Abstract
Background. Minimal change disease (MCD) and focal segmental glomerulosclerosis (FSGS) are the most prevalent histopathological lesions in idiopathic nephrotic syndrome (INS). The latter is associated with high morbidity and mortality due to symptomatic anasarca, bacterial infections, venous and arterial thromboembolism, and potential progression to end-stage renal disease in the case of FSGS. Traditionally, most patients are treated with corticosteroids, cyclophosphamide (CTX) or calcineurin-inhibitors (C-I). Unfortunately, many patients become steroid or C-I dependent, with the inherent risk of long-term side effects, or are resistant to both. The aim of this paper is to report on our experience with a new protocol of a combination of immunosuppressive agents added sequentially to improve the response of steroid and C-I refractory or resistant-INS and to minimize the long-term side-effects of single-agent treatment. Methods. Twenty-one patients with corticosteroid-resistant and C-I refractory INS (6 with MCD and 15 with FSGS) were treated prospectively over 6 and a half years. Our protocol consisted of an initial regimen of C-I followed by the addition of mycophenolate mofetil (MMF) and then by monthly intravenous CTX for 3 consecutive months. Dose reduction of C-I or/and MMF was attempted afterwards at 4-months intervals. Patients who remained refractory to the previously mentioned protocol were treated with an additional course of pulse Solu-Medrol given for 3 days followed by oral corticosteroids tapered over 6 months in addition to a second course of intravenous CTX given for 3 consecutive months. Results. With the initial regimen, two patients with MCD, remained in complete remission (CR) without any therapy after the course of CTX. Fifteen patients had variable response to C-I and MMF, but they achieved CR after CTX and their initial dosage of C-I and MMF were reduced to nearly one half. The remaining four patients had refractory form of FSGS even after the initial regimen, yet responded with CR to the additional course of steroid/CTX. However, no success with dose-reduction, in C-I and MMF, was achieved in the latter four patients. Conclusion. Our study represents the first clinical trial with prospective and adequate follow-up of combination therapy of immunosuppressive agents in INS. This method is effective and safe for treatment of patients who are refractory to the conventional single-agent therapy.
Introduction
Idiopathic nephrotic syndrome (INS) is a disease of the glomeruli characterized by nephrotic range proteinuria, hypoalbuminemia, generalized edema (up to anasarca), and hypercholesterolemia without an underlying etiology.Citation[1] Minimal change disease (MCD) and focal segmental glomerulosclerosis (FSGS) are the most prevalent histopathological lesions in both children and adults.Citation[2&3] Idiopathic nephrotic syndrome is associated with high morbidity and mortality due to its symptomatic anasarca, bacterial infections, and venous and arterial thromboembolisms, as well as the inherent complications of immunosuppressive therapy.Citation[1] Moreover, FSGS has a tendency for progressive renal disease and is a major cause of end-stage renal disease.Citation[4] Interestingly, FSGS can be overlooked in the initial renal biopsy, especially if the juxtamedullary glomeruli were not sampledCitation[5&6] and a transition from MCD to FSGS was a major concern particularly in treatment-resistant cases.Citation[7] For the latter reasons, the response to steroid therapy carried a greater prognostic weight than did the histological features seen on the initial biopsy.Citation[8] Hence two types of INS were defined: steroid-responsive and steroid-resistant. Traditionally, a 12-week course of corticosteroids (starting at 2 mg/day) is sufficient for steroid-sensitive INS and can be used for future infrequent relapses.Citation[1] A large proportion of frequently relapsing and steroid-dependent patients with INS were treated with 12 weeks of oral cyclophosphamide (CTX) or chlorambucil with adequate remission up to 2 years.Citation[9] In the past few years, large amount of research supported the use of cyclosporin A (CyA), and even tacrolimus (Prograf), in the treatment of patients who manifested relapses after adequate corticosteroid or cytotoxic therapy and even those who manifested steroid-resistance at the start.Citation[10&11] Unfortunately, a large percentage of such patients, were rendered CyA-dependent with the inherent risk of its long-term nephrotoxicityCitation[12&13] or were found to be CyA–resistant with a prevalence as high as 50% in steroid-resistant FSGS.Citation[14] The aim of this paper is to report on our experience with a new protocol of a combination of immunosuppressive agents added sequentially to improve the response of steroid and CyA refractory- or resistant-INS and to minimize the long-term side-effects of single-agent treatment.
Patients and Methods
Selection Criteria
In the period between January 1998 and April 2004, 21 patients with MCD or primary FSGS resistant to corticosteroids were selected for the study. Patients were included if they satisfied the following criteria: 1) histological diagnosis was made by adequate renal histology during their initial nephrotic presentation and none had collapsing glomerulopathy; 2) normal creatinine clearance at start; 3) secondary causes of NS were excluded after clinical, laboratory, radiological, and appropriate serological testing; and 4) INS was resistant to 8 weeks of treatment with corticosteroids at a dose of 2 mg/kg/day and refractory (frequently relapsing or dependent) to calcineurin-inhibitors (C-I).
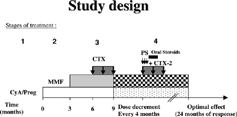
Study Design
As shown in , the initial therapy was with one of the C-I, i.e. CyA or Prograf (stage 1). Cyclosporine A (CyA) was the starting drug at this stage except for female patients who were mostly on Prograf from the start to avoid the skin darkening and hirsutism associated with long-term CyA therapy. Moreover, a few male patients were switched from CyA to Prograf after they had developed intolerable skin changes, hirsutism, or gum hyperplasia. The CyA was started at 3 mg/kg/day in two divided doses. Subsequently, the dose was further adjusted every 2 weeks to achieve a 12 hour trough level of 75–125 ng/mL. On the other hand, Prograf was started at 0.15 mg/kg/d in 2 divided doses and was further adjusted, at 2 week intervals, to achieve a 12 hour trough level of 5–10 ng/mL. The CyA assay was done using fluorescent polarization (TDx) while that of Prograf was done using microparticle enzyme immunoassay (MEIA, Abbott Laboratories, Abbott Park, IL). Patients who did not achieve complete remission (CR) after 12 weeks of C-I therapy, were further treated with mycophenolate mofetil (MMF) (stage 2). The latter was started at a dose of 250 mg every 12 hours and was adjusted gradually higher up to a maximum of 1 g twice daily, provided that the total peripheral leukocyte count remained above 4 × 109/L. Furthermore, if combined C-I and MMF treatment for 3 months did not result in complete remission of INS, a monthly intravenous therapy with CTX was given for 3 consecutive months (stage 3). The later was given at doses between 5–10 mg/kg intravenously and diluted in 100–200 mL of normal saline to run over 2 hours. In those who achieved CR, with or without CTX therapy, a trial was made every 4 months to decrease C-I or MMF to the minimum dose sufficient to maintain CR. Patients who failed to respond to the previously mentioned therapy were subjected to a second course of a combination of 3 monthly pulses of one gram of intravenous CTX in addition to 3 pulses of intravenous methylprednisolone, 1 g/1.73 m2, over 3 consecutive days followed by oral prednisone (1 mg/kg) for one month then gradual tapering over 2 more months (stage 4).
Periodic Assessment
Patients were seen at 2 week intervals for dose adjustment after addition of any drug. After establishment of CR, patients were seen at 1–2 months interval. In those visits, patients were assessed clinically for edema and side effects of therapy. Laboratory investigations included complete blood count and serum estimates of sugar, renal, liver, and lipid function tests as well as urine routine for proteinuria. Twenty-four-hour urine collections were done, every 2 months for assessment of creatinine clearance and protein excretion.
Definition of Response
Remission was considered complete (CR) if creatinine clearance remained normal and protein excretion decreased to < 200 mg/day in adults or (< 40 mg/m2/hour or protein:creatinine ratio < 200 mg/µmol) in children. Partial response (PR) was defined as a decrease in protein excretion to 50% of the initial value while creatinine clearance remains normal. Frequently relapsing (FR) INS was considered if the initial response was followed by ≥ 2 relapses per 6 months or ≥ 4 per 12 months while drug-dependent if relapses developed during dose tapering or 2 weeks after drug discontinuation. Nonresponders (NR) were defined as those who failed to achieve > 50% decrement in protein excretion within 12 weeks of treatment.
Results
As shown in , a total of 21 patients at different age groups were included in the study. Patients no. 1–6, had MCD and the rest had FSGS. Patients no. 16–21, had more significant mesangial cellular proliferation and sclerosis as well as interstitial infiltration with mononuclear cells.
Table 1. Clinical features and histology of the patients with steroid-resistant minimal change disease and focal segmental glomerulosclerosis (FSGS) treated with a combination of immunosuppressive agents
Response to Treatment
The overall response and sequence of management of individual patients are summarized in . In general, responders manifested significant improvement within 4–12 weeks from start or addition of any drug. Patients no. 1 and 2 had steroid-resistant MCD, and despite adequate C-I levels, they relapsed after 10 and 12 months, respectively. The addition of MMF resulted in a sustained remission, yet gradual reduction of the dose of C-I, 4 months later, was followed by relapses. The addition of CTX led to successful reduction of the dose of C-I to nearly one-half the original ones without relapse and at 12 months the MMF dose was reduced to one-half and was discontinued at 24 months. Patients no. 3–5 relapsed after 8–12 months of adequate doses of C-I. Hence, MMF was added. This resulted in CR, yet a trial to reduce the dose of C-I and/or MMF was not successful at 4 months. For this reason, CTX was added. Four months later, a trial to reduce the doses of C-I and MMF (even discontinuation in patients no. 4 and 5) was successful. Patient no. 6 had partial response to Prograf, yet CR after MMF. The same response, as the previous cases of MCD, was obtained after addition of CTX treatment. Patients with FSGS (no. 7–21) were either PR (no. 7–14) or NR (no. 15–21) to C-I. Addition of MMF resulted in no improvement in patients no. 7–11, while patients no. 12–14 achieved CR but with FR. Three patients (nos. 15–17) who failed to respond to C-I, became PR with the addition of MMF, while the remaining three did not respond. The addition of CTX, resulted in CR in all patients with FSGS except for the last four patients (nos. 18–21) who developed PR in three (nos. 18–20) and NR persisted in one (no. 21). The last four patients (nos. 18–21) were subjected to an additional course of steroid/CTX. The latter addition resulted in CR in those patients, yet the dose of C-I and MMF could not be lowered on further attempts. The final maintenance dose for each patient is shown in . The minimum period of follow-up was ≥ 24 months in all patients except for one patient (no. 20).
Table 2. Treatment and response of patients with steroid-resistant minimal change disease and focal segmental glomerulosclerosis treated with a combination of immunosuppressive agents
Side-Effects of Treatment
Patients no. 9, 10, and 15 could not tolerate the usual dose of Prograf due to extreme anxiety, tremors, and hyperactivity or gastrointestinal upsets, while patients no. 9, 10, and 16 had abdominal pains and/or diarrhea as well as significant anemia due to MMF. Hence, the dose of those two drugs was reduced and they tolerated that well. New-onset hypertension requiring antihypertensive therapy developed in all patients treated with C-I. However, it was mild in most patients, and decreased or disappeared after dose reduction or discontinuation of therapy with C-I. The CTX pulse treatment was almost invariably associated with nausea and body aches despite slow infusion, dilution with saline, and premedication with an IV anti-emetic; but the symptoms were mild, short lived, and tolerated by all patients.
Discussion
In this preliminary study, we evaluated the efficacy and safety of combined immunosuppressive therapy in a group of 21 patients with INS resistant to corticosteroids and/or refractory to C-I. We believe that our results are important as they represent the first clinical trial with prospective and adequate follow-up.
Overall the medications were tolerated by our patients and those who could not be treated with CyA for its side effects, especially females, were shifted to Prograf with similar efficacy.Citation[15] Moreover, those who could not tolerate Prograf or MMF, for their gastrointestinal upsets, were treated with a minimum dose of the drugs and the addition of CTX resulted in CR without long-term suffering.
Our protocol was tailored to include the use of C-I for treatment of steroid-refractory or steroid-resistant INS. Cyclosporine A was shown to be effective and safe on short-term follow-up.Citation[10] The addition of MMF was due to its potent immunosuppressive effect and antiproliferative action.Citation[16] In our opinion, its effect was limited in induction of immunosuppression, yet proved to be valuable in maintaining remission produced by other agents and CTX in particular. For this reason we elected to give MMF after C-I and for at least 3 months prior to CTX therapy. This may explain the dramatic response to the short course of CTX and our ability to reduce the C-I dose while maintaining CR, especially in patients with a disease that shows cellular proliferation such as FSGS. In fact, failure to completely withdraw C-I and/or MMF after CTX therapy is an indication of their effective immunosuppressive potentials as maintenance therapy. The maintenance doses of CyA, Prograf, and MMF may seem to be very high in children, yet their blood levels were within the therapeutic range. Furthermore, laboratory markers were within normal range (renal and liver function in patients on C-I as well as peripheral leukocyte count in patients on MMF). The latter is consistent with previous reports of higher metabolism of such drugs in children.Citation[17&18] Patients no. 18-21, were NR to the previous basic treatment and, hence, a more aggressive induction modality was added. They were treated with an additional course of 3 days of pulse Medrol followed by oral corticosteroids tapered over 6 months in addition to a second 3 grams of CTX. The addition of pulse Medrol in this setting was for its rapid cytotoxic effect especially on the highly active T-lymphocytes.Citation[19] In fact, we propose the use of other modalities to induce remission in more resistant cases in the future such as plasmapheresis or -exchange or -adsorption with or without a third course of CTX. The latter methods were reported to be useful in steroid-resistant FSGSCitation[20] and in disease recurrence after kidney transplantation.Citation[21] With regards to maintenance immunosuppressive therapy, new medications such as Sirolimus (rapamycin) can be included in future protocols to lower the dosage of CyA or MMF, due to its potent action on the growth factor receptor and hence its ability to suppress both T and B lymphocytes even at later stages of the immune response, i.e., even after immune stimulation.Citation[22] In our study, the additive effect of a limited course of CTX led to discontinuation or significant reduction of the dose of C-I and/or MMF in most patients with steroid-resistant disease. This may alleviate the concern of their long-term side effects, especially nephrotoxicity of C-I,Citation[13&14] gonadal failure with CTX, or malignant potentials of MMF and CTX.Citation[23] In our study, we did not repeat kidney biopsies in those who remained on a small therapeutic dose and even later on a low maintenance dose of C-I, since the expected incidence of CyA nephrotoxicity in this group is low.Citation[24] Moreover, creatinine clearance measurement remained normal at follow up. Patients no. 18–21 will have repeat biopsies, despite remaining in CR for 2 years, for the possibility of adding a further immunosuppressive modality (Vida supra) if histological evidence of chronic C-I toxicity exists.
Idiopathic nephrotic syndrome, and FSGS in particular, is a histological expression of diverse processes affecting the renal glomeruli.Citation[25] There is a complex interplay between genetic predisposition and environmental factors on the final phenotype expression and, hence, its response to pharmacological interventions.Citation[26-29] This heterogeneity of INS can be the result of different mediators of permeability, cellular proliferation, or inflammation on different target cells or the possibility of different and/or alternative pathways of action that are not completely blocked by the known single immunosuppressive agents.Citation[30-32] This hypothesis is the basis for our protocol of combined immunosuppressive therapy. The heterogeneity of INS is best seen in four phenomena. First, is the different response to immunosuppressive agents in different patients with seemingly the same histopathological lesion and in children vs. adults.Citation[33&34] Second, is the existence of late responders to the same agent, i.e., corticosteroids.Citation[35] Third, is the previous success with limited combination therapy.Citation[20], Citation[36&37] Fourth, is the genetic predisposition to recurrence of FSGS in the transplanted kidneys. This occurs more in the living-related than in cadaveric transplants, which are more miss-matched with recipients.Citation[38]
In conclusion, we have to admit that our present understanding of the pathogenesis of INS is inadequate and, hence, many types of interventions are likely to emerge. Until a specific therapy can be fashioned, it is essential for clinicians caring for such patients to provide nontoxic and effective measures to control this disease. We believe that our humble work is a step in this direction and it may appear to be useful in other forms of glomerulonephritis.
References
- Kaysen, G A. Nephrotic syndrome. In Current Therapy in Nephrology and Hypertension; Glassock, R J., Ed.; Mosby-Year Book: St. Louis, 1998; 223–230.
- Dember, L M.; Salant, D J. Minimal change disease. Therapy in Nephrology and Hypertension: A Companion to Brenner and Rector's The Kidney; Brady, H R., Wilcox, C S., Ed.; WB Saunders: Philadelphia, PA, 1999; 165–177.
- Korbet, S M. Primary focal segmental glomerulosclerosis. Therapy in Nephrology and Hypertension: A Companion to Brenner and Rector's The Kidney; Brady, H R., Wilcox, C S., Eds.; WB Saunders: Philadelphia, PA, 1999; 178–188.
- U.S. Renal Data System, USRDS 1998 Annual Data Report; National Institute of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, April 1998; 23–35.
- Rich, A R. A hitherto undescribed vulnerability of the juxtamedullary glomeruli in lipoid nephrosis. Bull. John Hopkins Hosp. 1957, 100, 173–183. [CSA]
- Habib, R. Focal glomerular sclerosis. Kidney Int. 1973, 4, 355–361. [PUBMED], [INFOTRIEVE], [CSA]
- Tejani, A. A morphological transition in minimal change nephrotic syndrome. Nephron 1985, 39, 157–159. [PUBMED], [INFOTRIEVE], [CSA]
- Broyer, M.; Meyrier, A.; Niaudet, P.; , et al. Minimal changes and focal segmental glomerulosclerosis. In Oxford Textbook of Clinical Nephrology, 2nd Ed.; Davison, A M., Cameron, S., Grunfield, J P., et al., Eds.; Oxford University Press: Oxford, 1997; 493–536.
- Meyrier, A. Minimal change disease and focal segmental glomerulosclerosis. In The Treatment of Glomerulonephritis; Pusey, C D., Ed.; Kluwer Academic Publishers, 1999; 39–54.
- El-Reshaid, K.; Amer, E.; Madda, J P.; Kapoor, M. Long-term cyclosporin A treatment in adults with refractory nephrotic syndrome. Ren. Fail. 1995, 17, 695–703. [PUBMED], [INFOTRIEVE], [CSA]
- Loeffler, K.; Gowrishankar, M.; Tiu, V. Tacrolimus therapy in pediatric patients with treatment-resistant nephritic syndrome. Pediatr. Nephrol. 2004, 19, 281–287. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Niaudet, P.; Broyer, M.; Habib, R. Serial renal biopsies in children with idiopathic nephrosis receiving cyclosporine. In Cyclosporin in the Therapy of Renal Disease; Tejani, A., Ed.; Karger: Basel, 1995; 78–83.
- Melcoton, T L.; Kamil, E S.; Cohen, A H.; Fine, R N. Long-term cyclosporine A treatment of steroid-resistant and steroid-dependant nephrotic syndrome. Am. J. Kidney Dis. 1991, 18, 583–588. [CSA]
- Niaudet, P.; Habib, R. Cyclosporine in treatment of idiopathic nephrosis. J. Am. Soc. Nephrol. 1994, 5, 1049–1056. [PUBMED], [INFOTRIEVE], [CSA]
- Aradhye, S.; Turka, L A. Immunosuppressive therapy in immunologic renal disease and transplantation. Therapy in Nephrology and Hypertension: A Companion to Brenner and Rector's The Kidney; Brady, H R., Wilcox, C S., Eds.; WB Saunders: Philadelphia, PA, 1999; 81–88.
- Gellermann, J.; Querfeld, U. Frequently relapsing nephritic syndrome: treatment with mycophenolate mofetil. Pediatr. Nephrol. 2004, 19 (1), 101–104. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Shapiro, R.; Scantlebury, V P.; Jordan, M L.; , et al. Tacrolimus in pediatric renal transplantation. Transplantation 1996, 62 (12), 1752–1758. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Virji, M.; Carter, J E.; Lirenman, D S. Single-center experience with mycophenolate mofetil in pediatric renal transplant recipients. Pediatr. Transplant. 2001, 5 (4), 293–296. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Mendoza, S A.; Reznik, M V.; Griswold, R W.; Krensky, M A.; Yorgin, P D.; Tune, M B. Treatment of steroid-resistant focal segmental glomerulosclerosis with pulse methylprednisolone and alkylating agents. Pediatr. Nephrol. 1990, 4, 303–307. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Mitwalli, A H. Adding plasmapheresis to corticosteroids and alkylating agents: does it benefit patients with focal segmental glomerulosclerosis? Nephrol. Dial. Transplant. 1988, 13, 1524–1528. [CSA], [CROSSREF]
- Dantal, J.; Bigot, E.; Bogers, W.; , et al. Effects of plasma protein adsorption on protein excretion in kidney-transplant recipients with recurrent nephrotic syndrome. N. Engl. J. Med. 1994, 300, 7–14. [CSA], [CROSSREF]
- Chung, J.; Kuo, C J.; Crabtree, G R. Rapamycin-FKBP specifically blocks growth dependent activation of and signaling by the 70 kd S6 protein kinases. Cell 1992, 69, 1227–1236. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Klipple, J H. Cyclophosphamide: ovarian and other toxicities. Lupus 1995, 4, 1–2. [CSA]
- Meyrier, A.; Noel, L H.; Auriche, P.; Callard, P. Long-term renal tolerance to cyclosporin A treatment in adult idiopathic nephrotic syndrome. Kidney Int. 1994, 45, 1446–1456. [PUBMED], [INFOTRIEVE], [CSA]
- Bolton, W K.; Abdel-Rahman, E. Pathogenesis of focal glomerulosclerosis. Nephron 2001, 88, 6–13. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Frishberg, Y.; Toledano, H.; Becker-Cohen, R.; Feigin, E.; Halle, D. Genetic polymorphism in paraoxonase is a risk factor for childhood focal segmental glomerulosclerosis. Am. J. Kidney Dis. 2000, 36, 1253–1261. [PUBMED], [INFOTRIEVE], [CSA]
- Futrakul, N.; Butthep, P.; Patumraj, S.; Tipprukmas, N.; Futrrakul, P. Enhanced tumor necrosis factor in the serum and renal hypoperfusion in nephrosis associated with focal segmental glomerulosclerosis. Ren. Fail. 2000, 22, 213–217. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Briggs, W A.; Gimenez, L F.; Samaniego-Picota, M.; Choi, M J.; Nadasdy, T.; Eustace, J.; Scheel, P J., Jr. Relationship between lymphocyte and clinical steroid responsiveness in focal segmental glomerulosclerosis. J. Clin. Pharmacol. 2000, 40, 115–123. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Alexopoulos, E.; Stangou, M.; Papagianni, A.; Papadimitriou, M. Factors influencing the course and the response to treatment in primary focal segmental glomerulosclerosis. Nephrol. Dial. Transplant. 2000, 15, 1348–1356. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Schwartz, M M. The role of podocyte injury in the pathogenesis of focal segmental glomerulosclerosis. Ren. Fail. 2000, 22, 663–684. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Sharma, R.; Sharma, M.; McCarthy, E T.; Ge, X L.; Savin, V J. Components of normal serum block the FSGS factors activity in vitro. Kidney Int. 2000, 58, 1973–1979. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Houck, K A.; Ferrara, N.; Winer, J.; , et al. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol. Endocrinol. 1991, 5, 1806–1814. [PUBMED], [INFOTRIEVE], [CSA]
- ISKDC (International Study of Kidney Disease in Children). Ten years of activity: a report for the International Study of Kidney Disease in Children. In Glomerulnephritis; Batsforrd, S R.,, et al., Eds.; John Wiley & Sons: New York, 1977; 201–209.
- Meyrier, A.; Simon, P. Treatment of corticoresistant idiopathic nephrotic syndrome in adults: minimal change disease and focal segmental glomerulosclerosis. Adv. Nephrol. 1988, 17, 127–150. [CSA]
- Pie, Y.; Cattran, D.; Delmore, T.; Katz, A.; Lang, A.; Rance, P. Evidence suggesting under-treatment in adults with idiopathic focal segmental glomerulosclerosis. Am. J. Med. 1987, 82, 938–944., [CSA], [CROSSREF]
- Niaudet, P.; French Society of Pediatric Nephrology. Treatment of childhood steroid-resistant idiopathic nephrosis with a combination of cyclosporine and prednisone. J. Pediatr. 1994, 125, 981–986. [PUBMED], [INFOTRIEVE], [CSA]
- Norona, B.; Valentin, M.; Gutierrez, E.; Praga, M. Treatment of steroid-dependent minimal change-nephrotic syndrome with mycophenolate mofetil. Nefrologia 2004, 24, 79–82. [PUBMED], [INFOTRIEVE], [CSA]
- Abbott, K C.; Sawyer, E S.; Oliver, J D.; III; Kop, C W.; Kirk, A D.; Welch, P G.; , et al. Graft loss due to recurrent focal segmental glomerulosclerosis in renal transplant recipients in the United States. Am. J. Kidney Dis. 2001, 37, 366–373. [PUBMED], [INFOTRIEVE], [CSA]