Abstract
Background. Cisplatin (CP)-induced kidney damage and effects of DL-buthionine-(S,R)-sulfoximine (BSO) on it are species- and age-different. It remains unclear whether CP-induced cytotoxicity in renal proximal tubular epithelial cells (RTEC), the main target cells of CP, is also species- and age-different; and whether CP-induced cytotoxicity varies with the difference in age and species, if any, is one of the questions. In the present study, the effects of BSO on CP-induced cytotoxicity in primary cultures of RTEC isolated from monkeys and different age and sex rats were studied. Methods. The RTEC were isolated from 3-week-old, 2-month-old, or 5-month-old rats, and 6-8 year-old monkeys. After subculturing, RTEC was inoculated into type I collagen-coated 96-well culture plates; after preincubation, 40 µM BSO was added, 16 hours later, varying concentrations of CP were added. At that time, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assays were performed to test cell viability. Results. The concentrations of CP that inhibited 50% cell growth (IC50) of RTEC from rats and monkeys were 1.11 and 3.03 mM at 8 hours, and 0.51 and 1.24 mM at 24 hours, respectively. The BSO made the IC50s of RTEC from rats and monkeys lower, down to 0.07 and 0.48 mM at 8 hours, and 0.02 and 0.11 mM at 24 hours, respectively. The IC50s of RTEC from different sex and age rats were almost same. Conclusion. These results suggested that CP-induced cytotoxicity was concentration- and time-dependent, with species-dependent differences, rat RTEC were more susceptible to CP than monkey RTEC, rat RTEC were more dependent on glutathione (GSH) during the stress state were than monkey cells; CP-induced cytotoxicity was without sex- and age-dependent differences in rat RTEC.
Introduction
Cisplatin (CP) is an important anticancer drug used to treat solid tumors, such as germ cell tumors, head and neck tumors, bladder cancer, lung cancer, and ovarian cancer.Citation[1] The nephrotoxicity of CP is recognized as the most important dose-limiting factor.Citation[2] The nephrotoxicity of CP has no sex difference, but it has age difference in rats.Citation[3] The main target cells are renal proximal tubular epithelial cells (RTEC),Citation[4] and the mechanism by which CP kills RTEC has been the focus of intense investigation for many years. Reactive oxygen and nitrogen metabolites have been implicated in CP nephrotoxicity.Citation[5-10]
Reduced glutathione (GSH) is well-established as a regulator of intracellular redox status. Protection against CP nephrotoxicity by administration of glutathione ester,Citation[11] or N-acetylcysteine,Citation[12&13] a precursor of GSH synthesis, has been reported. Glutathione is synthesized from its precursor amino acids (L-glutamate, L-cysteine, and glycine), and catalyzed by γ-glutamylcysteine synthetase (GCS) and GSH synthetase. The GCS is the rate-limiting enzyme in the GSH biosynthesis process and DL-buthionine-(S,R)-sulfoximine(BSO) is a potent and selective inhibitor of GCS.Citation[14] In theory, decreased renal GSH concentration by BSO will enhance CP nephrotoxicity. However, conflicting results were observed on the effects of BSO on CP nephrotoxicity in different ages and species of animals. In the mice model, depletion of GSH by treatment with BSO enhanced CP toxicity,Citation[15&16] but in rat models, it is complicated. Protective effects of BSO were observed by Mayer et al.,Citation[17&18] but Appenroth et al. found that BOS enhanced CP nephrotoxicity in 10-day-old rats, but had no effect in 55-day-old rats,Citation[13] Suzuki and Cherian also found BSO did not affect CP nephrotoxicity.Citation[19]
Based on in vivo results, we wanted to know if age-difference and species-difference in BSO effects are due to a difference in cytotoxicity. In the present study, we used primary culture of RTEC as an in vitro model to compare the effects of BSO on CP-induced cytotoxicity in RTEC from monkeys and rats of different ages and sex.
Material and Methods
Reagents
Cisplatin (CP), MTT, hyaluronidase, DL-buthionine-(S,R)-sulfoximine (BSO), and bovine serum albumin V(BSA) were purchased from the Sigma Chemical Co. Fetal calf serum (FCS), Dulbecco's modified eagle medium (DMEM), glutamine, Hanks balanced salt solution (HBSS), Ca,Mg-free Hanks balanced salt solution [HBSS(−)]. Dulbecco's phosphate buffer saline (D-PBS), EGTA, and HEPES were purchased from Gibco Co. Collagenase was purchased from Wako Co, Japan.
Animals
Male Sprague-Dawley rats (Nippon SLC Company, Shizuoka, Japan), 3-weeks-old, 2-months-old, or 5-months-old; female rats, 2-months-old; male Cynomolgus monkeys (Shin Nippon Biomedical Laboratories, Kagoshima, Japan), approx. 6–8 years old, body weight approx. 5.5–6.5 kg, were housed in the Nippon Roche Research Center Vivarium, were allowed access to food and water freely, and were kept in a room on a 12-hour light-dark cycle. In each study, 5 rats or 3 monkeys were used.
Isolation and Culture of RTEC
Isolation and culture of RTEC were described in details previously.Citation[20] Cells were washed with HBSS and resuspended in the culture medium at the concentration of 2 × 105 cells/mL, they were inoculated into type I collagen-coated 96-well culture plates at 100 µL/well. After overnight culture, cell monolayers were formed.
Preparation of Drugs
The CP was dissolved in dimethyl sulfoxide (DMSO) at 500 mM as a stock solution, diluted with fresh medium to 5 mM. The amount of DMSO in culture medium was 1%, at that concentration the DMSO didn't show any cytotoxicity (data not shown), and then the CP was diluted twice with culture medium containing 1% DMSO until the dilution became 0.01 mM. The BSO solution was prepared in culture medium at 40 µM, when co-incubated with CP, CP was prepared in 40 µM BSO solution containing 1% DMSO. Thus, both groups were subjected to 1% DMSO and comparable. All solutions were prepared before use.
Cell Treatment and Cytotoxicity Measurement
In type I collagen-coated 96-well culture plates, cells were incubated for 16 hours in the presence or absence of 40 µM of BSO, and then varying concentrations of CP were added in the presence or absence of BSO. After 8 or 24 hours, MTT assays were performed to evaluate cytotoxicity.Citation[21] In brief, the cell monolayers were incubated for 1 hour with MTT at a final concentration of 0.5 mg/mL in the culture medium, and then extracted with isopropanol containing 2% HCl. The concentration of formazan dye was determined spectrophotometrically at 545 nm with a reference wavelength 630 nm using a well reader (SK601, Seikagaku Corporation, Japan).
Statistical Analyses
For statistical analyses of the data, analysis of variance or if appropriate Student's t-test have been applied. The IC50 (drug concentration at 50% cell growth inhibition) was calculated by the Bliss method.
Results
Effects of BSO on CP-Induced Cytotoxicity in Primary Culture of RTEC From Rats with Different Ages and Sex
The RTEC from rats was inoculated into collagen-coated 96-well plates and incubated at 37°C, 16 hours prior to CP, BSO (40 µM) was added. After 24 h of CP treatment, MTT assays were performed to evaluate cell viabilities. BSO was not cytotoxic (data not shown).
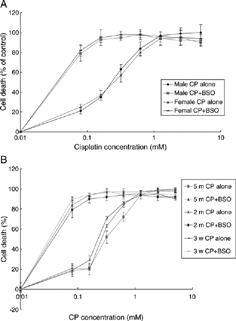
The CP-induced cytotoxicity in primary culture of RTEC is dose-dependent, BSO potentiated cytotoxicity dramatically. The inflections of concentration-response were almost similar. Cells from rats with different ages and sex did not show any difference in CP cytotoxicity and BSO enhancement (, ).
Figure 1 Effects of BSO on CP-cytotoxicity in primary culture of RTEC from different sex (A) and age (B) rats. Cells were seeded into 96-well plates at 2 × 104cell/well. After monolayers were formed, a series of CP were added. BSO(40 µM) was added 16 hours prior to CP. At 24 h after addition of CP, MTT assays were performed to evaluate cytotoxicity. Means ± SEM, n = 5. CP: cisplatin, BSO: DL-buthionine-(S,R)-sulfoximine, RTEC: renal tubular epithelial cells.
Effects of BSO on CP Cytotoxicity in Primary Culture of RTEC From Rats and Monkeys at Different Times
Time- and concentration-dependent cytotoxicity, potential effects of BSO were observed in both rat and monkey RTEC at different times. Rat RTEC, which were 2.8-fold (3.03/1.11) at 8 hours, 2.4-fold (1.24/0.51) at 24 hours, and 9.4-fold (0.66/0.07) at 48 hours, were more susceptible to CP than monkey RTEC, respectively. The BSO made rat RTEC 16.8-fold (1.11/0.066) at 8 hours, 25.5-fold (0.51/0.02) at 24 hours, and 10-fold (0.07/0.007) at 48 hours more susceptible to CP, and made monkey RTEC 6.3-fold (3.03/0.48) at 8 hours, 11.3-fold (1.24/0.11) at 24 hours, and 22-fold (0.66/0.03) more susceptible to CP, respectively ().
Table 1. Effects of BSO on CP cytotoxicity (IC50, mM) in RTEC from rats and monkeys at different times
Discussion
Isolated cells highly facilitate toxicological experiments by allowing a large number of parallel incubations with cells from a single animal, thus minimizing the change of false interpretations of data originating from several animals. In addition, they can be maintained for several days without redifferentiation.Citation[22] The RTEC in culture retains active transport systems for organic ions and D-glucose as well as many enzyme systems, like GGT, cysteinayl-glycinase, beta-lyase, and acrylase, which are essential for the expression of toxicity in the PTC, primary cultures faithfully reflect the toxicological susceptibilities that occur in freshly isolated cells and presumably, in the whole animal.Citation[23] The earlier information makes primary cell cultures the systems of our choice to investigate the difference in CP toxicity in intact animals. Conflicting results have been previously reported about effects of pretreatment with BSO on CP-induced nephrotoxicity. Mayer and coworkers pretreated BSO in rats and found that CP-induced nephrotoxicity was inhibited,Citation[17&18] but Ishikawa et al. found that CP-induced nephrotoxicity was enhanced by pretreatment of BSO to mice,Citation[15&16] Suzuki and Cherian's data showed only a slight change in CP-induced nephrotoxicity under treatment with BSO.Citation[19] The previous results were obtained from in vivo studies. The discrepancies may result from complexity of intact animal model systems. In the present study, isolated and cultivated RTEC model systems were used to make the experimental systems simple and comparable, and consistent results were obtained in both rat and monkey RTEC showing that CP-induced cytotoxicity was enhanced by the treatment of BSO.
The CP-induced kidney damage in intact animals is different by species. Treatment of rats with 5–6 mg/kg body weight elicits obvious kidney damage,Citation[24] but for mice a dose of 12 mg/kg body weight is needed.Citation[25] In the present study, CP-induced cytotoxicity is different between rat RTEC and monkey RTEC, and the enhancement of BSO is different, too. Therefore, CP-induced cytotoxicity in vitro with difference in species may be one of the reasons that CP-induced nephrotoxicity is species-different in intact animals. It seemed that rat RTEC was more dependent on intracellular GSH during the stress state than monkey RTEC, presumably difference in susceptibility to CP between the two species may be due to the different level of intracellular GSH.
Appenroth et al. found that CP-induced kidney damage in intact animals is age-different,Citation[3] and BSO enhanced CP-induced nephrotoxicity exists only in 10-day-old rats, no effect was found in 55-day-old rats.Citation[13] But in the present study, CP-induced cytotoxicity in RTEC from different age rats was not different. It suggested that difference in CP-induced nephrotoxicity in vivo with difference in age is not due to different cytotoxicity, therefore, there must be another reason. BSO enhanced CP-induced cytotoxicity without difference in age, which also suggested another reason involved. In fact, Appenroth et al.Citation[13] interpreted the enhanced CP-induced nephrotoxicity in young rats as the addition of BSO and CP toxic effects. Like in vivo results, there was no sex difference in CP-induced cytotoxicity.
In summary, CP-induced cytotoxicity in primary culture of RTEC without sex- and age-dependent difference in rat, but was species-dependent different. Rat RTEC were more susceptible to CP than monkey RTEC. The BSO enhanced CP-induced cytotoxicity without sex- and age-dependent differences but with species-dependent differences. The different results between intact animals and in primary cells suggested that there must be another reason involved rather than direct cytotoxicity.
References
- Rosenberg, B. Fundamental studies with CP. Cancer 1985, 55, 2303–2316. [PUBMED], [INFOTRIEVE], [CSA]
- Pinzani, V.; Bressolle, F.; Haug, I J.; Galtier, M.; Blayac, J P. CP induced renal toxicity and toxicity-modulating strategies: a review. Cancer Chemother. Pharmacol. 1994, 35, 1–9. [PUBMED], [INFOTRIEVE], [CSA]
- Appenroth, D.; Braunlich, H. Age differences in cisplatinum nephrotoxicity. Toxicology 1984, 32 (4), 343–353. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Dobya, D C.; Levi, J.; Jacobs, C.; Kosck, J.; Weiner, M W. Mechanism of cisplatin nephrotoxicity: II. Morphologic observations. J. Pharmacol. Exp. Ther. 1980, 213, 551–556. [CSA]
- Sugihara, K.; Nakaro, S.; Koda, M.; Tanaka, K.; Fukuishi, N.; Gemba, M. Stimulatory effect of CP on production of lipid peroxidation in renal tissue. Jpn. J. Pharmacol. 1987, 43, 246–252. [CSA]
- Hannemann, J.; Baumann, K. CP-induced lipid peroxidation and decrease of gluconeogenesis in rat kidney cortex: different effect of antioxidant and radical scavengers. Toxicology 1988, 51, 119–132. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Zhong, L F.; Zhang, J G.; Zhang, M.; Ma, S L.; Xia, Y X. Protection against CP-induced lipid peroxidation and kidney damage by procaine in rat. Arch. Toxicol. 1990, 64, 599–600. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Zhang, J G.; Zhong, L F.; Zhang, M.; Xia, Y X. Protection effects of procaine on oxidative stress and toxicities of renal cortical slices from rats caused by CP in vitro. Arch. Toxicol. 1992, 66, 354–358. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Srivastava, R C.; Farookh, A.; Ahmad, N.; Misra, M.; Hasan, S K.; Husain, M M. Evidence for the involvement of nitric oxide in cisplatin-induced toxicity in rats. BioMetals 1996, 9 (2), 139–142. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Saad, S Y.; Najjar, T A.; Daba, M H.; Al-Rikabi, A C. Inhibition of nitric oxide synthase aggravates cisplatin-induced nephrotoxicity: effect of 2-amino-4-methylpyridine. Chemotherapy 2002, 48 (6), 309–315. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Anderson, M E.; Naganuma, A.; Meister, A. Protection against cisplatin toxicity by administration of glutathione ester. FASEB J. 1990, 4 (14), 3251–3255. [PUBMED], [INFOTRIEVE], [CSA]
- Appenroth, D.; Winnefield, K.; Schroter, H.; , et al. Beneficial effect of acetylcysteine on CP nephrotoxicity in rats. J. Appl. Toxicol. 1993, 13, 189–192. [PUBMED], [INFOTRIEVE], [CSA]
- Appenroth, D.; Winnefield, K. Role of glutathione for CP nephrotoxicity in young and adult rats. Ren. Fail. 1993, 15, 135–139. [PUBMED], [INFOTRIEVE], [CSA]
- Lash, L H. Glutathione and other antioxidant defense mechanisms. In Comprehensive Toxicology; Renal Toxicology; Goldstein, R S., Ed.; Elsevier Science Ltd.: Oxford, 1997; Vol. 7, 403–428.
- Ishikawa, M.; Takayanagi, Y.; Sasaki, K. The deleterious effect of buthionine sulfoximine, a glutathione-depleting agent, on the CP toxicity in mice. Jpn. J. Pharmacol. 1990, 52 (4), 652–655. [PUBMED], [INFOTRIEVE], [CSA]
- Ishikawa, M.; Takayanagi, Y.; Sasaki, K. Enhancement of CP toxicity by buthionine sulfoximine, a glutathione-depleting agent, in mice. Res. Commun. Chem. Pathol. Pharmacol. 1990, 67 (1), 131–141. [PUBMED], [INFOTRIEVE], [CSA]
- Mayer, R D.; Lee, K E.; Cockett, A T. Inhibition of CP-induced nephrotoxicity in rats by buthionine sulfoximine, a glutathione synthesis inhibitor. Cancer Chemother. Pharmacol. 1987, 20 (3), 207–210. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Mayer, R D.; Lee, K E.; Cockett, A T. Improved use of buthionine sulfoximine to prevent CP nephrotoxicity in rats. J. Cancer Res. Clin. Oncol. 1989, 115 (5), 418–422. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Suzuki, C A.; Cherian, M G. The interactions of cis-diamminedichloroplatinum with metallothionein and glutathione in rat liver and kidney. Toxicology 1990, 64 (2), 113–127. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Lu, Y.; Kawashima, A.; Horii, I.; Zhong, L. Effects of BSO and L-cysteine on drug-induced cytotoxicity in primary cell cultures: drug-, cell type-, and species-specific difference. Drug Chem. Toxicol. 2004, 27 (3)269–280. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application of proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Boogaard, P J.; Zoeteweij, J P.; Van Berkel, T J.C.; Van't Noordende, J M.; Mulder, G J.; Nagelkerke, J F. Primary culture of proximal tubular cells from normal rat kidney as an in vitro model to study mechanisms of nephrotoxicity: study mechanisms of nephrotoxicants at low concentrations during prolonged exposure. Biochem. Pharmacol. 1990, 39 (8), 1335–1345. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Lash, L H. Use of freshly isolated and primary cultures of proximal tubular and distal tubular cells from rat kidney. In Methods in Renal Toxicology; Zalups, Lash, L H. Eds.; CRC Press, Inc.: Boca Raton, 1996; 190–215.
- Zhou, H.; Kato, A.; Yasuda, H.; , et al. The induction of cell cycle regulatory and DNA repair proteins in cisplatin-induced acute renal failure. Toxicol. Appl. Pharmacol. 2004, 200 (2), 111–120. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Mishra, J.; Mori, K.; Ma, Q.; Kelly, C.; Barasch, J.; Devarajan, P. Neutrophil gelatinase-associated lipocalin: a novel early urinary biomarker for cisplatin nephrotoxicity. Am. J. Nephrol. 2004, 24 (3), 307–315. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]