Abstract
Objective. Acute rejection, chronic allograft nephropathy, and cyclosporine (CsA) toxicity remain serious problems for renal transplant recipients and may lead to graft loss. We retrospectively analyzed 34 patients whose biopsies revealed acute and/or chronic allograft rejection, or CsA nephrotoxicity, and who converted from CsA to tacrolimus. Patients and Methods. From July 1996 through September 2003, CsA was converted to tacrolimus in 34 renal transplant recipients (26 male, 8 female) with renal biopsy at our hospital. Blood pressure and serum creatinine levels were checked monthly and serum cholesterol, triglyceride, and glutamic-pyruvic transaminase (GPT) levels were checked every three months. Results. A consistently stable and better function after conversion was obtained in a significant portion (24, 71%) of patients. A statistically significant decline in serum creatinine and an improvement in the glomerular filtration rate were found at 3 m, 6 m, 12 m, 36 m, and 72 m after tacrolimus conversion. In 85.7% (12/14) of patients with acute rejection and in 35.7% (5/14) of patients with chronic allograft nephropathy (concomitant with acute rejection in 5), improved or stabilized graft function was noted. In addition, the systolic blood pressure and diastolic BP dropped significantly (P< 0.05), while there was no significant change in cholesterol, triglyceride, and GPT levels. Conclusion. The beneficial effect of tacrolimus conversion on patients with acute rejection, chronic allograft nephropathy, or CsA nephrotoxicity was demonstrated in long-term follow up. The improvement in both renal function and blood pressure may be of paramount importance in reducing long-term cardiovascular morbidity and mortality.
Introduction
Following the introduction of cyclosporine (CsA) in 1980, both renal allograft and patient survival rates after kidney transplantation improved dramatically.Citation[1&2] However, the use of CsA is associated with significant side effects, including nephrotoxicity, hepatotoxicity, neurotoxicity, hypertension, hyperlipidemia, hyperglycemia, and hirsutism.Citation[3&4] Although CsA improves the results of renal transplantation significantly, chronic rejection, acute rejection, and recurrent glomerulonephritis remain major problems which may lead to graft loss.Citation[5] Tacrolimus (FK506) is a calcineurin inhibitor with a much higher potency than CsA.Citation[6] In a recent study, a consistently better post-transplant graft function was observed in tacrolimus-treated patients as compared to their CsA-treated counterparts.Citation[7&8] It is now a common practice to convert CsA to tacrolimus in patients who fail to respond to CsA, and previous studies have shown beneficial effects following this conversion.Citation[9&10] In such instances, lipid profiles and blood pressure control have been greatly improved. In a previous study,Citation[11] we showed that in a small portion of patients with chronic rejection, the graft function may be stabilized after tacrolimus rescue therapy. Whether this is a long-lasting effect needs to be investigated with a longer duration of follow-up. The goal of the present study was to analyze the long-term effect of tacrolimus on renal transplant patients previously treated with CsA.
Patients and Methods
Study Population
Between July 1996 and September 2003, 34 patients (26 male, 8 female) with a mean age of 44.4 ± 12.5 years (range 16 to 67 years) who were under CsA-based immunosuppression were converted to tacrolimus due to acute rejection, chronic allograft nephropathy, or CsA nephrotoxicity proven by renal biopsy. Before 1996, the immunosuppressive regimen was mainly cyclosporine-based. After 1996 CsA- or tacrolimus-based regimens were used individually. The initial dosage of CsA was 10 mg/kg/day and adjusted to reach a target whole blood trough concentration of 100–150 ng/mL after 4 months. All patients also received prednisolone, with or without azathioprine or mycophenolate mofetil. The maintenance dosage of prednisolone was 5 mg/day, azathioprine was 50 mg/day, and mycophenolate mofetil was 1000 mg/day. Pulse therapy with 500 mg methylprednisolone intravenously for 3 days was prescribed initially for acute rejection. Antilymphocyte globulin was added if methylprednisolone alone failed to halt rejection. The definition of acute rejection, chronic allograft nephropathy or CsA nephrotoxicity was based on the pathological finding according to the Banff (1997) criteria. The definition of improved renal function was a decrease in serum creatinine level of more than 20% at the end of the study compared to baseline, stabilized renal function was defined as serum creatinine level within 20% above or below baseline, and definition of worsened renal function was an increase in serum creatinine level of more than 20% of baseline.
Tacrolimus Dosing and Monitoring
Tacrolimus was administered orally at a dose of 0.2–0.3 mg/kg/day in two divided doses. The dosage was adjusted according to the whole blood trough level of tacrolimus, which was performed by using a microparticle enzyme immunoassay (IMX tacrolimus assay, Abbott Laboratories). The target blood level was set at around 5–8 ng/mL according to clinical and biochemical parameters. A lower trough level of 2–4 ng/mL was allowed in patients with evidence of CsA nephrotoxicity.
Laboratory Monitoring
Blood pressure, serum creatinine, and tacrolimus trough levels were checked at every monthly visit. The abbreviated Modification of Diet in Renal Disease (MDRD) equation {[GFR (mL/min/1.73m2) = 186 × (serum creatinine [mg/dL])− 1.154× (age years)− 0.203× (0.742 if female)]}Citation[12-14] was used for estimation of the glomerular filtration rate (GFR). Other laboratory examinations including complete blood count, liver function test, triglyceride, cholesterol, electrolytes, and fasting blood sugar were checked at least every 3 months.
Statistical Analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences program (SPSS Inc., Chicago, IL, USA). Results are expressed as mean and standard deviation. A paired t-test or Chi-square test with Yate's correction for continuity was used to compare baseline (before conversion) and follow-up data. P values less than 0.05 were considered statistically significant.
Results
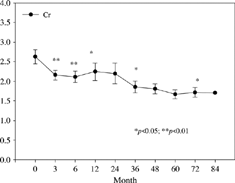
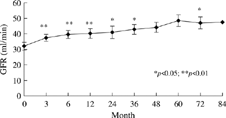
The characteristics of the 34 patients are listed in . The follow-up duration was 6.3 ± 3.7 years and the duration of tacrolimus use was 3.0 ± 2.2 years. Donors were mainly cadaveric (31 patients, 91%). The incidence of hepatitis B virus infection was 14.7% (5 patients) and incidence of hepatitis C virus infection was 20.6% (7 patients). The reasons for tacrolimus conversion were refractory acute rejection in 14 patients (41.2%), chronic allograft nephropathy in 9 (26.5%), both acute rejection and chronic allograft nephropathy in 5 (14.7%), and CsA nephrotoxicity in 6 (17.6%). A comparison of the systolic blood pressure (SBP), diastolic blood pressure (DBP), serum cholesterol, triglyceride, and glutamic-pyruvic transaminase (GPT) levels before and after tacrolimus conversion is presented in . There was a significant decrease in SBP (137.4 ± 2.2 vs. 128.6 ± 2.4 mm Hg, P = 0.011), DBP (84.0 ± 1.8 vs. 70.2 ± 1.6 mm Hg, P = 0.019), whereas serum cholesterol, triglyceride, and GPT levels were not significantly different. The baseline serum creatinine value was 2.6 ± 0.2 mg/dL, which declined significantly after conversion at 3 m (2.2 ± 0.1 mg/dL), 6 m (2.1 ± 0.1 mg/dL), 12 m (2.3 ± 0.2 mg/dL), 36 m (1.9 ± 0.2 mg/dL), and 72 m (1.7 ± 0.1 mg/dL) (). The serial estimated GFR is shown in . The outcomes of tacrolimus conversion in patients with confirmed pathological diagnosis are summarized in . There were no statistically significant differences in the numbers of patients using antihypertensive agents (P = 0.98) and lipid-lowering agents (P = 0.59) between before and after tacrolimus conversion. The incidence of posttransplantation diabetic mellitus (PTDM) in the CsA-treated phase was 8.8% (n = 3), which was doubled after conversion to 17.6% (n = 6), although it did not reach statistical significance (p = 0.48). Common side effects such as gingival hypertrophy, hirsutism, and tremor were significantly less frequent after conversion, while the prevalence of tremor did not change significantly (). When patients were stratified according to baseline serum creatinine and 3 mg/dL was used as a cut-off point, the outcome was not significantly different ().
Table 1. Patients' clinical characteristics
Table 2. Change in blood pressure and biochemical data after conversion
Table 3. Renal outcome after conversion of biopsy-proven patients
Table 4. Comparison of common side effects: before vs. after conversion
Table 5. Renal outcome in terms of baseline serum creatinine
Discussion
The results of the present study show a significantly favorable response in terms of blood pressure and graft function after tacrolimus conversion. Most of our patients (28, 82.3%) had either refractory acute rejection or chronic allograft nephropathy, and without aggressive intervention, a slow decline of graft function was expected. However, a consistently stable and better function after conversion was obtained in a significant portion (24, 71%) of patients. The exact mechanism of the beneficial effect of tacrolimus is unknown, although several factors may be implicated. First, tacrolimus is much more potent than CsA. Refractory acute rejection that fails to respond to CsA may be rescued after conversion to tacrolimus.Citation[15&16] Although the use of tacrolimus remains controversial, it has been shown to be effective in improving or stabilizing patients with chronic allograft nephropathy, at least temporarily.Citation[17&18] Second, although tacrolimus is nephrotoxic, as is CsA, individual responses to either agent are variable and when nephrotoxicity develops with one agent, conversion to the other immunosuppressive drug may diminish nephrotoxicity.Citation[19] Third, while both tacrolimus and cyclosporine have the potential to induce fibrotic lesions, tacrolimus has been associated with significantly less expression of TGF-β1 compared with cyclosporine.Citation[20-22] Fourth, several studies have demonstrated that tacrolimus, but not CsA, can suppress IL-10 and the down-regulation of functional CD8 T-cell and NK-cell infiltration by means of decreased release of toxic factors.Citation[23-26] Fifth, we targeted tacrolimus at a lower level (2–4 ng/mL) for those patients with biopsy-proven CsA nephrotoxicity. This may also partially explain the beneficial effect of conversion.
The Pittsburgh trial of tacrolimus rescue therapy in renal allograft recipients demonstrated that lower pretreatment serum creatinine values (< 3 mg/dL) were associated with a better result.Citation[27] In our series, six cases lost their graft at the end of follow-up. The baseline serum creatinine was ≧ 3 mg/dL in three (mean 4.13 ± 0.09 mg/dL) and < 3 mg/dL in the other three (mean 2.03 ± 0.03 mg/dL) (p = 0.046). Further data are provided in , which demonstrate that a cut-off point of 3 mg/dL of serum creatinine did not cause differentiation among patients with different outcomes. Although the number of cases in our series was too small to reveal statistical significance, other factors may be of relevance. While the initial level of graft function is an important prognostic indicator, the underlying graft disease is also important. In the present study, the baseline serum creatinine was ≧3 mg/dL in 11 cases. Of these, five had acute rejection, three had chronic allograft nephropathy, two had both acute rejection and chronic allograft nephropathy, and one had CsA nephrotoxicity. Graft loss occurred in 0% of patients with acute rejection, in two (2/9 or 22%) patients with graft loss or chronic rejection, and three (3/5 or 60%) with both acute and chronic rejection, which highlights the important influence of pathological changes on renal outcomes following tacrolimus conversion.
It is interesting to note that five out of 14 cases (35.7%) with chronic allograft nephropathy (concomitant with acute rejection in five) had either improved or stabilized graft function (). This suggests that tacrolimus has some effect on decreased progression of chronic allograft nephropathy. Because this was a retrospective, uncontrolled study with a relatively small population, a definitive conclusion cannot be reached at present. Nevertheless, the distinctive pharmacological feature of tacrolimus, as described previously, may contribute to the favorable outcome in some patients with chronic allograft nephropathy.
Improvement in patients with hypertension, gingival hypertrophy, and hirsutism after tacrolimus conversion was confirmed in this study. As expected, the incidence of DM increased after conversion, but not significantly. The impact on long-term graft survival should be noted.
In summary, we have demonstrated that tacrolimus conversion may result in an improvement of graft function in renal transplant recipients with either acute or chronic rejection. This beneficial effect is yet to be proved with a larger population and longer follow-up period.
Acknowledgments
We thank the Biostatistics Task Force of Taichung Veterans General Hospital, Taichung, Taiwan, R.O.C., for their assistance with the statistical analysis.
References
- Cecka, J.; Cho, Y.; Terasaki, P. Analysis of the UNOS scientific renal transplant registry at three years—early events affecting transplant success. Transplantation 1992, 53, 59–64. [PUBMED], [INFOTRIEVE], [CSA]
- Frey, D.; Matas, A.; Gillingham, K J.; Canafax, D.; Payne, W D.; Dunn, D L.; , et al. Sequential therapy—a prospective randomized trial of MALG versus OKT3 for prophylactic immunosuppression in cadaver renal allograft recipients. Transplantation 1992, 54, 50–56. [PUBMED], [INFOTRIEVE], [CSA]
- Graham, R. Cyclosporine: mechanisms of action and toxicity. Clevel. Clin. J. Med. 1994, 61, 308–313. [CSA]
- Kohnle, M.; Zimmermann, U.; Lutkes, P.; Albrecht, K H.; Philipp, T.; Heemann, U. Conversion from cyclosporine A to tacrolimus after kidney transplantation due to hyperlipidemia. Transplant. Int. 2000, 13(Suppl.)S345–S348. [CSA], [CROSSREF]
- Tanabe, K.; Ishikawa, N.; Tokumoto, K.; Takahashi, K.; Oshima, T.; Fuchinoue, S.; , et al. Factors affecting long-term renal allograft survival in cyclosporine-treated kidney transplants. Transplant. Proc. 1998, 30, 1805–1809. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Goto, T.; Kino, T.; Hatanaka, H.; Okuhara, M.; Kohsaka, M.; Aoki, H.; , et al. FK506: historical perspectives. Transplant. Proc. 1991, 23, 2713–2717. [PUBMED], [INFOTRIEVE], [CSA]
- Kaplan, B.; Schold, J D.; Meier-Kriesche, H U. Long-term graft survival with neoral and tacrolimus: a paired kidney analysis. J. Am. Soc. Nephrol. 2003, 14, 2980–2984. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Kelly, D.; Jara, P.; Rodeck, B.; Lykavieris, P.; Burdelski, M.; Becker, M.; , et al. Tacrolimus and steroids versus cyclosporine microemulsion, steroids, and azathioprine in children undergoing liver transplantation: randomized European multicenter trial. Lancet 2004, 364, 1054–1061. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Urbizu, J M.; Zarraga, S.; Gomez-Ullate, P.; Amenabar, J J.; Gainza, F I.; Lampreabe, I.; , et al. Safety and efficacy of tacrolimus rescue therapy in 55 kidney transplant patients treated with cyclosporine. Transplant. Proc. 2003, 35, 1704–1705. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Peters, D H.; Fitton, A.; Plosker, G L.; Faulds, D. Tacrolimus: a review of its pharmacology and therapeutic potential in hepatic and renal transplantation. Drugs 1993, 46, 746–794. [PUBMED], [INFOTRIEVE], [CSA]
- Shu, K H.; Cheng, C H.; Wu, M J.; Lian, J D.; Huang, C C.; Chu, S H.; , et al. A multicenter trial of FK506 as rescue therapy for renal transplant recipients in Taiwan. Transplant. Proc. 1998, 30, 3584–3586. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Lo, J C.; Chertow, G M.; Go, A S.; Hsu, C Y. Increased prevalence of subclinical and clinical hypothyroidism in persons with chronic kidney disease. Kidney Int. 2005, 67, 1047–1052. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Levey, A S.; Greene, T.; Kusek, J W.; Beck, G J. A simplified equation to predict glomerular filtration rate from serum creatinine. J. Am. Soc. Nephrol. 2000, 11, 155A. [CSA]
- Levey, A S.; Bosch, J P.; Lewis, J B.; , et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann. Intern. Med. 1999, 130, 461–470. [PUBMED], [INFOTRIEVE], [CSA]
- Woodle, E S.; Perdrizet, G A.; So, S.; Jendrisak, M D.; White, H M.; Marsh, J W. FK 506 rescue therapy: early conversion improves efficacy. Transplant. Proc. 1993, 25, 1990–1991. [PUBMED], [INFOTRIEVE], [CSA]
- Jordan, M L.; Naraghi, R.; Shapiro, R.; Smith, D.; Vivas, C A.; Scantlebury, V P.; , et al. Tacrolimus for rescue of refractory renal allograft rejection. Transplant. Proc. 1998, 30, 1257–1260. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Manu, M A.; Tanabe, K.; Ishikawa, N.; Tokumoto, T.; Oshima, T.; Shinmura, H.; , et al. Tacrolimus rescue for resistant rejection, chronic rejection and immunoglobulin A nephropathy of renal allografts under primary cyclosporine A immunosuppression. Transplant. Proc. 1999, 31, 2853–2855. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Budde, K.; Smettan, S.; Fritsche, L.; Waiser, J.; Neumayer, H H. Five-year outcome of tacrolimus rescue therapy in late rejection after renal transplantation. Transplant. Proc. 2002, 34, 1594–1596. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Morrissey, P E.; Gohh, R.; Shaffer, D.; Crosson, A.; Madras, P N.; Sahyoun, A I.; , et al. Correlation of clinical outcomes after tacrolimus conversion for resistant kidney rejection or cyclosporine toxicity with pathologic staging by the Banff criteria. Transplantation 1997, 63, 845–848. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Olyaei, A J.; de Mattos, A M.; Bennett, W M. Nephrotoxicity of immunosuppressive drugs: new insight and preventive strategies. Curr. Opin. Crit. Care 2001, 7, 384–389. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Vieira, J M.J.; Noronha, I L.; Malheiros, D M.; Burdmann, E A. Cyclosporine-induced interstitial fibrosis and arteriolar TGF-beta expression with preserved renal blood flow. Transplantation 1999, 68, 1746–1753. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Baan, C C.; van Riemsdijk-van Overbeeke, I C.; Balk, A H.; Vantrimpont, P M.; Mol, W M.; Knoop, C J.; , et al. Conversion from cyclosporine A to tacrolimus is safe and decreases blood pressure, cholesterol levels and TGF-β1 type I receptor expression. Clin. Transplant. 2001, 15, 276–283. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Ebbs, A.; Pan, F.; Wynn, C.; Erickson, L.; Kobayashi, M.; Jiang, H. Tacrolimus treats ongoing allograft rejection by inhibiting interleukin-10 mediated functional cytotoxic cell infiltration. Transplant. Proc. 2002, 34, 1378–1381. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Jiang, H.; Yang, X F.; Wynn, C.; Soriano, R.; Krishnan, K.; Fujimura, T.; , et al. IL-10: a tacrolimus-specific cytotoxic mediator in ongoing allograft rejection. Transplant. Proc. 2001, 33, 510–513. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Jiang, H.; Yang, X.; Soriano, R.; Fujitus, T.; Kobayashi, M. Inhibition of IL-10 by FK 506 may be responsible for overcoming ongoing allograft rejection in the rat. Transplant. Proc. 1999, 31, 1203–1205. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Jiang, H.; Wynn, C.; Pan, F.; Ebbs, A.; Erikson, L M.; Kobayashi, M. Tacrolimus and cyclosporine differ in their capacity to overcome ongoing allograft rejection as a result of their differential abilities to inhibit interleukin-10 production. Transplantation 2002, 73, 1808–1817. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Jordan, M L.; Shapiro, R.; Vivas, C A.; Scantlebury, V P.; Rhandhawa, P.; Carrieri, G.; , et al. FK 506 “rescue” for resistant rejection of renal allografts under primary cyclosporine immunosuppression. Transplantation 1994, 57, 860–865. [PUBMED], [INFOTRIEVE], [CSA]