Abstract
Mesangial proliferation and deposition of immunoglobulins and complement components within glomerular mesangium was one of the important pathological features of lupus nephritis. Autoantibodies against human mesangial cells could be detected in the sera of patients with IgA nephropathy (IgAN) and Henoch-Schöenlein nephritis. We speculated that autoantibodies against human glomerular mesangial cells might play a role in the development of lupus nephritis. Objective. To screen autoantibodies against human glomerular mesangial cells in sera from patients with lupus nephritis and to identify their target antigens. Methods. Sera were collected from 96 patients with lupus nephritis as well as 25 patients with IgAN and 20 patients with idiopathic membranous nephropathy (IMN). Cell lysates of in vitro cultured human glomerular mesangial cells were used as antigens in Western-blot analysis to detect autoantibodies against human mesangial cells in sera from patients with lupus nephritis as well as IgAN and IMN. The clinical and pathological significance of the autoantibodies were further investigated. Results. Autoantibodies against human mesangial cells could be detected in 94/96 (97.9%) of the sera from patients with lupus nephritis in Western-blot analysis. Twelve protein bands could be blotted by the sera from patients with lupus nephritis. The prevalence of autoantibodies against human mesangial cells in IgAN was 14/25 (56.0%) and only seven protein bands could be blotted. Five autoantibodies (anti-18, 24, 36, 46, and 91 kD) could be detected only in sera from patients with lupus nephritis. In patients with lupus nephritis, some autoantibodies might have some relationship with gender, hematuria, ANA, anti-dsDNA or anti-ENA antibodies. Conclusions. There are autoantibodies directly against heterogeneous antigens of human glomerular mesangial cells in sera from patients with lupus nephritis, and some of them might be associated with different clinical manifestations.
Introduction
Systemic lupus erythematosus (SLE) is one of the most common autoimmune diseases in China and lupus nephritis is one of the leading causes of patients' mortality. Glomerular mesangial proliferation and deposition of immunoglobulins and complement components within glomerular mesangium are important pathological features of lupus nephritis. Previous studies have suggested that anti-DNA antibodies might play an important role in the deposition of the immune complex in glomerular mesangium, but whether glomerular mesangial cells act as autoantigens and contribute to the in situ immune complex formation in lupus nephritis was not investigated. It has been reported that autoantibodies against human glomerular mesangial cells could be detected in sera from patients with IgA nephropathy (IgAN) and Henoch-Schöenlein nephritis.Citation[1-3] The aim of the current study was to detect autoantibodies directed against human- glomerular mesangial cells in sera from patients with lupus nephritis and further identify their autoantigens.
Subjects and Methods
Patients and Sera
Ninety-six hospitalized patients with SLE in our hospital were enrolled in this study. All the patients were fulfilled the 1982 ACR criteria for SLE and all of them had renal biopsy proven lupus nephritis. There were 14 males and 82 females with an average age of 30.0 (14–58) years. According to the International Society of Nephrology/Renal Pathology Society (ISN/RPS) 2003 classification of lupus nephritis,Citation[4] 21/96 were classified as mesangial proliferative lupus nephritis (class II), 7/96 were classified as focal lupus nephritis (class III), 49/96 were classified as diffuse lupus nephritis (class IV), 18/96 had membranous lupus nephritis (class V), and the remaining patient had advanced sclerosis lupus nephritis (class VI). The sera were collected one day before renal biopsy and were stored at − 30° until use. Twenty-five sera from 25 patients with primary IgAN and 20 sera from 20 patients with idiopathic membranous nephropathy (IMN) were also employed. Twenty sera from healthy blood donors were collected as normal controls.
Active renal pathology was considered if one or more of the followings were observed on renal histology: 1) glomerular hypercellularity; 2) segmental glomerular fibrinoid necrosis; 3) wire loop formation; 4) glomerular karyorrhexis; 5) cellular crescents; 6) glomerular leukocyte infiltration; 7) glomerular hyaline thrombi; 8) vasculitis; 9) severe interstitial edema and/or inflammatory cell infiltration; and; 10) large electron-dense deposits in subendothelial and/or mesangial sites were determined by electron-microscope examination.
Clinical activity was evaluated according to the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI).Citation[5]
Preparation of In Vitro Cultured Human Primary Glomerular Mesangial Cells
Normal human cortical renal tissue was obtained from patients with nephrectomy due to renal malignancy. Mesangial cells were cultured from nephrectomized kidneys according to established methods.Citation[6] Briefly, cells derived from collagenase-treated glomeruli were maintained in RPMI 1640 medium supplemented with 20% (vol/vol) fetal calf serum (FCS), glutamine (2 mM), transferrin (5 µg/mL), insulin (5 µg/mL), sodium selenite (5 ng/mL), penicillin (100 IU/mL), and streptomycin (100 µg/mL). Mesangial cells were characterized by their stellate morphology, ability to form hillocks, and immunohistochemical staining (positive for vimentin; negative for cytokeratin and von Willebrand factor). Mesangial cells were passaged at a split ratio of 1:3, and growth-arrested cells of the four through seventh passage were used in experiments.
Preparation of Soluble Proteins from Primary Mesangial Cells
The primary mesangial cells were counted and lysed by 1% Triton X-100 (108 cells/mL) with 1 mmol/L phenylmethylsulfonyl fluoride (PMSF) at 0°C for 30 min in gentle agitation. After centrifugation, the supernatant was collected as soluble proteins.
Western-Blot Analysis
Soluble proteins from mesangial cells were electrophoresed at nonreducing condition on 10% sodium dodecylsulfate (SDS) polyacrylamide gel (20 mA, 60 minutes). Then the proteins were transferred to PVDF membranes by an electrophoretic semidry blotting system (Amersham Pharmacia) at 0.8 mA/cm2 for 70 minutes. The PVDF membranes were cut into strips and were blocked in room temperature for 30 minutes with 2% skimmed milk in 10 mmol/L Tris/HCl buffer (pH 7.5) with 0.1% Tween-20 (TBST). Patients' sera and control sera were diluted 1:50 in TBST with 2% skimmed milk and incubated for 4 hours at room temperature in gentle agitation. Three washes with TBST followed, 10 minutes each time. Then the strips were incubated with alkaline phosphatase–labeled affinity purified goat anti-human IgG (Sigma, St. Louis, MO, USA) and diluted 1:6000 with TBST with 2% skimmed milk for 1 hour at room temperature in gentle agitation. After three 10 minute washes with TBST, the reaction was revealed by addition of an appropriate substrate: nitro-blue-tetrazoleum (NBT) and 5 bromo-4 chloro-3 indolyl phosphate (BCIP) (Sigma, St. Louis, MO, USA). The reaction was stopped with distilled water after 5–15 minutes.
Statistical Analysis
Chi-square analysis was used to compare the prevalences of autoantibodies against different target antigens in patients with and without various clinical and pathological parameters, including skin rash, arthralgia, photosensitivity, oral ulcer, hematuria, proteinuria, anemia, SLEDAI, mesangial proliferation, and active renal pathological indices, etc.
Results
The sera from 94/96 (97.9%) patients with lupus nephritis could recognize heterogeneous antigens of human mesangial cells and 12 different bands could be blotted. In sera from patients with IgAN, the prevalence of autoantibodies against glomerular mesangial cell was only 14/25 (56.0%) and only seven different bands could be blotted (, and ). No protein band could be blotted by 20 sera from patients with IMN and 20 sera from normal controls.
Table 1. The prevalences of autoantibodies against mesangial cells and their target antigens in Western blot analysis
Figure 1 Western blot analysis of autoantibodies against mesangial cell in sera of patients with lupus nephritis (1). Lane 1: blank control; Lane 2: normal control; Lanes 3 and 4: sera from two patients with lupus nephritis recognized a 101 kD band; Lanes 5 and 9: sera from two patients with lupus nephritis recognized a 91 kD band; Lanes 6 and 7: sera from two patients with lupus nephritis recognized a 69 kD band; Lane 8: sera from a patients with lupus nephritis recognized 96 and 79 kD bands.
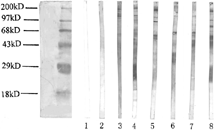
Figure 2 Western blot analysis of autoantibodies against mesangial cell in sera of patients with lupus nephritis (2). Lane 1: blank control; Lane 2: normal control; Lanes 3 to 5: sera from three patients with lupus nephritis recognized a 74 kD band; Lane 6: sera from a patients with lupus nephritis recognized 63 and 24 kD bands; Lane 7: sera from a patients with lupus nephritis recognized a 63 kD band; Lane 8: sera from a patients with lupus nephritis recognized 63, 52 and 46 kD bands; Lane 9: sera from a patients with lupus nephritis recognized 63, 52 and 36 kD bands; Lane 10: sera from a patients with lupus nephritis recognized 63, 36, 24 and 18 kD bands; Lane 11: sera from a patients with lupus nephritis recognized a 63 kD band; Lane 12: sera from a patients with lupus nephritis recognized 52, 24 and 18 kD bands; Lane 13: sera from a patients with lupus nephritis recognized 36 and 18 kD band.
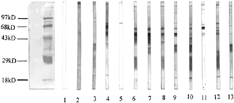
Correlations Between Autoantibodies and Clinicopathological Parameters
In patients with lupus nephritis, review of the clinical and pathological data revealed that the prevalence of anti-63 kD autoantibodies positive samples was significantly higher in female patients than it was in male patients (59.8% vs. 21.4%, P < 0.01) and was also significantly higher in patients with positive antinuclear antibody (ANA) than the prevalence in patients without ANA (67.6% vs. 16.7%, P < 0.001). The prevalences of anti-74 kD, 46 kD, and 36 kD autoantibodies were significantly higher in patients with hematuria than those in patients without hematuria (36.8% vs. 5.0%, P < 0.01; 34.2% vs. 10.0%, P < 0.05; 31.6% vs. 5.0% P < 0.05, respectively). The prevalence of anti-18 kD autoantibodies was significantly higher in patients with anti-dsDNA antibodies than that in patients without (61.5% vs. 34.4%, P < 0.05).
The relationship between the autoantibodies against human mesangial cells and the pathological type of lupus nephritis is listed in . There is no correlation between different autoantibodies and the different pathological types.
Table 2. Associations between autoantibodies against mesangial cell and the pathological type of lupus nephritis
The relationship between other clinical and pathological parameters and autoantibodies are listed in .
Table 3. Associations between autoantibodis against mesangial cell and the clinicopathological parameters in lupus nephritis
Discussion
Autoantibodies against human glomerular mesangial cells were first described by Ballardie and coworkers (1988) in patients with primary IgAN.Citation[1] Other author also found autoantibodies against human mesangial cells in sera from patients with primary IgAN and Henoch-Schöenlein nephritis.Citation[2&3] The prevalences were 18%–67% and 36%–70%, respectively, and the molecular weight of the target antigens ranged from 48 kD to 55 kD.Citation[1-3] It was found by O'Donoghue et al.,Citation[2] in a cross-sectional study, that the patients' -urinary erythrocyte excretion and proteinuria correlated with the levels of IgG autoantibodies against glomerular mesangial cells. During a follow-up study the episodes of nephritis were associated with high levels of circulating IgG autoantibodies.Citation[2] It was also found by Fornasieri et al.Citation[3] that in patients with IgAN, there was a close correlation between the levels of autoantibodies against mesangial cells, the presence of a recent nephritis episode, and with the levels of circulating IgA immune complexes.
Systemic lupus erythmatosus is a systemic autoimmune disease characterized by the presence of autoantibodies against nuclear antigens. Our previous studies and others had revealed that a variety of autoantibodies have been found in sera from patients with SLE, including ANA and antineutrophil cytoplasmic autoantibodies (ANCA),Citation[7-13] anti-endothelial cell autoantibodies (AECA),Citation[14-18] etc. Mesangial proliferation- and deposition of immune complex within glomerular mesangium are features of lupus nephritis. Previous studies suggested that autoantibodies against nuclear components such as anti-DNA autoantibodies might play an important role in the pathogenesis of lupus nephritis. There are three hypothesized mechanisms to explain the deposition of anti-DNA antibodies containing immune complexes in kidneys. Immune complexes formed in blood circulation could be trapped passively within glomeruli; or the cross-reactive anti-DNA antibodies directly bound to glomerular antigens and thus lead to immune deposit formation; or intracellular antigens that were released into circulation after cell death and then bound to certain sites within the glomerulus served as antigen determinants to which anti-DNA antibodies could bind, initiating immune deposit formation.Citation[19] However, patients with autoantibodies against nuclear antigens did not always develop lupus nephritis, which suggested that other factors might also be involved in the pathogenesis of lupus nephritis.
A recent studyCitation[20] found that anti-DNA antibodies from patients with SLE were capable of binding to human mesangial cells and such binding was associated with renal involvement and disease activity. After DNase treatment of human mesangial cell membranes, such binding remained unaffected, indicating that the binding was not mediated by DNA components on human mesangial cell membranes. But the exact mechanism of anti-DNA antibodies binding to human mesangial cell remained unclear. It was reasonable to speculate that there might be autoantibodies directly against human mesangial cells in the sera of patients with lupus nephritis. In the current study, cell lysates of in vitro cultured human glomerular mesangial cells were used as antigens; Western-blot analysis was employed to confirm the existence of heterogeneous autoantibodies directly against glomerular mesangial cells in sera from patients with lupus nephritis. Sera from patients with IgAN and IMN were also employed to detect whether the target antigens were specific for lupus nephritis. The result showed that autoantibodies against glomerular mesangial cells could be detected only in sera from patients with lupus nephritis or IgAN but not in sera from patients with IMN or healthy controls. The prevalence was much higher in lupus nephritis than in IgAN (97.9% vs. 56.0%). The target antigens of lupus nephritis were different from those of IgAN and five autoantibodies (anti-18, 24, 36, 46, and 91 kD autoantibodies) could be detected only in sera from patients with lupus nephritis. Further investigation suggested that some of the autoantibodies in sera from patients with lupus nephritis might be associated with gender, hematuria, ANA, anti-dsDNA antibodies, or antibodies against extractable nuclear antigens (ENA) of patients with lupus nephritis.
We speculated that autoantibodies against human glomerular mesangial cells might directly react with glomerular mesangial cells to form an immune complex in situ and further activate complements to induce glomerular damage. The relationship of the autoantibodies against glomerular mesangial cells found in the current study and anti-DNA antibodies has been undergoing further investigation. Characterization of the target antigens of glomerular mesangial cells also need to be further clarified.
Acknowledgment
This study was supported in part by a research grant from the China National Education Committee.
References
- Ballardie, F W.; Brenchley, P E.; Williams, S.; O'Donoghue, D J. Autoimmunity in IgA nephropathy.Lancet 1988, 10, 588–592. [CSA]
- O'Donoghue, D J.; Darvill, A.; Ballardie, F W. Mesangial cell autoantigens in immunoglobulin A nephropathy and Henoch-Schonlein purpura.J. Clin. Invest. 1991, 88, 1522–1530. [PUBMED], [INFOTRIEVE], [CSA]
- Fornasieri, A.; Pinerolo, C.; Bernasconi, P.; Li, M.; Armelloni, S.; Gibelli, A.; D'Amico, G. Anti-mesangial and anti-endothelial cell antibodies in IgA mesangial nephropathy.Clin. Nephrol. 1995, 44, 71–79. [PUBMED], [INFOTRIEVE], [CSA]
- Weening, J J.; D'Agati, V D.; Schwartz, M M..; Seshan, S V.; Alpers, C E.; Appel, G B.; Balow, J E.; Bruijn, J A.; Cook, T.; Ferrario, F.; Fogo, A B.; Ginzler, E M.; Hebert, L.; Hill, G.; Hill, P.; Jennette, J C.; Kong, N C.; Lesavre, P.; Lockshin, M.; Looi, L M.; Makino, H.; Moura, L A.; Nagata, M.. The classification of glomerulonephritis in systemic lupus erythematosus revisited.J. Am. Soc. Nephrol. 2004, 15, 241–250. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Bombardier, C.; Gladman, D D.; Urowitz, M B.; Caron, D.; Chang, C H. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE.Arthritis Rheum. 1992, 35, 630–640. [PUBMED], [CSA]
- Thomas, G J.; Mason, R M.; Davies, M. Characterization of proteoglycans synthesized by human adult glomerular mesangial cells in culture.Biochem. J. 1991, 277, 81–88. [PUBMED], [INFOTRIEVE], [CSA]
- Zhao, M H.; Liu, N.; Zhang, Y K.; Wang, H Y. Antineutrophil cytoplasmic autoantibodies (ANCA) and their target antigens in Chinese patients with lupus nephritis.Nephrol. Dial. Transplant. 1998, 13, 2821–2824. [PUBMED], [CSA], [CROSSREF]
- Fu, H L.; Hsu, T C.; Chang, C C.; Tsay, G J. Antigenic specificity of anti-neutrophil cytoplasmic antibody.J. Formos. Med. Assoc. 2001, 100, 35–39. [PUBMED], [INFOTRIEVE], [CSA]
- Manolova, I.; Dancheva, M.; Halacheva, K. Antineutrophil cytoplasmic antibodies in patients with systemic lupus erythematosus: prevalence, antigen specificity, and clinical associations.Rheumatol. Int. 2001, 20, 197–204. [PUBMED], [INFOTRIEVE], [CSA]
- Chin, H J.; Ahn, C.; Lim, C S.; Chung, H K.; Lee, J G.; Song, Y W.; Lee, H S.; Han, J S.; Kim, S S.; Sang Lee, J S. Clinical implications of antineutrophil cytoplasmic antibody test in lupus nephritis.Am. J. Nephrol. 2000, 20, 57–63. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Molnar, K.; Kovacs, L.; Kiss, M.; Husz, S.; Dobozy, A.; Pokorny, G. Antineutrophil cytoplasmic antibodies in patients with systemic lupus erythematosus.Clin. Exp. Dermatol. 2002, 27, 59–61. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Chikazawa, H.; Nishiya, K.; Matsumori, A.; Hashimoto, K.. Immunoglobulin isotypes of anti-myeloperoxidase and anti-lactoferrin antibodies in patients with collagen diseases.J. Clin. Immunol. 2000, 20, 279–286. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Chen, M.; Zhao, M.-H.; Zhang, Y.; Wang, H. Antineutrophil autoantibodies and their target antigens in systemic lupus erythematosus.Lupus 2004, 13, 584–589. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Zhang, Y K.; Geng, H.; Zhao, M H.; Zou, W E.J.; Zheng, X.; Wang, H. The significance of anti-endothelial cell antibodies in patients with lupus nephritis and immunoblotting analysis of the target components.Chin. Med. J. 1999, 112, 597–602. [PUBMED], [INFOTRIEVE], [CSA]
- van der Zee, J M.; Siegert, C E.; de Vreede, T A.; Daha, M R.; Breedveld, F C. Characterization of anti-endothelial cell antibodies in systemic lupus erythematosus (SLE).Clin. Exp. Immunol. 1991, 84, 238–244. [PUBMED], [INFOTRIEVE], [CSA]
- Li, J S.; Liu, M F.; Lei, H Y. Characterization of anti-endothelial cell antibodies in the patients with systemic lupus erythematosus: a potential marker for disease activity.Clin. Immunol. Immunopathol. 1996, 79, 211–216. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Chan, T M.; Cheng, I K.P. A prospective study on anti-endothelial cell antibodies in systemic lupus erythematosus.Clin. Immunol. Immunopathol. 1996, 78, 41–46. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Perry, G J.; Elston, T.; Khouri, N A.; Chan, T M.; Cameron, J S.; Frampton, G. Anti-endothelial cell antibodies in lupus: correlation with renal injury and circulating markers of endothelial damage.Q. J. Med. 1993, 86, 727–734. [PUBMED], [INFOTRIEVE], [CSA]
- Foster, M H.; Cizma, B.; Madaio, M P. Biology of disease. Nephritogenic autoantibodies in systemic lupus erythematosus: immunochemical properties, mechanisms of immune deposition, and genetic origin.Lab. Invest. 1993, 494, 69. [CSA]
- Chan, T M.; Leung, J K.; Ho, S K.; Yung, S. Mesangial cell-binding anti-DNA antibodies in patients with systemic lupus erythematosus.J. Am. Soc. Nephrol. 2002, 13, 1219–1229. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]