Abstract
This study was performed to investigate the potential relationship between left ventricular hypertrophy (LVH) and proinflammatory cytokines in hemodialysis (HD) patients and the effect of HD on cytokine production. Serum interleukin 1 beta (IL-1 β), interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α) measurements and echocardiographic studies were performed in 35 stable HD patients. A variety of probable risk factors for LVH including age, HD duration, blood pressure (BP), body mass index, lipid profile, hemoglobin, albumin, parathormone and homocysteine levels were also investigated. Additionally, the effect of HD procedure on cytokine levels was evaluated. Predialysis serum levels of IL-1β, IL-6, TNF-α, and homocysteine in HD patients were compared with 12 healthy subjects. Left ventricular hypertrophy was demonstrated in 20 (57%) of HD patients by echocardiography. Left ventricular mass index (LVMI) was correlated positively with systolic BP (r = 0.556, p = 0.001), diastolic BP (r = 0.474, p = 0.004), and serum levels of TNF-α (r = 0.446, p = 0.009).Multiple regression analysis showed that systolic BP and TNF-α levels were significant independent predictors of LVH. No relationship was observed between LVH and other parameters. The mean predialysis serum level of IL-6 was significantly higher in HD patients compared to healthy controls (15.7 ± 8.7 vs. 7.3 ± 0.7 pg/mL, p = 0.001). Predialysis serum levels of TNF-α in HD patients were higher when compared to healthy subjects, but the difference was not statistically significant (8.3 ± 3 vs. 7 ± 1.45 pg/mL, respectively, p > 0.05). However, serum levels of IL-6 and TNF-α significantly elevated after HD, when compared to predialysis levels (from 15.7 ± 8.7 to 17.8 ± 9.5 pg/mL, p = 0.001 and from 8.3 ± 3.0 to 9.9 ± 3.5 pg/mL p = 0.004, respectively). As a conclusion, in addition to BP, proinflammatory cytokines, TNF-α in particular, seem to be associated with LVH in ESRD patients.
Introduction
Cardiovascular disease is the major cause of death in patients with end stage renal disease (ESRD).Citation[1&2] Left ventricular hypertrophy, as determined by echocardiography was documented with a very high frequency in HD patients, which is proposed as one of the most important predictors for cardiovascular mortality of these patients.Citation[3-5] Several traditional risk factors including hypertension, age, gender, increased body mass index (BMI), and diabetes mellitus have a role in development of LVH in uremic and nonuremic patients.Citation[6&7] However, extraordinarily high prevalence of LVH in patients with ESRD appears to be multifactorial in etiology. Uremia and HD, per se have additional peculiar risk factors for development of LVH including anemia, presence of arteriovenous fistulae, secondary hyperparathyroidism, and insufficient dialysis.Citation[8] Recent studies focused on the role of other mechanisms for LVH, which are commonly associated with cardiovascular disease both in uremic and nonuremic patients. Of note, metabolic abnormalities such as hyperhomocysteinemia and inflammatory process, evidenced by elevated circulating levels of IL-6, TNF-α, and C-reactive protein (CRP) are frequently seen in patients with ESRD and have been considered as risk factors for atherosclerosis.Citation[9-17] These factors also appear to be related to development of LVH.Citation[18-22]
The aim of this study was to investigate the frequency of LVH by using echocardiography and to analyze various probable risk factors for development of LVH, including IL-1β, IL-6, TNF-α levels, age, duration of HD, blood pressure, hemoglobin, total cholesterol (t-chol), low density lipoprotein cholesterol (LDL-chol), high density lipoprotein cholesterol (HDL-chol), albumin, parathormone, and homocysteine levels in stable HD patients. Moreover, the effect of HD on serum levels of IL-1β, IL-6, TNF-α, and homocysteine was also investigated.
Materials and Methods
Sixty-eight HD patients older than 18 years were recruited from our unit. Inclusion criteria were as follows: Treatment by maintenance HD three times a week for at least 6 months; no acute cardiovascular symptoms, no evidence of systemic disease to be associated with cardiac disease such as diabetes mellitus and systemic amyloidosis, no active or chronic infection, no evidence of acute or chronic inflammatory disease, lack of malignancy and no ongoing immunosuppressive therapy. Thirty-three patients were excluded according to these criteria. The remaining 35 patients constituted the participants of this study. The study was approved by the Ethical Committee on Studies Involving Human Beings at Gazi University Faculty of Medicine and informed consent was obtained from each participant. The etiology of the renal failure in the analyzed group was glomerulonephritis in seven patients, pyelonephritis in three, vascular disease in six, nephrolithiasis in three, and other causes in three. The etiology was unknown in 13 of the patients.
All of the patients treated with a standard HD procedure via A-V fistulae, each session lasted 4 hours, three times a week, using bicarbonate-containing dialysate and hemophan membrane. The blood flow rate ranged from 250 to 300 mL/min and dialysate flow rate was 500 mL/min. Twenty-two patients were receiving erythropoietin. Fourteen patients were taking antihypertensive drugs: five on monotherapy with angiotensin converting enzyme inhibitors, angiotensin II subtype I receptor antagonists, calcium channel blockers, or alpha blockers; six on double; and three on triple therapy with various combinations of these drugs. Twelve age and sex-matched (43 ± 10 years; 8 men, 4 women) healthy hospital staff served as the control group.
Blood Pressure Measurements
Predialysis blood pressures of patients were calculated as the average values of 12 measurements that were taken during a period of 1 month.
Laboratory Parameters
Blood samples were obtained from arteriovenous fistula before initiation of HD, following an overnight fast. Pre- and postdialysis blood urea nitrogen (BUN), predialysis creatinine, albumin, t-chol, LDL-chol, HDL-chol, hemoglobin (Hb), calcium (Ca), phosphate (PO4), and intact parathormone (iPTH) measurements were made by using standard methods in the routine clinical laboratory. Pre- and postdialysis serum total homocysteine (tHcy) levels (Axis-ELISA Kit) and levels of IL-1β, IL-6, and TNF-α (Biosource-ELISA kit) were measured by enzyme linked immune sorbent assay. The urea reduction rate (URR) was used as a surrogate measurement of urea clearance obtained by dialysis and calculated by the formula: (predialysis BUN-postdialysis BUN)/predialysis BUN) X 100.
Echocardiography
All echocardiographic measurements were performed within 60 minutes after termination of the dialysis during when patients were at their dry weight. The same observer from the Echocardiography Laboratory in the Department of Cardiology at Gazi University made the measurements. Two dimensional, Doppler, and M mode measurements were performed on an ultrasound machine (General Electric, Vivid 3, USA) using an output frequency of 1.7 mHz with secondary harmonic imaging. The patients were examined in the left lateral decubitus position. Parasternal long axis, short axis, apical four chamber, and two chamber views have been examined. Left ventricular end-systolic dimension (LVESD), left ventricular end-diastolic dimension (LVEDD), interventricular septum (IVS) and posterior wall thickness (PWT) were measured using two dimensionally guided M-mode echocardiography as recommended by American Society of Echocardiography.Citation[23] End-diastolic and end-systolic volumes, and stroke volume, ejection fraction values were calculated as well. Left ventricular mass was calculated using Devereux equation: LV mass (in grams)= 0,8 × 1.04[(LVID + IVST + PWT)3-(LVID)3] g.Citation[24] Left ventricular mass was corrected for body surface area and left ventricular hypertrophy was defined as LVMI > 131 g/m2 for men, > 100 g/m2 for women.Citation[25]
Statistical Analysis
Results are expressed as mean ± SD. Differences between groups were tested by t test, unpaired t test (Mann Whitney U test) or by Chi-square test, as appropriate.
Univariate correlation was established by Pearson's correlation coefficient. To assess the influence of tested parameters on LVMI as the dependent variable, multiple regression analysis was performed. Since the number of patients in the study group was relatively low, only parameters correlated significantly with LVMI were included in the subsequent multiple regression models. All statistical calculations were performed using the Statistical Package for the Social Sciences (SPSS version 11). Significance defined as a p value less than 0.05.
Results
The mean age of 35 (25 men/10 women) patients was 45 ± 14 (range 18–68) years and the mean duration of HD was 39 ± 34 (range 6–122) months. Twenty patients (57%) had LVH. Mean values of left ventricular parameters are shown in . Left ventricular mass index, PWT, IVS, LVEDD, and LVESD were significantly greater in patients with LVH, compared to the patients without LVH (p< 0.05). Mean values of ejection fraction and fractional shortening were similar between those with and without LVH (p > 0.05). Eleven patients with LVH (3 on monotherapy, 5 on double, and 3 on triple therapy), and three patients without LVH were on (2 on monotherapy, 1 on double therapy) antihypertensive therapy.
Table 1. Left ventricular characteristics of hemodialysis patients by echocardiography
Comparison of Patients with and without LVH
There was no significant difference in age, gender, HD duration, EPO administration, BMI, URR, mean interdialytic weight gain, systolic BP, and diastolic BP, between the patients with and without LVH (p > 0.05) (). However, antihypertensive drug administration was significantly higher in patients with LVH, compared to the patients without LVH (p = 0.04). Laboratory parameters including Hb, ferritin, albumin, Ca, PO4, iPTH, t-chol, LDL-chol, HDL chol, IL-1 β, IL-6, TNF-α, and tHcy levels were not different between the two groups (p > 0.05) ().
Table 2. Clinical and laboratory characteristics of HD patients with and without LVH
Cytokine and tHcy Levels
Predialysis serum levels of IL-6 and tHcy were significantly higher in HD patients compared to controls (15.7 ± 8.7 vs. 7.3 ± 0.7 pg/mL p = 0.001, and 24.2 ± 7.6 vs. 8.7 ± 1.6, respectively, p = 0.001) (). Despite higher mean serum TNF-α level in HD patients than healthy controls, the difference was not significant (8.3 ± 3 vs. 7 ± 1.45 pg/mL, respectively, p > 0.05). Serum IL-1 β level was also found to be similar between the two groups. After HD, serum IL-1 β level did not change, serum IL-6 and TNF-α levels significantly elevated (from 15.7 ± 8.7 to 17.8 ± 9.5 pg/mL, p = 0.001 and from 8.3 ± 3 to 9.9 ± 3.5 pg/mL p = 0.004, respectively), whereas tHcy level significantly decreased (from 24.2 ± 7.6 to 18.9 ± 5.9 pg/mL, p = 0.001) compared to predialysis levels ().
Table 3. Mean serum levels of IL-1 β, IL-6, TNF-α, and tHcy in HD patients (before and after HD) and healthy subjects
Determinants of LVMI
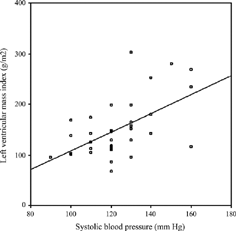
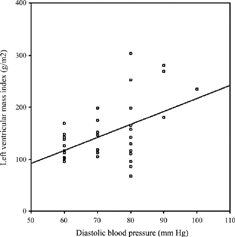
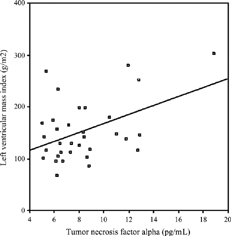
No correlation was found between LVMI and age, HD duration, URR, interdialytic weight gain, predialysis serum Hb, ferritin, albumin, Ca, PO4, iPTH, t-chol, HDL chol, tHcy, and IL-1β levels (p > 0.05). However, there were significant correlations between LVMI and systolic BP (r = 0.556, p = 0.001) (), diastolic BP (r = 0.474, p = 0.004) () or predialysis serum level of TNF-α(r = 0.446, p = 0.009) (). The correlation between predialysis IL-6 and LVMI showed a borderline significance (r = 0.337, p = 0.063).
Modeling significant factors including systolic BP, diastolic BP, and TNF-α by multiple regression showed systolic BP (β = 0.597; p = 0.001) and TNF-α (β = 0.368; p = 0.006) as independent predictors of LVMI.
Discussion
Left ventricular hypertrophy is a common cardiac abnormality of patients with ESRD, occurring in 39%-74% of patients starting dialysisCitation[26-28] and in 68%-82 % of patients during maintenance dialysis therapy.Citation[29-31] In this study, LVH was detected in 57% of HD patients. Decreased frequency of LVH in ESRD patients by years was correlated with intensified treatment of hypertension, fluid overload, and uremia.Citation[32]). Better control of the aforementioned conditions might explain the lower frequency of LVH seen in this study in comparison to the previous reports. Additionally, younger age of patients in the studied group and exclusion of diabetic and amyloidosis patients would be other explanations of lower LVH prevalence.
Since LVH is a strong independent risk factor for mortality in dialysis patients, it is important to define and even correct any reversible risk factor for development of LVH in these patients. There are several reversible risk factors for LVH in chronic uremia, including anemia, hypertension, hypoalbuminemia, and hyperparathyroidism Citation[8] Treatment of anemia by recombinant human erythropoietin,Citation[33&34] reducing PTH level by calcitriol therapy,Citation[35] and lowering BP either by antihypertensive drugs, especially by ACE inhibitors,Citation[36] or by strict volume controlCitation[37] have induced regression of LVH in HD patients. Besides, the importance of serum albumin level for LVH has also been demonstrated.Citation[31] In the present study, levels of Hb, albumin, and iPTH were comparable among the patients with and without LVH and no correlation was found between LVMI and these parameters. The importance of either isolated systolic BP or combined systolic and diastolic BP for development of LVH has been well established both in uremic and nonuremic patients.Citation[38-41] In the current study of the patients with ESRD, a significant association between systolic/diastolic blood pressures and LVMI supports importance of BP for development of LVH. However, demonstration of LVH in normotensive HD patientsCitation[42] and progression of LVH in uremic animals despite normalization of BPCitation[43] suggest factors other than hemodynamic overload that might contribute to the development of LVH.
Chronic inflammation, demonstrated by altered cytokine levels, is highly prevalent in uremic patients.Citation[16], Citation[44&45] It might be due to underlying uremic condition, reduced renal clearance of cytokines, or co-morbidity such as chronic heart failure.Citation[46&47] Moreover, various factors associated with HD procedure would also contribute to the inflammation observed in HD patients. Activation of complement cascade during HD by bioincompatible as well as biocompatible membrane material and/or contamination of dialysate by endotoxin fragments and other bacterial toxins may result in production of IL-1β, IL-6, and TNF-α.Citation[12&13], Citation[44], Citation[45] In the current study, significant elevation in serum IL-6 and TNF-α levels after HD therapy supports the effect of HD on cytokine production. Several studies have demonstrated that these cytokines promote atherosclerotic cardiovascular disease and mortality in patients with ESRD.Citation[10-12], Citation[14]
Recently, the association of proinflammatory cytokines with myocardial hypertrophy received increased attention.Citation[18-21] Park et al. demonstrated a positive correlation between LVMI and IL-6 level in HD patients.Citation[48] An association between IL-6 promoter polymorphism -174G/C and high blood pressure and LVH both in HD patients and healthy subjects has also been observed.Citation[49&50] Moreover, regression of LVH by ACE inhibitors was shown to be associated with not only lowering BP but also normalizing myocardial IL-6 expression.Citation[51] In the present study, correlation of IL-6 and LVMI did not reach significance. In part, this may be due to the small size of the study group, which is a limitation of the study. However, an interesting finding of the current study was the positive correlation of TNF-α with increment of LV mass, in addition to demonstrating TNF-α as an independent risk factor for LVH. This is in line with the finding of Espinoza et al.,Citation[22] who demonstrated a relationship between TNF-α and LVH in continuous ambulatory peritoneal dialysis patients. Moreover, in the recent literature, several experimental studies indicate that TNF-α may have a role in development of LVH. Tumor necrosis factor alpha has been shown to provoke a hypertrophic growth response in cultured cardiac myocytesCitation[21] and prolonged TNF-α stimulation may contribute to the development of cardiac hypertrophyCitation[21],Citation[52] and fibrosis.Citation[53] Additionally, TNF-α was also shown to be produced in the heart under various forms of stress and capable of inducing hypertrophy and cardiomyopathy in experimental animals.Citation[19&20] Finally, in patients with hypertrophic obstructive cardiomyopathy, TNF-α has been suggested as playing a pathogenetic role in the development of hypertrophy.Citation[54] Another limitation of our study is that the duration of the high TNF-α levels after HD was not investigated and it remains unknown if TNF-α elevation is sustained long enough to produce these cardiac effects in HD patients. To the best of our knowledge, there is no such study for TNF-α in HD patients investigating the duration of an elevated level of TNF-α after hemodialysis. However. Caglar et al. demonstrated an increase in IL-6 and fibrinogen concentrations during HD, which is exacerbated during the 2-hour period following the termination of HD and suggested that other cytokines, such as IL-1β and TNF-α could also be involved in this inflammatory cascade.Citation[13]
Homocysteine is another possible risk factor for development of LVH. Hyperhomocysteinemia has been shown as a common metabolic disturbance and an independent risk factor for cardiovascular disease in ESRD patients.Citation[55&56] A positive correlation between homocystene (Hcy) and LVH has also been demonstrated both in uremic and nonuremic patients.Citation[57&58] In the present study, despite higher level of tHcy in HD patients compared to healthy subjects, no significant relationship was observed between tHcy level and LVH. Similar results have been reported by Moon et al.Citation[31]
In conclusion, our study suggests the importance of BP and a role of TNF-α in the development of LVH in HD patients. Furthermore, increase in IL-6 and TNF-α levels after an HD session has also been demonstrated. Confirmatory studies are needed to clarify the relationship between HD-induced TNF-α production and LVH in HD patients.
Acknowledgments
This study was supported by grants from Gazi University Scientific Research Projects Department. The authors thank hemodialysis staff of Gazi University for their assistance.
References
- Foley, R N.; Parfrey, P S.; Sarnak, M J. Epidemiology of cardiovascular disease in chronic renal disease. J. Am. Soc. Nephrol. 1998, 9 (12 Suppl.), S16–S23. [PUBMED], [INFOTRIEVE], [CSA]
- Brown, J H.; Hunt, L P.; Vites, N P.; Short, C D.; Gokal, R.; Mallick, N P. Comparative mortality from cardiovascular disease in patients with chronic renal failure. Nephrol. Dial. Transplant. 1994, 9 (8), 1136–1142. [PUBMED], [INFOTRIEVE], [CSA]
- Silberberg, J S.; Barre, P E.; Prichard, S S.; Sniderman, A D. Impact of left ventricular hypertrophy on survival in end stage renal disease. Kidney Int. 1989, 36, 286–290. [PUBMED], [INFOTRIEVE], [CSA]
- Nakamura, S.; Sasaki, O.; Nakahama, H.; Inenaga, T.; Kimura, G. Left ventricular hypertrophy is a risk factor independent of hypertension in survival of hemodialyzed patients. Ren. Fail. 2002, 24 (2), 175–186. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Foley, R N.; Parfrey, P S.; Harnett, J D.; Kent, G M.; Murray, D C.; Barre, P E. The prognostic importance of left ventricular geometry in uremic cardiomyopathy. J. Am. Soc. Nephrol. 1995, 5, 2024–2031. [PUBMED], [INFOTRIEVE], [CSA]
- Amann, K.; Rychlik, I.; Miltenberger-Milteny, G.; Ritz, E. Left ventricular hypertrophy in renal failure. Kidney Int. 1998, 54 (Suppl. 68), S78–S85. [CSA], [CROSSREF]
- London, G M. Left ventricular hypertrophy: why does it happen? Nephrol. Dial. Transplant. 2003, 18 (Suppl. 8), viii2–viii6., [PUBMED], [INFOTRIEVE], [CSA]
- London, G M.; Pannier, B.; Guerin, A P.; Parfrey, P S. Cardiac disease in chronic uremia: Pathogenesis. Adv. Renal Replace. Ther. 1997, 4, 194–211. [CSA]
- Stompor, T.; Pasowicz, M.; Sullowicz, W.; Dembinska- Kiec, A.; Janda, K.; Wojcik, K.; Tracz, W.; Zdzienicka, A.; Klimeczek, P.; Janusz-Grzybowska, E. An association between coronary artery calcification score, lipid profile, and selected markers of chronic inflammation in ESRD patients treated with peritoneal dialysis. Am. J. Kidney Dis. 2003, 41 (1), 203–211. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Santoro, A.; Manchini, E. Cardiac effects of chronic inflammation in dialysis patients. Nephrol. Dial. Transplant. 2002, 17 (Suppl. 8), 10–15. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Wanner, C.; Zimmermann, J.; Shwedler, S.; Metzger, T. Inflammation and cardiovascular risk in dialysis patients. Kidney Int. 2002, 61 (Suppl. 80), S.99–S.102. [CSA]
- Kaysen, G A. The microinflammatory state in uremia; Causes and potential consequences. J. Am. Soc. Nephrol. 2001, 12, 1549–1557. [PUBMED], [INFOTRIEVE], [CSA]
- Caglar, K.; Peng, Y.; Pupim, L B.; Flakoll, P J.; Levenhagen, D.; Hakim, R M.; Ikizler, T A. Inflammatory signals associated with hemodialysis. Kidney Int. 2002, 62 (4), 1408–1416. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Stenvinkel, P.; Barany, P.; Heimburger, O.; Pechoits-Filho, R.; Lindholm, B. Mortality, malnutrition, and atherosclerosis in ESRD: what is the role of interleukin-6? Kidney Int. Suppl. 2002, 80, 103–108. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Yudkin, J S.; Kumari, M.; Humphries, S E.; Mohamed-Ali, V. Inflammation, obesity, stres and coronary heart disease: is interleukin-6 the link? Atherosclerosis 2000, 148 (2), 209–214. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Descamps-Latscha, B.; Herbelin, A.; Nguyen, A T.; Roux-Lombard, P.; Zingraff, J.; Moynot, A.; Verger, C.; Dahmane, D.; de Groote, D.; Jungers, P. Balance between IL-1β, TNF-α and their specific inhibitors in chronic renal failure and maintenance dialysis. J. Immunol. 1995, 154 (2), 882–892. [PUBMED], [INFOTRIEVE], [CSA]
- Erren, M.; Reinecke, H.; Junker, R.; Fobker, M.; Schulte, H.; Schurek, J O.; Kropf, J.; Kerber, S.; Breithardt, G.; Assmann, G.; Cullen, P. Systemic inflammatory parameters in patients with atherosclerosis of the coronary and peripheral arteries. Arterioscler. Thromb. Vasc. Biol. 1999, 19 (10), 2355–2363. [PUBMED], [INFOTRIEVE], [CSA]
- Hirote, H.; Yoshida, K.; Kishimoto, T.; Taga, T. Continuous activation of gp 130, a signal-transducing receptor component for interleukin-6 related cytokines, causes myocardial hypertrophy in mice. Proc. Natl. Acad. Sci. U. S. A. 1995, 92, 4862–4866. [CSA]
- Bryant, D.; Becker, L.; Richardson, J.; Shelton, J.; Franco, F.; Peshock, R.; Thompson, M.; Giroir, B. Cardiac failure in transgenic mice with myocardial expression of tumor necrosis factor alpha. Circulation 1998, 97 (14), 1375–1381. [PUBMED], [INFOTRIEVE], [CSA]
- Bozkurt, B.; Kribbs, S.; Clubb, M., Jr.; Michael, L H.; Didenko, V V.; Hornsby, P J.; Seta, Y.; Oral, H.; Spinale, F G.; Mann, D L. Pathophysiologically relevant concentrations of tumor necrosis factor-α promote progressive left ventricular dysfunction and remodeling in rats. Circulation 1998, 97, 1382–1391. [PUBMED], [INFOTRIEVE], [CSA]
- Yokoyama, T.; Nakano, M.; Bednarzczyk, J L.; McIntyre, B W.; Entman, M.; Mann, D L. Tumor necrosis factor-alpha provokes a hypertrophic growth response in adult cardiac myocytes. Circulation 1997, 95 (5), 1247–1252. [PUBMED], [INFOTRIEVE], [CSA]
- Espinoza, M.; Aguilera, A.; Auxiliadora, B M.; Codoceo, R.; Caravaca, E.; Cirugeda, A.; del Peso, G.; Hevia, C.; Selgas. Tumor necrosis factor alpha as a uremic toxin: correlation with neuropathy, left ventricular hypertrophy, anemia and hypertriglyceridemia in peritoneal dialysis patients. Adv. Perit. Dial. 1999, 15, 82–86. [PUBMED], [INFOTRIEVE], [CSA]
- Sahn, D J.; DeMaria, A.; Kisslo, J.; Weyman, A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation 1978, 58, 1072–1083. [PUBMED], [INFOTRIEVE], [CSA]
- Devereux, R B.; Alonso, D R.; Lutas, E M.; Gottlieb, G J.; Campo, E.; Sachs, I.; Reichek, N. Echocardiographic assesment of left ventricular hypertrophy: Comparison to necropsy findings. Am. J. Cardiol. 1986, 57, 450–458. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Savage, D D.; Garrison, R J.; Kannel, W B Levy, D.; Anderson, S J.; Stokes, J., III; Feinleib, M.; Castelli, W P. The spectrum of left ventricular hypertrophy in a general population sample: the Framingham Study. Circulation 1987, 75 (1 Pt. 2), I26–I33. [PUBMED], [INFOTRIEVE], [CSA]
- Levin, A.; Singer, J.; Thompson, C R.; Ross, H.; Lewis, M. Prevalent left ventricular hypertrophy in the predialysis population: identifying opportunities for intervention. Am. J. Kidney Dis. 1996, 27 (3), 347–354. [PUBMED], [INFOTRIEVE], [CSA]
- Parfrey, P S.; Foley, R N.; Harnett, J D.; Kent, G.M.; Martin, C J.; Murray, D C.; Barre, P E. Clinical and echocardiographic disease in patients starting end-stage renal disease. Kidney Int. 1995, 47, 186–192. [PUBMED], [INFOTRIEVE], [CSA]
- McMahon, L P.; Roger, S D.; Levin, A. Slimheart Investigators Group. Development, prevention, and potential reversal of left ventricular hypertrophy in chronic kidney disease. J. Am. Soc. Nephrol. 2004, 15 (6), 1640–1647. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Harnett, J D.; Kent, G M.; Barre, P E.; Taylor, R.; Parfrey, P S. Risk factors for the development of left ventricular hypertrophy in a prospectively followed cohort of dialysis patients. J. Am. Soc. Nephrol. 1994, 4, 1486–1490. [PUBMED], [INFOTRIEVE], [CSA]
- Covic, A.; Goldsmith, D J.A.; Georgescu, G.; Venning, M C.; Ackrill, P. Echocardiographic findings in long term, long hour hemodialysis patients. Clin. Nephrol. 1996, 45 (2), 104–110. [PUBMED], [INFOTRIEVE], [CSA]
- Moon, K H.; Song, I S.; Yang, W S.; Shin, Y T.; Kim, S B.; Song, J K.; Park, J S. Hypoalbuminemia as a risk factor for progressive left-ventricular hypertrophy in hemodialysis patients. Am. J. Nephrol. 2000, 20, 396–401. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Bech-Hanssen, O.; Wallentin, I.; Larsson, O.; Nyberg, G. Reduced left ventricular hypertrophy in type 1 diabetic patients with end-stage renal failure. A comparison between groups investigated 1977-80 and 1991-93. Nephrol. Dial. Transplant. 1996, 11 (8), 1547–1552. [PUBMED], [INFOTRIEVE], [CSA]
- Low-Friedrich, I.; Grutzmacher, P.; Marz, W.; Bergmann, M.; Schoeppe, W. Therapy with recombinant human erythropoietin reduces cardiac size and improves heart function in chronic hemodialysis patients. Am. J. Nephrol. 1991, 11 (1), 54–60. [PUBMED], [INFOTRIEVE], [CSA]
- Silberberg, J.; Racine, N.; Barre, P.; Sniderman, A D. Regression of left ventricular hypertrophy in dialysis patients following correction of anemia with recombinant human erythropoietin. Can. J. Cardiol. 1990, 6 (1), 1–4. [PUBMED], [INFOTRIEVE], [CSA]
- Park, C W.; Oh, Y S.; Shin, Y S.; Kim, C M.; Kim, Y S.; Kim, S Y.; Choi, E J.; Chang, Y S.; Bang, B K. Intravenous calcitriol regresses myocardial hypertrophy in hemodialysis patients with secondary hyperparathyroidism. Am. J. Kidney Dis. 1999, 33 (1), 73–81. [PUBMED], [INFOTRIEVE], [CSA]
- Cannella, G.; Paoletti, E.; Delfino, R.; Cannella, G.; Paoletti, E.; Delfino, R.; Peloso, G.; Molinari, S.; Traverso, G B. Regression of left ventricular hypertrophy in hemodialysed uremic patients on long-term antihypertensive therapy. Kidney Int. 1993, 44, 881–886. [PUBMED], [INFOTRIEVE], [CSA]
- Ozkahya, M.; Ok, E.; Cirit, M.; Ozkahya, M.; Ok, E.; Cirit, M.; Aydin, S.; Akcicek, F.; Basci, A.; Dorhout Mees, E J. Regression of left ventricular hypertrophy in haemodialysis patients by ultrafiltration and reduced salt intake without antihypertensive drugs. Nephrol. Dial. Transplant. 1998, 13 (6), 1489–1493. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- de Lima, J J.; Abensur, H.; Krieger, E M.; Pileggi, F. Arterial blood pressure and left ventricular hypertrophy in haemodialysis patients. J. Hypertens. 1996, 14 (8), 1019–1024. [PUBMED], [INFOTRIEVE], [CSA]
- Ganau, A.; Devereux, R B.; Pickering, T G.; , et al. Relation of left ventricular hemodynamic load and contractile performance to left ventricular mass in hypertension. Circulation 1990, 81 (1), 25–36. [PUBMED], [INFOTRIEVE], [CSA]
- Tucker, B.; Fabbian, F.; Giles, M.; Thuraisingham, R C.; Raine, A E.G.; Baker, L R.I. Left ventricular hypertrophy and ambulatory blood pressure monitoring in chronic renal failure. Nephrol. Dial. Transplant. 1997, 12, 724–728. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Nishikimi, T.; Minami, J.; Tamano, K.; Nishikimi, T.; Minami, J.; Tamano, K.; Takahashi, M.; Numabe, A.; Futoo, Y.; Honda, T.; Kobayashi, T.; Uetake, S.; Mori, Y.; Saito, T.; Matsuoka, H. Left ventricular mass relates to average systolic blood pressure, but not loss of circadian blood pressure in stable hemodialysis patients: an ambulatory 48-hour blood pressure study. Hypertens. Res. 2001, 24 (5), 507–514. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Lin, Y P.; Chen, C H.; Yu, W C.; Hsu, T L.; Ding, P Y.; Wang, W C. Left ventricular mass and hemodynamic overload in normotensive hemodialysis patients. Kidney Int. 2002, 62, 1828–1838. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Rambausek, M.; Ritz, E.; Mall, G.; Mehls, O.; Katus, H. Myocardial hypertrophy in rats with renal insufficiency. Kidney Int. 1985, 28, 775–782. [PUBMED], [INFOTRIEVE], [CSA]
- Singh, N P.; Banal, R.; Thakur, A.; Kohli, R.; Bansal, R C.; Agarval, S K. Effect of membrane composition on cytokine production and clinical symptoms during hemodialysis: a crossover study. Ren. Fail. 2003, 25 (3), 411–430. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Sitter, T.; Bergner, A.; Schiffl, H. Dialysate related cytokine induction and response to recombinant erythropoietin in haemodialysis patients. Nephrol. Dial. Transplant. 2000, 15 (8), 1207–1211. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Niebauer, J.; Volk, H D.; Kemp, M.; Dominguez, M.; Schumann, R R.; Rauchhaus, M.; Poole-Wilson, P A.; Coats, A J.; Anker, S D. Endotoxin and immune activation in chronic heart failure: a prospective cohort study. Lancet 1999, 353 (9167), 1838–1842. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Kato, A.; Odamaki, M.; Takita, T.; Maruyama, Y.; Kumagai, H.; Hishida, A. Association between interleukin-6 and carotid atheroslerosis in hemodialysis patients. Kidney Int. 2002, 61 (3), 1143–1152. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Park, C W.; Shin, Y S.; Kim, C M.; Lee, S Y.; Yu, S E.; Kim, S.Y.; Choi, E J.; Chang, Y S.; Bang, B K. Increased C-reactive protein following hemodialysis predicts cardiac hypertrophy in chronic hemodialysis patients. Am. J. Kidney Dis. 2002, 40 (6), 1230–1239. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Losito, A.; Kalidas, K.; Santoni, S.; Jeffery, S. Association of interleukin-6 − 174 − G/C promoter polymorphism with hypertension and left ventricular hypertrophy in dialysis patients. Kidney Int. 2003, 64, 616–622. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Humphries, S E.; Luong, L A.; Ogg, M S.; Hawe, E.; Miller, G J. The interleukin-6 174 G/C polymorphism associated with risk of coronary artery disease and systolic blood pressure in healthy men. Eur. Heart J. 2001, 22, 2219–2220. [CSA], [CROSSREF]
- Jeron, A.; Straub, R H.; Kaiser, T.; Riegger, G A.; Muders, F. Systemic immunosuppression fails to suppress cardiac cytokine induction in pressure overload hypertrophy in rats. Immunobiology 2002, 205 (1), 51–60. [PUBMED], [INFOTRIEVE], [CSA]
- Nakamura, K.; Mihara, K. Inhibitory effects of neonatal rat cardiac myocyte hypertrophy induced by tumor necrosis factor-alpha and angiotensin II. Circulation 1998, 98, 794–799. [PUBMED], [INFOTRIEVE], [CSA]
- Liu, J Y.; Brass, D M.; Hoyle, G W.; Brody, A R. TNF alpha receptor knockout mice are protected from the fibroproliferative effects of inhaled asbestosis fibers. Am. J. Pathol. 1998, 153, 1839–1847. [PUBMED], [INFOTRIEVE], [CSA]
- Nagueh, S F.; Stetson, S J.; Lakkis, N M.; Killip, D.; Perez-Verdia, A.; Entman, M L.; Spencer, W H., III; Torre-Amione, G. Decreased expression of tumor necrosis factor-alpha and regression of hypertrophy after nonsurgical septal reduction therapy for patients with hypertrophic obstructive cardiomyopathy. Circulation 2001, 103 (14), 1844–1850. [PUBMED], [INFOTRIEVE], [CSA]
- Moustapha, A.; Naso, A.; Nahlawi, M.; Gupta, A.; Arheart, K L.; Jacobsen, D W.; Robinson, K.; Dennis, V W. Prospective study of hyperhomocysteinemia as an adverse cardiovascular risk factor in end-stage renal disease. Circulation 1998, 97 (2), 138–141. [PUBMED], [INFOTRIEVE], [CSA]
- Manns, B J.; Burgess, E D.; Hyndman, M E.; Parsons, H G.; Schaefer, J P.; Scott-Douglas, N W. Hyperhomocyst(e)inemia and the prevalence of atherosclerotic vascular disease in patients with end-stage renal disease. Am. J. Kidney Dis. 1999, 34 (4), 669–677. [PUBMED], [INFOTRIEVE], [CSA]
- Blacher, J.; Demuth, K.; Guerin, A P.;Blacher, J.; Demuth, K.; Guerin, A P.; Vadez, C.; Moatti, N.; Safar, M E.; London, G M. Association between plasma homocysteine concentrations and cardiac hypertrophy in end-stage renal disease. J. Nephrol. 1999, 22, 248–255. [CSA], [CROSSREF]
- Franke, S.; Muller, A.; Sommer, M.; Busch, M.; Kientsch-Engel, R.; Stein, G. Serum levels of total homocysteine, homocysteine metabolites and glycation end products in patients after renal transplantation. Clin. Nephrol. 2003, 59 (2), 88–97. [PUBMED], [INFOTRIEVE], [CSA]