anchique-Santos, M. Supervia, K. Turk-Adawi, R. R. Britto,Dawn C. Scantlebury, Felipe Gonzalez Graciela, and Benaim, Briseida. 2021. Cardiac rehabilitation availability and characteristics in Latin America and the Caribbean: A global Comparison. American Heart Journal 240:16–27. doi:10.1016/j.ahj.2021.05.010.
- Chaves, G., K. Turk-Adawi, M. Supervia, C. Santiago, A. de Pio, A. H. Abu-Jeish, T. Mamataz, S. Tarima, F. Lopez Jimenez, and S. L. Grace. 2020. Cardiac Rehabilitation Dose Around the World: Variation and Correlates. Circulation Cardiovascular Quality and Outcomes 13 (1):e005453. doi:10.1161/CIRCOUTCOMES.119.005453.
- Chen, W., Q. Sun, X. Chen, G. Xie, H. Wu, and C. Xu. 2021. Deep learning methods for heart sounds classification: A systematic review. Entropy (Basel) 23 (6):667. doi:10.3390/e23060667.
- Chong, M. S., J. W. H. Sit, K. Karthikesu, and S. Y. Chair. 2021. Effectiveness of technology-assisted cardiac rehabilitation: A systematic review and meta-analysis. International Journal of Nursing Studies 124:104087. doi:10.1016/j.ijnurstu.2021.104087.
- Chowdhury, M., F. A. Heald, J. C. Sanchez-Delgado, M. Pakosh, A. M. Jacome-Hortua, and S. L. Grace. 2021. The effects of maintenance cardiac rehabilitation: A systematic review and Meta-analysis, with a focus on sex. Heart & Lung 50 (4):504–24. doi:10.1016/j.hrtlng.2021.02.016.
- Christopoulou, F., T. T. Tran, S. K. Sahu, M. Miwa, and S. Ananiadou. 2020. Adverse drug events and medication relation extraction in electronic health records with ensemble deep learning methods. Journal of the American Medical Informatics Association: JAMIA 27 (1):39–46. doi:10.1093/jamia/ocz101.
- Davenport, T., and R. Kalakota. 2019. The potential for artificial intelligence in healthcare. Future Healthcare Journal 6 (2):94–98. doi:10.7861/futurehosp.6-2-94.
- De Canniere, H., F. Corradi, C. J. P. Smeets, M. Schoutteten, C. Varon, C. Van Hoof, S. Van Huffel, W. Groenendaal, and P. Vandervoort. 2020. Wearable monitoring and interpretable machine learning can objectively track progression in patients during cardiac rehabilitation. Sensors (Basel) 20 (12):3601. doi:10.3390/s20123601.
- De Cannière, H., F. Corradi, C. J. Smeets, M. Schoutteten, C. Varon, C. Van Hoof, S. Van Huffel, W. Groenendaal, and P. Vandervoort. 2020. Wearable monitoring and interpretable machine learning can objectively track progression in patients during cardiac rehabilitation. Sensors 20 (12):3601. doi:10.3390/s20123601.
- Diciolla, M., G. Binetti, T. Di Noia, F. Pesce, F. P. Schena, A. M. Vagane, R. Bjorneklett, H. Suzuki, Y. Tomino, and D. Naso. 2015. Patient classification and outcome prediction in IgA nephropathy. Computers in Biology and Medicine 66:278–86. doi:10.1016/j.compbiomed.2015.09.003.
- Duan, Y., J. S. Edwards, and Y. K. Dwivedi. 2019. Artificial intelligence for decision making in the era of big data–evolution, challenges and research agenda. International Journal of Information Management 48:63–71. doi:10.1016/j.ijinfomgt.2019.01.021.
- Edwards, K., N. Jones, J. Newton, C. Foster, A. Judge, K. Jackson, N. K. Arden, and R. Pinedo-Villanueva. 2017. The cost-effectiveness of exercise-based cardiac rehabilitation: A systematic review of the characteristics and methodological quality of published literature. Health Economics Review 7 (1):37. doi:10.1186/s13561-017-0173-3.
- Falter, M., W. Budts, K. Goetschalckx, V. Cornelissen, and R. Buys. 2019. Accuracy of apple watch measurements for heart rate and energy expenditure in patients with cardiovascular disease: Cross-sectional study. JMIR Mhealth Uhealth 7 (3):e11889. doi:10.2196/11889.
- Falter, M., M. Scherrenberg, and P. Dendale. 2020. Digital health in cardiac rehabilitation and secondary prevention: A search for the ideal tool. Sensors (Basel) 21 (1):12. doi:10.3390/s21010012.
- Francis, T., N. Kabboul, V. Rac, N. Mitsakakis, P. Pechlivanoglou, J. Bielecki, D. Alter, and M. Krahn. 2019. The effect of cardiac rehabilitation on health-related quality of life in patients with coronary artery disease: A meta-analysis. The Canadian Journal of Cardiology 35 (3):352–64. doi:10.1016/j.cjca.2018.11.013.
- General Authority for Statistics. 2022. Population Estimates. https://www.stats.gov.sa/en (last accessed January 12 2023).
- Gensini, G. F., C. Alderighi, R. Rasoini, M. Mazzanti, and G. Casolo. 2017. Value of telemonitoring and telemedicine in heart failure management. Card Fail Review 3 (2):116–21. doi:10.15420/cfr.2017:6:2.
- Gevaert, A. B., V. Adams, M. Bahls, T. S. Bowen, V. Cornelissen, M. Dorr, D. Hansen, H. M. Kemps, P. Leeson, E. M. Van Craenenbroeck, et al. 2020. Towards a personalised approach in exercise-based cardiovascular rehabilitation: How can translational research help? a ’call to action’ from the section on secondary prevention and cardiac rehabilitation of the European Association of preventive cardiology. European Journal of Preventive Cardiology. 27(13):1369–85. doi:10.1177/2047487319877716.
- Ghisi, G. L., P. Polyzotis, P. Oh, M. Pakosh, and S. L. Grace. 2013. Physician factors affecting cardiac rehabilitation referral and patient enrollment: A systematic review. Clinical cardiology 36 (6):323–35. doi:10.1002/clc.22126.
- Grace, S. L., K. Kotseva, and M. A. Whooley. 2021. Cardiac rehabilitation: under-utilized globally. Current Cardiology Reports 23 (9):118. doi:10.1007/s11886-021-01543-x.
- Grace, S. L., K. L. Russell, R. D. Reid, P. Oh, S. Anand, J. Rush, Karen Williamson, Milan Gupta, David A. Alter, and Donna E. Stewart. 2011. Effect of cardiac rehabilitation referral strategies on utilization rates: A prospective, controlled study. Archives of Internal Medicine. 171(3):235–41. doi:10.1001/archinternmed.2010.501.
- Grace, S. L., S. Shanmugasegaram, S. Gravely-Witte, J. Brual, N. Suskin, and D. E. Stewart. 2009. Barriers to cardiac rehabilitation: Does age make a difference? Journal of Cardiopulmonary Rehabilitation and Prevention 29 (3):183–87. doi:10.1097/HCR.0b013e3181a3333c.
- Grace, S. L., K. I. Turk-Adawi, A. Contractor, A. Atrey, N. Campbell, W. Derman, Gabriela L Melo Oldridge, Neil Sarkar, Bidyut K Yeo, Tee Joo. 2016. Cardiac rehabilitation delivery model for low-resource settings. Heart. 102(18):1449–55. doi:10.1136/heartjnl-2015-309209.
- Guha, S., R. Sethi, S. Ray, V. K. Bahl, S. Shanmugasundaram, P. Kerkar, et al. 2017. Cardiological society of India: Position statement for the management of ST elevation myocardial infarction in India. Indian Heart Journal 69(1):S63–97. Suppl. doi:10.1016/j.ihj.2017.03.006.
- Gunn, A. A. 1976. The diagnosis of acute abdominal pain with computer analysis. Journal of the Royal College of Surgeons of Edinburgh 21 (3):170–72. Retrieved from. https://www.ncbi.nlm.nih.gov/pubmed/781220.
- Guo, J., and B. Li. 2018. The application of medical artificial intelligence technology in rural areas of developing countries. Health Equity 2 (1):174–81. doi:10.1089/heq.2018.0037.
- Hibler, B. P., Q. Qi, and A. M. Rossi. 2016. Current state of imaging in dermatology. Seminars in Cutaneous Medicine and Surgery 35 (1):2–8. doi:10.12788/j.sder.2016.001.
- Huang, K. S., D. X. He, D. J. Huang, Q. L. Tao, X. J. Deng, B. Zhang, G. Mai, and D. Guha-Sapir. 2021. Changes in ischemic heart disease mortality at the global level and their associations with natural disasters: A 28-year ecological trend study in 193 countries. Plos One 16 (7):e0254459. doi:10.1371/journal.pone.0254459.
- Jiang, F., Y. Jiang, H. Zhi, Y. Dong, H. Li, S. Ma, Y. Wang, Q. Dong, H. Shen, and Y. Wang. 2017. Artificial intelligence in healthcare: Past, present and future. Stroke Vascular Neurology 2 (4):230–43. doi:10.1136/svn-2017-000101.
- Jin, K., S. Khonsari, R. Gallagher, P. Gallagher, A. M. Clark, B. Freedman, T. Briffa, A. Bauman, J. Redfern, and L. Neubeck. 2019. Telehealth interventions for the secondary prevention of coronary heart disease: A systematic review and meta-analysis. European Journal of Cardiovascular Nursing: Journal of the Working Group on Cardiovascular Nursing of the European Society of Cardiology 18 (4):260–71. doi:10.1177/1474515119826510.
- Johnson, K. W., J. Torres Soto, B. S. Glicksberg, K. Shameer, R. Miotto, M. Ali, E. Ashley, and J. T. Dudley. 2018. Artificial intelligence in cardiology. Journal of the American College of Cardiology 71 (23):2668–79. doi:10.1016/j.jacc.2018.03.521.
- Kalix, P. 1988. Khat: A plant with amphetamine effects. Journal of Substance Abuse Treatment 5 (3):163–69. doi:10.1016/0740-5472(88)90005-0.
- Kaplan, M. S., J. T. Newsom, B. H. McFarland, and L. Lu. 2001. Demographic and psychosocial correlates of physical activity in late life. American Journal of Preventive Medicine 21 (4):306–12. doi:10.1016/s0749-3797(01)00364-6.
- Khalsa, R. K., A. Khashkhusha, S. Zaidi, A. Harky, and M. Bashir. 2021. Artificial intelligence and cardiac surgery during COVID-19 era. Journal of Cardiac Surgery 36 (5):1729–33. doi:10.1111/jocs.15417.
- Kong, G., D. -L. Xu, and J. -B. Yang. 2008. Clinical decision support systems: A review on knowledge representation and inference under uncertainties. International Journal of Computational Intelligence Systems, Cham. Available at: https://link.springer.com/chapter/10.1007/978-3-030-87687-6_36
- Konstam, M. A., J. A. Hill, R. J. Kovacs, R. A. Harrington, J. A. Arrighi, A. Khera, C, and Academic Cardiology Section Leadership Council of the American College of. 2017. The academic medical system: Reinvention to survive the revolution in health care. Journal of the American College of Cardiology 69 (10):1305–12. doi:10.1016/j.jacc.2016.12.024.
- Lara, J. S., J. Casas, A. Aguirre, M. Munera, M. Rincon-Roncancio, B. Irfan, E. Senft, T. Belpaeme, and C. A. Cifuentes. 2017. Human-robot sensor interface for cardiac rehabilitation. International Conference on Rehabilitation Robotics (ICORR), London, UK. Available at: https://ieeexplore.ieee.org/document/8009382
- Leon, A. S., B. A. Franklin, F. Costa, G. J. Balady, K. A. Berra, K. J. Stewart, Thompson, Paul D, Williams, Mark A and Lauer, Michael S. 2005. Cardiac rehabilitation and secondary prevention of coronary heart disease: An American heart association scientific statement from the council on clinical cardiology (subcommittee on exercise, cardiac rehabilitation, and prevention) and the council on nutrition, physical activity, and metabolism (subcommittee on physical activity), in collaboration with the American association of cardiovascular and pulmonary rehabilitation. Circulation. 111(3):369–76. doi:10.1161/01.CIR.0000151788.08740.5C.
- Leung, Y. W., J. Brual, A. Macpherson, and S. L. Grace. 2010. Geographic issues in cardiac rehabilitation utilization: A narrative review. Health & Place 16 (6):1196–205. doi:10.1016/j.healthplace.2010.08.004.
- Liang, H., B. Y. Tsui, H. Ni, C. C. S. Valentim, S. L. Baxter, G. Liu, Cai, Wenjia, Kermany, Daniel S, Sun, Xin and Chen, Jiancong. 2019. Evaluation and accurate diagnoses of pediatric diseases using artificial intelligence. Nature medicine. 25(3):433–38. doi:10.1038/s41591-018-0335-9.
- Linder, J. A., J. L. Schnipper, R. Tsurikova, T. Yu, L. A. Volk, A. J. Melnikas, M. B. Palchuk, M. Olsha-Yehiav, and B. Middleton. 2009. Documentation-based clinical decision support to improve antibiotic prescribing for acute respiratory infections in primary care: A cluster randomised controlled trial. Informatics in Primary Care 17 (4):231–40. doi:10.14236/jhi.v17i4.742.
- Main, C., T. Moxham, J. C. Wyatt, J. Kay, R. Anderson, and K. Stein. 2010. Computerised decision support systems in order communication for diagnostic, screening or monitoring test ordering: Systematic reviews of the effects and cost-effectiveness of systems. Health Technol Assess 14 (48):1–227. doi:10.3310/hta14480.
- Mathur, P., S. Srivastava, X. Xu, and J. L. Mehta. 2020. Artificial Intelligence, Machine Learning, and Cardiovascular Disease. Clinical Medicine Insights Cardiology 14:1179546820927404. doi:10.1177/1179546820927404.
- McGregor, G., R. Powell, P. Kimani, and M. Underwood. 2020. Does contemporary exercise-based cardiac rehabilitation improve quality of life for people with coronary artery disease? A systematic review and meta-analysis. BMJ Open 10 (6):e036089. doi:10.1136/bmjopen-2019-036089.
- McMahon, S. R., P. A. Ades, and P. D. Thompson. 2017. The role of cardiac rehabilitation in patients with heart disease. Trends in cardiovascular medicine 27 (6):420–25. doi:10.1016/j.tcm.2017.02.005.
- Medina Quero, J., M. R. Fernandez Olmo, M. D. Pelaez Aguilera, and M. Espinilla Estevez. 2017. Real-time monitoring in home-based cardiac rehabilitation using wrist-worn heart rate devices. Sensors (Basel) 17 (12):2892. doi:10.3390/s17122892.
- Midence, L., A. Mola, C. M. Terzic, R. J. Thomas, and S. L. Grace. 2014. Ethnocultural diversity in cardiac rehabilitation. Journal of Cardiopulmonary Rehabilitation and Prevention 34 (6):437–44. doi:10.1097/HCR.0000000000000089.
- Miller, R. A. 1994. Medical diagnostic decision support systems–past, present, and future: A threaded bibliography and brief commentary. Journal of the American Medical Informatics Association: JAMIA 1 (1):8–27. doi:10.1136/jamia.1994.95236141.
- Murdoch, T. B., and A. S. Detsky. 2013. The inevitable application of big data to health care. JAMA 309 (13):1351–52. doi:10.1001/jama.2013.393.
- Naci, H., and J. P. Ioannidis. 2015. Comparative effectiveness of exercise and drug interventions on mortality outcomes: Metaepidemiological study. British Journal of Sports Medicine 49 (21):1414–22. doi:10.1136/bjsports-2015-f5577rep.
- Omura, J. D., S. A. Carlson, P. Paul, K. B. Watson, and J. E. Fulton. 2017. National physical activity surveillance: Users of wearable activity monitors as a potential data source. Preventive Medicle Rep 5:124–26. doi:10.1016/j.pmedr.2016.10.014.
- Parak, J., M. Salonen, T. Myllymaki, and I. Korhonen. 2021. Comparison of heart rate monitoring accuracy between chest strap and vest during physical training and implications on training decisions. Sensors (Basel) 21 (24):8411. doi:10.3390/s21248411.
- Patel, V. L., E. H. Shortliffe, M. Stefanelli, P. Szolovits, M. R. Berthold, R. Bellazzi, and A. Abu-Hanna. 2009. The coming of age of artificial intelligence in medicine. Artificial intelligence in medicine 46 (1):5–17. doi:10.1016/j.artmed.2008.07.017.
- Peláez-Aguilera, M. D., M. Espinilla, M. R. Fernandez Olmo, and J. Medina. 2019. Fuzzy linguistic protoforms to summarize heart rate streams of patients with ischemic heart disease. Complexity 2019:1–11. 2019. doi:10.1155/2019/2694126.
- Pérez-Robledo, F., A. S. Mendes, L. A. Silva, B. M. Bermejo-Gil, R. Llamas-Ramos, and I. Llamas-Ramos. 2021. CardioSafe: A platform for remote rehabilitation for patients with cardiological problems. International Conference on Disruptive Technologies, Tech Ethics and Artificial Intelligence, Cham. Available at: https://link.springer.com/chapter/10.1007/978-3-030-87687-6_361
- Piepoli, M. F., A. W. Hoes, S. Agewall, C. Albus, C. Brotons, A. L. Catapano, M. -T. Cooney, U. Corrà, B. Cosyns, C. Deaton, et al. 2016. 2016 European guidelines on cardiovascular disease prevention in clinical practice: The sixth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts)developed with the special contribution of the European Association for cardiovascular prevention & rehabilitation (EACPR). European Heart Journal. 37(29):2315–81. doi:10.1093/eurheartj/ehw106.
- Pisarchik, A. N., V. A. Maksimenko, and A. E. Hramov. 2019. From novel technology to novel applications: Comment on “An integrated brain-machine interface platform with thousands of channels” by Elon Musk and neuralink. Journal of Medical Internet Research 21 (10):e16356. doi:10.2196/16356.
- Pouke, M., and J. Hakkila. 2013. Elderly healthcare monitoring using an avatar-based 3D virtual environment. International Journal of Environmental Research and Public Health 10 (12):7283–98. doi:10.3390/ijerph10127283.
- Rashed, M., N. Theruvan, A. Gad, H. Shaheen, and S. Mosbah. 2020. Cardiac rehabilitation: Future of heart health in Saudi Arabia, a perceptual view. World Journal of Cardiovascular Diseases 10 (09):666–77. doi:10.4236/wjcd.2020.109064.
- Romiti, S., M. Vinciguerra, W. Saade, I. Anso Cortajarena, and E. Greco. 2020. Artificial intelligence (AI) and cardiovascular diseases: An unexpected alliance. Cardiology Research and Practice 2020:4972346. doi:10.1155/2020/4972346.
- Roshanov, P. S., N. Fernandes, J. M. Wilczynski, B. J. Hemens, J. J. You, S. M. Handler, Nieuwlaat, Robby, Souza, Nathan M, Beyene, Joseph and Van Spall, Harriette GC. 2013. Features of effective computerised clinical decision support systems: Meta-regression of 162 randomised trials. The BMJ. 346(feb14 1):f657. doi:10.1136/bmj.f657.
- Roth, G. A., G. A. Mensah, C. O. Johnson, G. Addolorato, E. Ammirati, L. M. Baddour, et al. 2020. Global burden of cardiovascular diseases and risk factors, 1990–2019. Journal of the American College of Cardiology. 76(25):2982–3021. doi:10.1016/j.jacc.2020.11.010.
- Ruano-Ravina, A., C. Pena-Gil, E. Abu-Assi, S. Raposeiras, A. van ‘t, H. E. Meindersma, E. I. Bossano Prescott, and J. R. Gonzalez-Juanatey. 2016. Participation and adherence to cardiac rehabilitation programs. A systematic review. International Journal of Cardiology 223:436–43. doi:10.1016/j.ijcard.2016.08.120.
- Samayoa, L., S. L. Grace, S. Gravely, L. B. Scott, S. Marzolini, and T. J. Colella. 2014. Sex differences in cardiac rehabilitation enrollment: A meta-analysis. The Canadian Journal of Cardiology 30 (7):793–800. doi:10.1016/j.cjca.2013.11.007.
- Santiago de Araujo Pio, C., T. M. Beckie, M. Varnfield, N. Sarrafzadegan, A. S. Babu, S. Baidya, Buckley, John, Chen, Ssu-Yuan, Gagliardi, Anna and Heine, Martin. 2020. Promoting patient utilization of outpatient cardiac rehabilitation: A joint International council and Canadian Association of cardiovascular prevention and rehabilitation position statement. International Journal of Cardiology 298:1–7. doi:10.1016/j.ijcard.2019.06.064.
- Santiago de Araujo Pio, C., G. S. Chaves, P. Davies, R. S. Taylor, and S. L. Grace. 2019. Interventions to promote patient utilisation of cardiac rehabilitation. Cochrane Database Syst Rev 2: CD007131. doi:10.1002/14651858.CD007131.pub4
- Santiago de Araujo Pio, C., S. Marzolini, M. Pakosh, and S. L. Grace. 2017. Effect of cardiac rehabilitation dose on mortality and morbidity: A systematic review and meta-regression analysis. Mayo Clinic Proceedings Mayo Clinic 92 (11):1644–59. doi:10.1016/j.mayocp.2017.07.019.
- Schork, N. J. 2019. Artificial intelligence and personalized medicine. Cancer Treatment and Research 178:265–83. doi:10.1007/978-3-030-16391-4_11.
- Shajrawi, A., M. Granat, I. Jones, and F. Astin. 2020. Physical activity and cardiac self-efficacy levels during early recovery after acute myocardial infarction: A Jordanian study. The Journal of Nursing Research: JNR 29 (1):e131. doi:10.1097/JNR.0000000000000408.
- Shameer, K., K. W. Johnson, B. S. Glicksberg, J. T. Dudley, and P. P. Sengupta. 2018. Machine learning in cardiovascular medicine: Are we there yet? Heart 104 (14):1156–64. doi:10.1136/heartjnl-2017-311198.
- Shanmugasegaram, S., L. Gagliese, P. Oh, D. E. Stewart, S. J. Brister, V. Chan, and S. L. Grace. 2012. Psychometric validation of the cardiac rehabilitation barriers scale. Clinical rehabilitation 26 (2):152–64. doi:10.1177/0269215511410579.
- Shields, G. E., A. Wells, P. Doherty, A. Heagerty, D. Buck, and L. M. Davies. 2018. Cost-effectiveness of cardiac rehabilitation: A systematic review. Heart 104 (17):1403–10. doi:10.1136/heartjnl-2017-312809.
- Smith, S. C., Jr., E. J. Benjamin, R. O. Bonow, L. T. Braun, M. A. Creager, B. A. Franklin, Gibbons, Raymond J, Grundy, Scott M, Hiratzka, Loren F and Jones, Daniel W. 2011. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: A guideline from the American heart association and American college of cardiology foundation. Circulation. 124(22):2458–73. doi:10.1161/CIR.0b013e318235eb4d.
- Soofi, M. A., and M. A. Youssef. 2015. Prediction of 10-year risk of hard coronary events among Saudi adults based on prevalence of heart disease risk factors. Journal Saudi Heart Association 27 (3):152–59. doi:10.1016/j.jsha.2015.03.003.
- Sotirakos, S., B. Fouda, N. A. Mohamed Razif, N. Cribben, C. Mulhall, A. O’byrne, B. Moran, and R. Connolly. 2021. Harnessing artificial intelligence in cardiac rehabilitation, a systematic review. Future cardiology 18 (2):154–64. doi:10.2217/fca-2021-0010.
- Sotirakos, S., B. Fouda, N. A. Mohamed Razif, N. Cribben, C. Mulhall, A. O’byrne, B. Moran, and R. Connolly. 2022. Harnessing artificial intelligence in cardiac rehabilitation, a systematic review. Future cardiology 18 (2):154–64. doi:10.2217/fca-2021-0010.
- Steinhubl, S. R., and E. J. Topol. 2015. Moving from digitalization to digitization in cardiovascular care: Why is it important, and what could it mean for patients and providers? Journal of the American College of Cardiology 66 (13):1489–96. doi:10.1016/j.jacc.2015.08.006.
- Stewart, J., J. Lu, A. Goudie, M. Bennamoun, P. Sprivulis, F. Sanfillipo, G. Dwivedi, and G. Bivona. 2021. Applications of machine learning to undifferentiated chest pain in the emergency department: A systematic review. Plos One 16 (8):e0252612. doi:10.1371/journal.pone.0252612.
- Suaya, J. A., D. S. Shepard, S. L. Normand, P. A. Ades, J. Prottas, and W. B. Stason. 2007. Use of cardiac rehabilitation by medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation 116 (15):1653–62. doi:10.1161/CIRCULATIONAHA.107.701466.
- Supervia, M., K. Turk-Adawi, F. Lopez-Jimenez, E. Pesah, R. Ding, R. R. Britto, B. Bjarnason-Wehrens, W. Derman, A. Abreu, A. S. Babu, et al. 2019. Nature of cardiac rehabilitation around the globe. EClinicalMedicine 13:46–56. doi:10.1016/j.eclinm.2019.06.006.
- Taylor, R. S., H. M. Dalal, and S. T. J. McDonagh. 2022. The role of cardiac rehabilitation in improving cardiovascular outcomes. Nature Reviews Cardiology 19 (3):180–94. doi:10.1038/s41569-021-00611-7.
- Thomas, R. J., A. L. Beatty, T. M. Beckie, L. C. Brewer, T. M. Brown, D. E. Forman, B. A. Franklin, S. J. Keteyian, D. W. Kitzman, J. G. Regensteiner, et al. 2019. Home-based cardiac rehabilitation: A scientific statement from the American association of cardiovascular and pulmonary rehabilitation, the American heart association, and the American college of cardiology. Circulation. 140(1):e69–89. doi:10.1161/CIR.0000000000000663.
- Tran, N. K., S. Albahra, L. May, S. Waldman, S. Crabtree, S. Bainbridge, and H. Rashidi. 2021. Evolving applications of artificial intelligence and machine learning in infectious diseases testing. Clinical Chemistry 68 (1):125–33. doi:10.1093/clinchem/hvab239.
- Turk-Adawi, K., N. Sarrafzadegan, and S. L. Grace. 2014. Global availability of cardiac rehabilitation. Nature Reviews Cardiology 11 (10):586–96. doi:10.1038/nrcardio.2014.98.
- Turk-Adawi, K., M. Supervia, F. Lopez-Jimenez, E. Pesah, R. Ding, R. R. Britto, B. Bjarnason-Wehrens, W. Derman, A. Abreu, A. S. Babu, et al. 2019. Cardiac rehabilitation availability and density around the globe. EClinicalMedicine 13:31–45. doi:10.1016/j.eclinm.2019.06.007.
- Visco, V., G. J. Ferruzzi, F. Nicastro, N. Virtuoso, A. Carrizzo, G. Galasso, C. Vecchione, and M. Ciccarelli. 2021. Artificial Intelligence as a business partner in cardiovascular precision medicine: An emerging approach for disease detection and treatment optimization. Current Medicinal Chemistry 28 (32):6569–90. doi:10.2174/0929867328666201218122633.
- Wang, T. J., B. Chau, M. Lui, G. T. Lam, N. Lin, and S. Humbert. 2020. Physical medicine and rehabilitation and pulmonary rehabilitation for COVID-19. American Journal of Physical Medicine & Rehabilitation / Association of Academic Physiatrists 99 (9):769–74. doi:10.1097/PHM.0000000000001505.
- Wang, H., Q. Zu, J. Chen, Z. Yang, and M. A. Ahmed. 2021. Application of artificial intelligence in acute coronary syndrome: A brief literature review. Advances in Therapy 38 (10):5078–86. doi:10.1007/s12325-021-01908-2.
- WHO. 1993. Rehabilitation after cardiovascular diseases, with special emphasis on developing countries: Report of a WHO expert committee. Geneva: World Health Organization.
- Widmer, R. J., T. G. Allison, R. Lennon, F. Lopez-Jimenez, L. O. Lerman, and A. Lerman. 2017. Digital health intervention during cardiac rehabilitation: A randomized controlled trial. American Heart Journal 188:65–72. doi:10.1016/j.ahj.2017.02.016.
- Wong, W. P., J. Feng, K. H. Pwee, and J. Lim. 2012. A systematic review of economic evaluations of cardiac rehabilitation. BMC Health Services Research 12 (1):243. doi:10.1186/1472-6963-12-243.
- Xia, T. L., F. Y. Huang, Y. Peng, B. T. Huang, X. B. Pu, Y. Yang, H. Chai, and M. Chen. 2018. Efficacy of different types of exercise-based cardiac rehabilitation on coronary heart disease: A network meta-analysis. Journal of General Internal Medicine 33 (12):2201–09. doi:10.1007/s11606-018-4636-y.
- Zhang, Y., H. Cao, P. Jiang, and H. Tang. 2018. Cardiac rehabilitation in acute myocardial infarction patients after percutaneous coronary intervention: A community-based study. Medicine (Baltimore) 97 (8):e9785. doi:10.1097/MD.0000000000009785.
- Zhao, Y., E. P. Wood, N. Mirin, S. H. Cook, and R. Chunara. 2021. Social determinants in machine learning cardiovascular disease prediction models: A systematic review. American Journal of Preventive Medicine 61 (4):596–605. doi:10.1016/j.amepre.2021.04.016.