Abstract
Background. This study was aimed at examining the effects of radiocontrast agents on selected membrane transport functions. Methods. The effect of diatrizoate sodium (DS), diatrizoate meglumine (DM), and diatrizoate compound (DC) on Na+/K+ pump activity and number, L-arginine, and choline transports were evaluated in erythrocytes of normal individuals and patients undergoing cardiac catheterization. Results. Normal individuals' erythrocytes potassium influxes were 1.50 ± 0.35, 1.32 ± 0.37, 1.28 ± 0.30, and 1.01 ± 0.25 mmol/L cell/h in control, DS, DM, and DC groups, respectively (P = 0.004; DC vs. control). Patients exposure to Hypaque M-76® significantly diminished Na+/K+ pump activity (1.40 ± 0.36 before, vs. 1.27 ± 0.40 mmol/L cell/h after; P = 0.039). The number of Na+/K+ pumps was reduced (156 ± 36 vs. 143 ± 34 pumps/erythrocyte; P = 0.015) in presence of DS. L-arginine and choline transports changed only at high radiocontrast concentrations. Conclusion. Selective changes in erythrocytes membrane transport function take place on exposure to radiocontrasts.
Introduction
Use of radiocontrast agents has been associated with decline in renal function, being implicated in 5% to 32% of all in-hospital cases of acute renal failure (ARF).Citation[1] Patients with previously impaired renal function are at particularly high risk.Citation[1] The molecular mechanisms responsible for the disorder remain thus far unknown. Tubular cells injury and changes in intrarenal hemodynamics appear to be factors playing important roles.Citation[2-4]
Erythrocytes are structurally simple cells, yet their membranes carry all the necessary transport machinery, behaving in a manner similar to that of more complex cells. The erythrocyte membrane is a simple, reliable, and, possibly, an ideal model to evaluate membrane transport systems.Citation[5]
Altered membrane transport systems have been described in several clinical situations.Citation[6-8] Decreased Na+/K+ transport, and increased lysine and choline membrane transport have been found in erythrocytes of uremic patients.Citation[9-12] Epithelial Na+/K+ pump exposure to radiocontrasts resulted in inhibited activity.Citation[4], Citation[13] Proximal tubule cells incubated with radiocontrast agents displayed metabolic alterations and histologic changes, besides the release of intracellular enzymes, indicating the presence of cell damage.Citation[14] In addition, changes in morphology and cell deformity characteristics have been verified in erythrocytes exposed to radiocontrasts.Citation[15&16]
Clinical use of radiocontrasts appears to be associated with significant changes in intrarenal hemodynamics.Citation[1], Citation[17&18] Sustained medullary vasoconstriction and increased preglomerular arteriolar resistance follow a brief cortical hyperemic phase.Citation[17], Citation[19] Reduced synthesis of vasodilatory substances may accompany radiocontrast nephrotoxicity in patients with vascular or kidney diseases.Citation[1], Citation[20&21]
Vasodilation partly depends on nitric oxide (NO) generation, having L-arginine as a precursor. Some evidences suggest that NO plays a role in radiocontrast-induced ARF.Citation[3], Citation[22] Administration of L-arginine protected against radiocontrast-induced vasoconstriction and ARF.Citation[3], Citation[20], Citation[23] In an experimental model of nephropathy in rats, prostanoids and NO appeared to exercise a protective effect on the renal vascular response to radiocontrast exposure.Citation[22] A role for L-arginine in the intrarenal hemodynamic changes induced by radiocontrasts has not yet been evaluated.
Choline transport changes have been associated with membrane dysfunction in several clinical situations.Citation[11&12], Citation[24], Citation[25] To evaluate the extent of the membrane changes induced by radiocontrasts, choline transport was also assessed.
The aim of the study was to examine the effect of radiocontrast agents on selected membrane transport systems, using the erythrocyte membrane as model.
Subjects and Methods
Normal volunteers and patients undergoing cardiac catheterization participated in the study. Informed consent was obtained, and the study was approved by the hospital Ethics Committee.
Erythrocytes from the normal individuals were incubated with saline or radiocontrasts solutions—diatrizoate sodium (DS), diatrizoate meglumine (DM), or diatrizoate compound (DC). In addition, Na+/K+ pump activity experiments were performed on erythrocytes isolated from patients undergoing cardiac catheterization—15 minutes prior to and following radiocontrast administration (Hypaque M-76%® [Winthrop, Inc., Malvern, PA, USA]: 10% diatrizoate sodium (W/V), and diatrizoate meglumine, 66% (W/V) solution). Volumes infused during the procedures ranged from 70 to 90 mL.
Individuals with diabetes, kidney, thyroid or liver disease, neoplasia, in use of digitalis, or having been blood transfused in the last 3 months were excluded.
In all experiments, 10 mL of fasting blood was taken into heparinized tubes, centrifuged (3000 g) for 10 min, and plasma and buffy coat were discarded. The erythrocytes remaining were washed three times by sequential centrifugation and resuspension in ice-cold saline solution (NaCl 150 mM, KCI 5 mM, glucose 5 mM, MOPS 10 mM, pH 7.4) and kept in ice-cold saline solution, to a 10% hematocrit, until assayed. Subsequent cells washing, lysing, and protein precipitation were accomplished in ice-cold isotonic MgCl2 solution (MgCl2 107 mM, MOPS 10 mM, pH 7.4), 0.1% Triton-X 100 solution (0.5 mL), and 5% trichloroacetic acid (TCA) solution (0.5 mL), respectively.
Na+/K+ Pump Activity Assay
The K+ influx was measured according to previously described methods.Citation[10] Briefly, triplicate tubes containing 1 mL of the erythrocytes suspension were incubated at 37°C for 5 min, either in the presence or absence of ouabain (0.1 mM). To evaluate the influence of radiocontrasts on the Na+/K+ pump, erythrocytes were incubated for 15 min with solutions of DS (diatrizoate sodium 150 mM, KCl 5 mM, MOPS 10 mM, glucose 5 mM, pH 7.4), DM (diatrizoate meglumine 150 mM, KCl 5 mM, MOPS 10 mM, glucose 5 mM, pH 7.4), or DC (DM 11.42 mM, and DS 2.08 mM) to approximately replicate the in vivo concentrations of Hypaque M-76% achieved during cardiac catheterization, with or without ouabain. Tracer amounts of 86Rb+ were added to each tube, and incubation for 5 min at 37°C was performed—fluxes started and stopped on ice-bath. Cooled for 3 min, each tube was rapidly centrifuged (2000 g), and the supernatant (10 µL) retrieved for 86Rb+ counting. In sequence, erythrocytes were quickly centrifuged, washed three times, lysed, and the protein content precipitated. The cells suspension was pelleted down by centrifuging for 10 min (3000 g). The supernatant was collected by pipetting, and the intracellular 86Rb+ content was counted in a β-scintillation spectrophotometer (Beckman LS 6500, Beckman Coulter, Inc., Fullerton, CA, USA). The intracellular/extracellular 86Rb+ counts ratio, hematocrits, and measured K+ concentrations were used to calculate total K+ influx rate and its ouabain-sensitive component (Na+/K+ pump). It has been established that 86Rb+ is a valid congener for K+.Citation[26]
Na+/K+ Pumps Number Estimate
To evaluate the radiocontrast effect on the number of Na+/K+ pumps, cells were incubated for 15 min, in presence or absence of DS. The Na+/K+ pumps number was estimated according to techniques previously described.Citation[10] Tubes containing 1 mL of normal individual's erythrocytes suspension in saline or in DS were incubated at 37°C for 15 min, with or without unlabeled ouabain (0.1 mM). 3H-ouabain was then added to each tube, to a final concentration of approximately 440 nM. In sequence, the erythrocytes suspension was incubated at 37°C for 2 h. Cells were washed three times in ice-cold MgCl2. Bound 3H-ouabain was solubilized by the sequential addition of Triton-X 100 and TCA, followed by pelleting down. The supernatant was retrieved for radioactivity counting. Specific ouabain binding was obtained by subtraction of the bound 3H-ouabain in the presence of excess cold ouabain.
L-Arginine and Choline Transport
Blood samples used in experiments of L-arginine and choline transport were taken from normal patients and processed within 2 h.
The methods applied in erythrocytes transmembrane flux measurements have been previously described.Citation[9], Citation[11] Total erythrocyte L-arginine, or choline, uptake was measured by incubating red blood cells at 10% hematocrit, for 3 min at 37°C, in the presence of 500 µM L-arginine solution (or 250 µM choline). 14C-L-arginine, or 14C-choline, was used as tracer. Fluxes were started and interrupted in ice-cold water bath (3 min). Erythrocytes were washed free of extracellular radioactivity, resuspended in ice-cold saline, and centrifuged three times (14,000 g), followed by lysis, protein precipitation, and pelleting down. Supernatants were added to scintillation fluid, and the intracellular radioactivity counted for 5 min.
Two series of flux experiments were performed. In the first (n = 10), blood was previously incubated in Hypaque M-76 solutions of increasing concentration (0, 3.5, 7, 14, 28, 70, and 112 µM) for 15 min. In the second (n = 5 for L-arginine, and n = 6 for choline), blood was preincubated in Hypaque M-76 solution (14 µM) to approximate in vivo concentrations achieved during cardiac catheterization—for 0, 5, 15, and 30 min. Control fluxes were performed in saline solution.
Statistical Analysis
Data are presented as mean ± standard deviation (SD). Comparisons between two groups were performed by two-tailed paired Student's t test. Differences among multiple groups were analyzed by repeated measures analysis of variance (ANOVA); and post hoc comparisons performed by Dunnett's multiple comparisons test (comparison against control) or Tukey's multiple comparisons test (pairwise comparisons). The effect size for changes in Na+/K+ pump activity between experimental groups and control group was also determined.Citation[27] P values ≤ 0.05 were considered significant. A GraphPad Prism 3.03 (GraphPad Software, Inc., San Diego, CA, USA) software was used in statistical analyses.
Results
Na+/K+ pump activity was examined in erythrocytes collected from 8 normal individuals (female: 7; age: 55 ± 11 years; range: 44–74 years) and from 15 patients undergoing cardiac catheterization (female: 9; age: 56 ± 9 years; range: 44–74 years). The effect of different radiocontrast solutions on Na+/K+ pump activity is depicted in .
Table 1. Effect of radiocontrasts on erythrocytes Na+/K+ pump activity (n = 8)
Potassium influx was diminished in DS, DM, and DC, in comparison with the control group, yet significantly and with a large effect size (1.63) only in DC.
Results of flux experiments performed in erythrocytes of 15 patients (female: 9; age: 56 ± 9 years; range: 44–74 years)—before and after Hypaque M-76 infusions—were 1.40 ± 0.36 and 1.27 ± 0.40 mmol/L cell/h, respectively (P = 0.039). Patient's weight was 69 ± 10 kg, and the radiocontrast dose was 1.03 ± 0.16 mL/kg.
The number of Na+/K+ pumps was significantly reduced in presence of DS (156 ± 36 vs. 143 ± 34 pumps/erythrocytes for control and DS, respectively; P = 0.015).
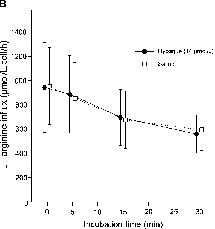
L-arginine influx was evaluated in erythrocytes obtained from 10 normal adult individuals (female: 6; age: 28 ± 7 years; range: 23–50 years). L-arginine uptake, at increasing concentrations of Hypaque M-76, was significantly lower only at 70 and 112 µM, in comparison with saline incubation (P < 0.0001, repeated measures ANOVA; P = 0.010, post hoc Dunnett's multiple comparison test). depicts influx at 500 µM extracellular L-arginine. The influence of radiocontrast exposition time on L-arginine uptake was also evaluated in cells from normal individuals (n = 5). Results are shown in . Progressively decreasing L-arginine uptake with time and no difference between groups was observed (P = 0.996, repeated measures ANOVA).
Figure 1. (A) Erythrocytes L-arginine influx at 500 µM extracellular L-arginine concentration. Cells were exposed to progressively increased concentrations of Hypaque M-76®. L-arginine uptake was significantly lower at 70 and 112 µM, compared with saline incubation (P = 0.0001, repeated measures analysis of variance [ANOVA]; P = 0.010, post hoc Dunnett's multiple comparison test). (B) Erythrocytes L-arginine influx on saline (open squares) or 14 µM Hypaque M-76 (solid circles), at 500 µM extracellular L-arginine concentration. Progressively decreased L-arginine uptake with time—in both groups—with no differences between groups (P = 0.996, repeated measures ANOVA). Each point represents mean and standard deviation.
![Figure 1. (A) Erythrocytes L-arginine influx at 500 µM extracellular L-arginine concentration. Cells were exposed to progressively increased concentrations of Hypaque M-76®. L-arginine uptake was significantly lower at 70 and 112 µM, compared with saline incubation (P = 0.0001, repeated measures analysis of variance [ANOVA]; P = 0.010, post hoc Dunnett's multiple comparison test). (B) Erythrocytes L-arginine influx on saline (open squares) or 14 µM Hypaque M-76 (solid circles), at 500 µM extracellular L-arginine concentration. Progressively decreased L-arginine uptake with time—in both groups—with no differences between groups (P = 0.996, repeated measures ANOVA). Each point represents mean and standard deviation.](/cms/asset/2b9b2260-4bf8-4d82-b44c-dd798e710f43/irnf_a_124307_uf0001_b.gif)
Choline transport assays were performed in erythrocytes from 10 normal adult individuals (female: 5; age: 29 ± 9 years; range: 24–50 years). depicts choline influx at 250 µM extracellular choline. A significant difference in choline uptake with increasing Hypaque M-76 concentrations, compared with saline incubation, appeared only at 112 µM (P = 0.0001, repeated measures ANOVA; P = 0.010, Dunnett's multiple comparisons test). The influence of radiocontrast exposition time on choline uptake was also evaluated (n = 6). Results are depicted in . No significant differences were found between groups or among different exposition times (P = 0.628 and P = 0.122, respectively; repeated measures ANOVA).
Figure 2. (A) Erythrocytes choline influx at 250 µM extracellular choline concentration. Cells were exposed to progressively increased concentrations of Hypaque M-76®. A significant difference in choline uptake was only apparent for fluxes performed at 112 µM, compared with saline incubation (P = 0.001, repeated measures analysis of variance [ANOVA]; P = 0.010, Dunnett's multiple comparisons test). (B) Erythrocytes choline influx on saline (open squares) or 14 µM Hypaque M-76 (solid circles), at 250 µM extracellular choline concentration. No significant differences between groups and exposition times (P = 0.628, and P = 0.122, respectively; repeated measures ANOVA). Each point represents mean and standard deviation.
![Figure 2. (A) Erythrocytes choline influx at 250 µM extracellular choline concentration. Cells were exposed to progressively increased concentrations of Hypaque M-76®. A significant difference in choline uptake was only apparent for fluxes performed at 112 µM, compared with saline incubation (P = 0.001, repeated measures analysis of variance [ANOVA]; P = 0.010, Dunnett's multiple comparisons test). (B) Erythrocytes choline influx on saline (open squares) or 14 µM Hypaque M-76 (solid circles), at 250 µM extracellular choline concentration. No significant differences between groups and exposition times (P = 0.628, and P = 0.122, respectively; repeated measures ANOVA). Each point represents mean and standard deviation.](/cms/asset/c1c89f18-c76f-46fe-a9ab-c9147be5e035/irnf_a_124307_uf0003_b.gif)
Discussion
The study demonstrated reduced Na+/K+ pump activity and decreased number of Na+/K+ pumps in erythrocytes incubated with radiocontrast agents. The effect was selective, as the other membrane transport systems were not affected, except at high concentrations. A similar reduction in pump activity took place in red blood cells from patients undergoing cardiac catheterization, following radiocontrast infusion. Data suggest that inhibition of the Na+/K+ pump may explain—at least in part—radiocontrast toxicity. It may be argued that the magnitude of reduction in the Na+/K+ pump activity and of decrease in the number of active pumps was not remarkable. Yet, comparatively, the changes were greater than those previously found in hypertension and in end-stage renal diseaseCitation[10], Citation[28&29])]. The choice of Hypaque M-76 instead of newer non-ionic contrast agents may also be questioned. Despite having been more often associated with decline on renal function, its worldwide use, mostly on economic grounds, persists.Citation[30]
In presence of radiocontrast, rabbit erythrocytes membranes exhibited increased permeability,Citation[31] releasing potassium. Salts of diatrizoate and iothalamate reversibly inhibited the active sodium transport in the urinary bladder of Colombian toads—a model for renal distal tubule epithelium—albeit the site of action was not identified.Citation[4] Bino et al.Citation[13] more recently suggested that inhibition of tubular cells ion transport by the Na+/K+ pump might represent an important mechanism in the pathophysiology of radiocontrast-induced ARF. Individuals with impaired renal function are at increased risk of developing ARF, following the use of radiocontrasts.Citation[17] Abnormalities in membrane transport systems, including the Na+/K+ pump, have been described in those patients.Citation[9], Citation[11&12] The risk for contrast nephropathy in patients with chronic renal failure may thus depend on the additive effect of toxicity on previously altered tubule cell transport systems. Different from findings in end-stage kidney disease—where increased lysine and choline transport have been described—radiocontrasts did not affect red blood cells membrane transport, except at concentrations no less than five times those achieved in blood, during cardiac catheterization.
Erythrocytes and kidney tubule cells are differently exposed to radiocontrast agents—other potentially damaging effects may take place in tubular cells, adding to the membrane dysfunction. It is not unconceivable that, on exposure to radiocontrast agents, similar transport changes occur in tubular cells. Contrast agents are almost exclusively removed by glomerular filtration, reaching the renal tubules at greatly increased concentrations.Citation[32] Therefore, it is possible that the changes in L-arginine and choline transport, evidenced at high radiocontrast concentrations, are relevant to what occurs in kidney tubules on clinical exposure.
There is suggestion that NO plays a role in radiocontrast-induced ARF. Inhibition of NO synthase by competitive inhibitors added no protection or caused even further deterioration of the renal function in salt-depleted rats.Citation[3] L-arginine attenuated the reduction in urinary guanosine 3′-5′-cyclic monophosphate and nitrate/nitrite excretion induced by radiocontrast administration in rats.Citation[3]
The study supports the hypothesis that inhibition of Na+/K+ membrane transport contributes to the cell dysfunctions accompanying radiocontrast exposition. Furthermore, other systems, such as L-arginine and choline transport, may be affected at high radiocontrast concentrations. The protection of L-arginine administration may be associated with nonspecific amino acid effects on the renal circulation—enhanced blood flow—and not necessarily by changes on the L-arginine/NO pathway.
In conclusion, radiocontrast agents present a selective inhibitory effect on erythrocytes membrane transport systems. A similar effect on tubular cells might be contributory in the pathophysiology of radiocontrast-induced ARF.
References
- Solomon R. Contrast-medium-induced acute renal failure. Kidney Int. 1998;53(1):230–242. [PUBMED], [INFOTRIEVE], [CSA]
- Erley C M, Heyne N, Burgert K, Langanke J, Risler T, Osswald H. Prevention of radiocontrast-induced nephropathy by adenosine antagonists in rats with chronic nitric oxide deficiency. J Am Soc Nephrol. 1997;8(7):1125–1132. [PUBMED], [INFOTRIEVE], [CSA]
- Schwartz D, Blum M, Peer G, , et al. Role of nitric oxide (EDRF) in radiocontrast acute renal failure in rats. Am J Physiol. 1994;267(3 pt 2):F374–F379. [PUBMED], [INFOTRIEVE], [CSA]
- Ziegler T W, Ludens J H, Fanestil D D, Talner L B. Inhibition of active sodium transport by radiographic contrast media. Kidney Int. 1975;7(2):68–76. [PUBMED], [INFOTRIEVE], [CSA]
- Stewart G W, Fricke B. The curious genomic path from leaky red cell to nephrotic kidney. Nephron Physiol. 2003;93(2):29–33. [CSA], [CROSSREF]
- Allen P D, Schmidt T A, Marsh J D, Kjeldsen H. Na,K-ATPase expression in normal and failing human left ventricle. Basic Res Cardiol. 1992;87(suppl. 1):87–94. [PUBMED], [INFOTRIEVE], [CSA]
- Aviv A, Lasker N. Defects in membrane transport of ions as possible pathogenic factors in hypertension. Curr Opin Nephrol Hypertens. 1992;1(1):68–72., [PUBMED], [INFOTRIEVE], [CSA]
- Lijnen P, Fenyvesi A, Bex M, Bouillon R, Amery A. Erythrocyte cation transport systems and membrane lipids in insulin-dependent diabetics. Am J Hypertens. 1993;6(9):763–770. [PUBMED], [INFOTRIEVE], [CSA]
- Fervenza F C, Harvey C M, Hendry B, Ellory J C. Increased lysine transport capacity in erythrocytes from patients with chronic renal failure. Clin Sci. 1989;76(4):419–422. [PUBMED], [INFOTRIEVE], [CSA]
- Fervenza F C, Hendry B M, Ellory J C. Effects of dialysis and transplantation on red cell Na pump function in renal failure. Nephron. 1989;53(2):121–128. [PUBMED], [INFOTRIEVE], [CSA]
- Fervenza F C, Meredith D, Ellory J C, Hendry B M. Abnormal erythrocyte choline transport in patients with chronic renal failure. Clin Sci. 1991;80(2):137–141. [PUBMED], [INFOTRIEVE], [CSA]
- Poli de Figueiredo C E, Ellory J C, Hendry B M. Erythrocyte choline uptake after renal transplantation. Lancet. 1992;339(8786):146–148. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Bino F G, Costa E R, Burth P, Sampaio J C, Castro-Faria M V, Younes-Ibrahim M. The effect of nonionic radiocontrast media on the activity of purified human kidney Na, K ATPase. In: Taniguchi K, Kaya S, eds. Na/K-ATPase and Related ATPases. Amsterdam: Elsevier, 2000;413–416.
- Humes H D, Hunt D A, White M D. Direct toxic effect of the radiocontrast agent diatrizoate on renal proximal tubule cells. Am J Physiol. 1987;252(2 pt 2):F246–F255. [PUBMED], [INFOTRIEVE], [CSA]
- Aspelin P, Nilsson P E, Schmid-Schönbein H, Schröder S, Simon R. Effect of four non-ionic contrast media on red cells in vitro. I. Morphology. Acta Radiol. 1987;370(suppl):79–83. [CSA]
- Aspelin P, Nilsson P E, Schmid-Schönbein H, Schröder S, Simon R. Effect of four non-ionic contrast media on red cells in vitro. III. Deformability. Acta Radiol. 1987;370(suppl):89–91. [CSA]
- Brezis M, Cronin R. Radiocontrast media-induced acute renal failure. In: Schrier R, Gottschalk C, eds. Diseases of the Kidney. 2nd ed. Boston: Little & Brown, 1997;1189–1202.
- Weisberg L S, Kurnik P B, Kurnik B R. Radiocontrast-induced nephropathy in humans: role of renal vasoconstriction. Kidney Int. 1992;41(5):1408–1415. [PUBMED], [INFOTRIEVE], [CSA]
- Deray G. Nephrotoxicity of contrast media. Nephrol Dial Transplant. 1999;14(11):2602–2606. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Andrade L, Campos S B, Seguro A C. Hypercholesterolemia aggravates radiocontrast nephrotoxicity: protective role of L-arginine. Kidney Int. 1998;53(6):1736–1742. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Tommaso C L. Contrast-induced nephrotoxicity in patients undergoing cardiac catheterization. Catheter Cardiovasc Diagn. 1994;31(4):316–321. [CSA]
- Agmon Y, Peleg H, Greenfeld Z, Rosen S, Brezis M. Nitric oxide and prostanoids protect the renal outer medulla from radiocontrast toxicity in the rat. J Clin Invest. 1994;94(3):1069–1075. [PUBMED], [INFOTRIEVE], [CSA]
- Campos S B, Ori M, Dórea E L, Seguro A C. Protective effect of L-arginine on hypercholesterolemia-enhanced renal ischemic injury. Atherosclerosis. 1999;143(2):327–334. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Chatterjee S. Gentamicin-induced alterations in phospholipid metabolism in cultured human proximal tubular cells. J Biochem Toxicol. 1987;2(Fall-Winter):181–201. [PUBMED], [INFOTRIEVE], [CSA]
- Deves R, Krupka R M. Inhibition of choline transport in erythrocytes by n-alkanols. Biochim Biophys Acta. 1990;1030(1):32–40. [PUBMED], [INFOTRIEVE], [CSA]
- Beaugé L, Lew V L. Passive fluxes of sodium and potassium across red cell membranes. In: Ellory C, Lew V, eds. Membrane Transport in Red Cells. London: Academic Press, 1977;39–51.
- Hopkins W G. A Scale of Magnitudes for Effect Statistics [Online]. Available at http://www.sportsci.org/resource/stats/effect.html. Accessed April 2002.
- Moreth K, Kuske R, Renner D, Schoner W. Blood pressure in essential hypertension correlates with the concentration of a circulating inhibitor of the sodium pump. Klin Wochenschr. 1986;64(5):239–244. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Izumo H, Izumo S, DeLuise M, Flier J S. Erythrocyte Na,K pump in uremia. Acute correction of a transport defect by hemodialysis. J Clin Invest. 1984;74(2):581–588. [PUBMED], [INFOTRIEVE], [CSA]
- Katholi R E, Taylor G J, Woods W T, , et al. Nephrotoxicity of noionic low-osmolality versus ionic high-osmolality contrast media: a prospective double-blind randomized comparison in human beings. Radiology. 1993;186(1):183–187. [PUBMED], [INFOTRIEVE], [CSA]
- Hayakawa K, Shimizu Y. Do iodinated contrast media increase serum potassium levels?. Radiology. 1996;200(2):407–411. [PUBMED], [INFOTRIEVE], [CSA]
- Haller C, Meyer M, Scheele T, Koch A, Forssmann W G, Kubler W. Radiocontrast-induced natriuresis associated with increased urinary urodilatin excretion. J Int Med. 1998;243(2):155–162. [CSA], [CROSSREF]