Abstract
Thyroid hormone has been reported to affect renal function. To investigate the effects of thyroid hormone on the progression of renal deterioration, thyroid hormone (dried thyroid) and an antithyroid drug (thiamazole) were administered to adriamycin (ADR)-induced renal failure rats. The rats were divided into four groups, including 1) ADR-DT, given dried thyroid and thiamazole; 2) ADR-T, given thiamazole; 3) ADR; and 4) control. The survival rate at the end of the study (22 weeks) was 62.5% in ADR-DT group and 100% in ADR-T, ADR, and control groups, respectively. There was a significant difference in the body weight and pulse rate between ADR-DT and ADR-T or ADR groups, except for the pulse rate at week 6 (P < 0.05). The creatinine clearance was greater in the ADR-T group than in the ADR or ADR-DT groups at week 22, and was significantly different between the ADR-T and the ADR-DT groups (P < 0.05). The fractional kidney weight and tubular changes were significantly greater in the ADR-DT group than in the ADR-T or ADR groups (P < 0.05). The interstitial volume was significantly greater in the ADR-DT group than in the ADR-T group (P < 0.05). We therefore conclude that a dried thyroid has an aggravative effect in the tubular changes and relative interstitial volume induced by ADR.
Introduction
Thyroid hormones such as T3 and T4 are suppressed in chronic renal failure, and chronic nonthyroidal diseases, including chronic renal failure, are characterized by low T3 syndrome.Citation[1] Thyroid hormones affect both the renal morphology and function. Thyroid deficiency results in a decreased renal plasma flow and glomerular filtration rate, and hyperthyroidism in turn increases the renal plasma flow and glomerular filtration rate.Citation[2] It has been commonly reported that changes in primary hypothyroidism include a decrease in the renal blood flow and glomerular filtration rate.Citation[3&4] The renal hemodynamics are almost always increased in hyperthyroid patients.Citation[2] A selective thyroidectomy has been shown to reduce proteinuria and chronic renal failure progression 1) by altering the immune response; 2) by reducing the glomerular capillary pressure or plasma flow; 3) by the attenuation of the tubular work, thereby lowering the increased cellular energy requirement in the residual nephrons and susceptibility to functional ischemic injury of the tubules; or 4) by means of some as yet unidentified metabolic consequence of hypothyroidism.Citation[5-8]
It is interesting to note the effect of the generation of free oxygen radicals on the thyroidal function because they have been shown to be immune suppressive in vitro.Citation[9] Graves' disease may cause increased oxidative stress as a result of increased free radical activity. The stimulatory effects of methimazole-inhibited oxygen radical generation.Citation[10] Because renal disease with thyroid disorders involves the immune system, two groups received a same dose of thiamazole in this experiment.
Adriamycin (ADR) is a commonly used antineoplastic antibiotic, which damages the renewal systems of highly proliferative cells. In addition, long-lasting toxic effects on the kidney have been observed in the rat.Citation[11] We have previously reported our findings on the long-term observations of ADR-treated rats, in which persistent heavy proteinuria, progressive increases in the serum creatinine level, and extensive renal deterioration were observed.Citation[12] Because the functional and histologic courses are similar to those in clinical focal glomerular sclerosis, this model should be useful for the study of chronic progressive renal diseases.
To investigate the effects of the thyroid function on the progression of renal deterioration, either thiamazole or dried thyroid was administered to ADR-induced progressive renal failure model rats. This experiment revealed that an impaired renal function and the histologic consequences in an experiment model of progressive focal glomerular sclerosis were aggravated by dried thyroid.
Materials and Methods
Experimental Design
ADR, 2 mg/kg, was intravenously administered twice at a 20-day interval, to Sprague-Dawley rats (Kyushu University Animal Center, Fukuoka, Japan), age 10 weeks, and weighing from 190 to 220 g.Citation[12] They were divided into four groups, according to the method reported previously:Citation[13] 1) a group of ADR-dried thyroid-thiamazole (ADR-DT) (dried thyroid 5–15 mg/day in their diet, Fujisawa Astra Co., Tokyo, Japan, Thiamazole 0.18–0.80 mg/day in their water, Chugai Pharmaceutical Co., Tokyo, Japan, n = 8), 2) a group of ADR-thiamazole (ADR-T) (thimazole 0.18–0.80 mg/day in their water, n = 8), 3) a group of ADR (n = 11), and 4) normal control (C, n = 10). All rats were fed standard chow containing 24.8% protein, 0.39% sodium, 1.25% calcium, and 1.06% phosphorus. In an attempt to maintain an equal caloric and protein intake, all groups were pair-fed with the group, which had minimal ingestion. Dried thyroid was mixed with powdered regular chow throughout the experiment. The study design and goals are an impaired renal function, the histologic consequences, and the death due to uremia in an experiment model of progressive focal glomerular sclerosis.
The body weight, systolic blood pressure, pulse rate, T4, blood urea nitrogen (BUN), serum creatinine, total protein, total cholesterol, triglyceride, and phosphate were examined at weeks 6, 12, and 22. The 24-h urinary protein excretion, creatinine clearance, and calcium levels were examined at weeks 12 and 22. The albumin and alkaline phosphatase (ALP) level was examined at week 22.
Analytical Methods
The systolic blood pressure and pulse rate were measured in a conscious state by the tail cuff method. The 24-h urinary protein excretion was measured by the sulfosalicylic acid method. The serum BUN was determined by the reduced nicotinamide adenine dinucleotide-couple reaction, serum and urinary creatinine by the Jaff reaction, total protein by the biuret reaction, the albumin level was determined by the use of bromcresol green, cholesterol and triglyceride by an enzymatic method, calcium by orthocresolphthalein, phosphate by the method of Fiske and Subbarow, and T4 by a radioimmunoassay. ALP measurements were performed spectrophotometrically using p-nitrophenol-phosphate as the substrate.
Morphologic Analysis
The kidney was fixed in 6% neutral buffered formalin and stained with periodic acid-Schiff reagent (PAS). A histologic evaluation was independently made by two investigators without any prior knowledge of the experimental groups.
A semiquantitative score was used to evaluate the degree of glomerular sclerosis according to the method of Raij et al.Citation[14] The severity of the lesions was examined in 50 glomeruli, graded from 0 to 4 points, according to the percentage of morphologic changes on each glomerulus, where 1 point represented an involvement of 25% and 4 points represented an involvement of 100% of the glomerulus.
The extent of tubular atrophy was expressed by the number of microscopic fields where unequivocal atrophy was found, while observing 50 consecutive microscopic fields in the cortex of each specimen with the aid of a × 40 objective.Citation[15]
To estimate the relative interstitial volume of the kidney, tissue sections were examined by light microscopy (magnification × 100), using a 121-point (100-square) eyepiece micrometer.Citation[16] Using the point-counting technique, representative sections from the entire cortex were analyzed to determine the relative interstitial volumes. Five sections (605 pints) were counted in all cases.
Statistical Analysis
The data are expressed as the mean ± SD. Any statistical differences were calculated using the one-way analysis of variance among three groups and the unpaired τ-test with Bonferroni's method and Student't t test between the ADR and C groups, or the ADR-T and ADR-DT groups. The mean survival time and differences in the survival were calculated using Kaplan-Meier method and log-rank test. A level of 0.05 was regarded as significant.
Results
Survival Rate
The survival rate at the end of the study (week 22) was 62.5% in the ADR-DT group, 100% in the ADR-T group, 100% in the ADR group, and 100% in the C group, respectively. Three rats that died showed uremic symptoms such as azotemia, anemia, and weight loss in the ADR-DT group. There was a significant difference in the survival rate between the ADR-DT group and the ADR-T group, ADR group, or C rat, respectively (P < 0.05).
T4, Body Weight, Systolic Blood Pressure, and Pulse Rate
As shown in , T4, the body weight, systolic blood pressure, and pulse rate were measured. The T4 were significantly lower in the ADR-T group than in the ADR group throughout the entire study (P < 0.05). It was significantly lower in the ADR group than in the C group (P < 0.05). The body weight was significantly lower and systolic blood pressure was significantly higher in the ADR-DT group than in the ADR-T or ADR groups at weeks 6, 12, and 22 (P < 0.05). The former was significantly lower at week 6 and the latter higher at week 22 in the ADR group than in the C group (P < 0.05). The pulse rates were significantly higher in the ADR-DT group than in the ADR-T or ADR groups at weeks 12 and 22 (P < 0.05) and in the ADR-DT group than in the ADR group at week 6 (P < 0.05). It was significantly higher in the ADR group than in the C group at week 22 (P < 0.05).
Table 1. T4, body weight, systolic blood pressure, and pulse rate
Urinary Protein Excretion and Creatinine Clearance
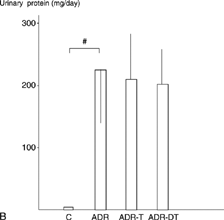
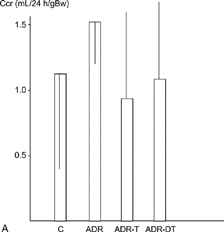
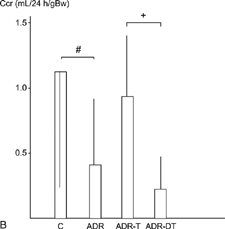
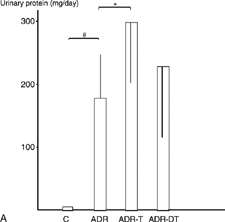
The urinary protein excretion was significantly higher in the ADR-T group (297 ± 78 mg/day) than in the ADR group (176 ± 74) at week 12 (P < 0.05). They were significantly higher in the ADR group than in the C group at weeks 12 and 22 (P < 0.05) (). The average values of creatinine clearance at week 12 were 0.62 mL/24 h/gBW in the C group, 1.5 ± 0.75 mL/24 h/gBW in the ADR group, 1.50 ± 0.68 mL/24 h/gBW in the ADR-T group, and 1.14 ± 0.65 mL/24 h/gBW in the ADR-DT group at week 12. It was higher in the ADR-T group (0.87 ± 0.62) than in the ADR-DT (0.23 ± 0.20) or ADR groups (0.37 ± 0.52) at week 22, and the difference was significant between the ADR-DT and ADR-T groups (P < 0.05). It was significantly lower in the ADR group than in the C group (1.19 ± 0.90) at weeks 22 (P < 0.05) ().
Figure 1. (A) Urinary protein execretion at week 12. (B) Urinary protein execretion at week 22. #P < 0.05 group ADR vs. group C. *P < 0.05 groups ADR-T or ADR-DT vs. group ADR. †P < 0.05 group ADR-T vs. group ADR-DT. Data are expressed as the mean ± SD.
Blood Chemistries
As shown in , the serum creatinine levels were significantly higher in the ADR-T group than in the ADR-DT group at weeks 6 and 12 (P < 0.05), and the BUN was significantly higher in the ADR-DT group than in the ADR-T or ADR groups at week 22 (P < 0.05). The total protein levels were significantly lower in the ADR-DT group than in the ADR-T or ADR groups at weeks 6, 12, and 22 (P < 0.05). Serum albumin was significantly lower in the ADR group than in the C group at week 22 (P < 0.05). The total cholesterol levels were significantly higher in the ADR-T group than in the ADR group at week 12 (P < 0.05) and the triglyceride in the ADR-T group than in the ADR group at week 6 (P < 0.05). They were significantly higher in the ADR group than in the C group throughout the entire study, except for triglyceride at week 12 (P < 0.05).
Table 2. Blood chemistries
Phosphate, Calcium, Calcium-Phosphate Product, and ALP
The bone mineral metabolism is shown in ; the phosphate levels were significantly higher in the ADR-DT group than in the ADR-T or ADR groups at weeks 6 and 22, except for the ADR-T group at week 22 (P < 0.05). The calcium were significantly lower in the ADR-DT group than in the ADR-T or ADR groups at weeks 12 and 22 (P < 0.05). The calcium-phosphate product (Ca × P) showed no significant difference at weeks 12 and 22 and ALP at week 22. The phosphate and Ca × P were significantly lower in the ADR group than in the C group at week 22 (P < 0.05).
Table 3. Phosphate, calcium, calcium-phosphate product, and ALP
Morphologic Study
Autopsies were performed on rats that died before the end of the experiment. The surviving rats were sacrificed at week 22 for histologic examination.
Microscopically segmental or global glomerular sclerosis with a collapsed or obliterated capillary lumen and an enlarged mesangial area was predominantly observed in the ADR group. Hyaline deposition in glomerular epithelium and adhesion of the glomerular tuft to Bowman's capsule were also observed. In the tubulointerstitial area, tubular epithelial atrophy, luminal dilatation with cast, and interstitial fibrosis with mononuclear cell infiltration were prominent findings in this group.
As shown in , the kidney weights were significantly greater in the ADR-DT group than in the ADR-T group, and the fractional kidney weight ([kidney weight/body weight] × 10− 3) was significantly greater in the ADR-DT group than in the ADR-T or ADR groups (P < 0.05). The glomerular sclerosis was more severe in the ADR-DT group than in the ADR or ADR-T groups, although the difference was not significant. The tubular changes were significantly greater in the ADR-DT group than in the ADR-T or ADR groups (P < 0.05), and the relative interstitial volumes were significantly higher in the ADR-DT group than in the ADR-T group (P < 0.05). The kidney weight, fractional kidney weight, glomerular sclerosis, tubular changes, and relative interstitial volume was significantly greater in the ADR group than in the C group (P < 0.05).
Table 4. Kidney weight, fractional kidney weight, glomerular sclerosis, tubular atrophy, and relative interstitial volume
Discussion
To investigate the effects of the thyroid function on the progression of renal deterioration, thyroid hormone was administered to ADR-induced progressive renal failure model rats. This experiment revealed that the impaired renal function and histologic consequences in the experimental model of progressive focal glomerular sclerosis were exaggerated due to the administration of dried thyroid.
The T4 was lower in the ADR group than in the C group throughout the experiment. This results was similar to those of a study by Beckett et al.Citation[17] in which hypothyroidism was observed in chronic renal failure patients. Systolic blood pressure and pulse rate were higher in the ADR group than in the C group at week 22. The kidney weight, fractional kidney weight, glomerular sclerosis, tubular changes, and relative interstitial volume were significantly more in the ADR group than in the C group. The renal histologic changes may thus be related to the observed blood pressure elevation.
Hypertension is an important factor accelerating the progress of renal disease.Citation[18] The systolic blood pressure was significantly higher in the ADR-DT group than in the ADR-T or in the ADR groups at weeks 6, 12, and 22. Three of the rats in the ADR-DT group died of uremia and showed marked renal deterioration. Because a diseased kidney is susceptible to hemodynamic changes and aggravated by hypertension,Citation[18] an elevation of the blood pressure might play some role in speeding up the progress of renal damage in the ADR-DT group.
The energy requirement increases in the remnant nephron in rats with renal mass reduction, and this is considered to be the cause for renal injury.Citation[19] Israel et al.Citation[20] also suggested that an elevation of the energy metabolism was closely related to the destruction of damaged cell. By increasing the energy metabolism, thyroid hormone might exert aggravative effects on the damaged epithelial cells. In this study, the creatinine clearance was significantly less in the ADR-DT group (0.23 ± 0.20 mL/24 h/gBw) than that in the ADR-T group (0.87 ± 0.62) at week 22.
It has been known for many years that the kidney weight decreases and a decrease in the length of the proximal tubule occurs during hypothyroidism.Citation[20&21] During hypothyroidism, there is an inhibition of kidney growth that affects all structures except the glomeruli in the cortex, the S3 segment of the proximal tubule, and the thick ascending limb in the outer medulla.Citation[13] However, the fractional kidney weight and relative interstitial volume were all significantly greater in the ADR-DT group than in the ADR-T group. Yoshida et al.Citation[22] reported that glomerular hypertrophy in an important step preceding glomerular sclerosis. In our previous study using the same ADR-induced rat model as in this study, we confirmed that a slow but progressive renal deterioration takes place in association with changes in the kidney weight, which reaches a peak at week 20 and then decreases thereafter.Citation[12] Thyroid hormone may thus increase the nephron segment size, which thus results in renal histologic or functional deterioration in the ADR-DT group.
Nephrocalcinosis with chronic interstitial inflammation is known to accelerate the deterioration of the renal function and the progression of renal diseases.Citation[23] The renal calcium content increases concomitantly with a decreased renal function, a fact that has been demonstrated in biopsy materials from a wide variety of renal diseases.Citation[24] An elevated serum phosphate level seems to be the most important factor for initiating the sequence necessary for the development of nephrocalcinosis. In this study, the phosphate was higher in the ADR-DT group than in the ADR-T group, but there was no significance in the calcium-phosphate product at week 22.
Conclusion
We therefore conclude that the administration of dried thyroid has a negative effect on the tubular changes and relative interstitial volume induced by ADR.
Acknowledgment
We thank Mr. B. Quinn for his comments on the manuscript.
References
- Schaefer F, Wiecek A, Ritz E. Endocined disorders. In: Davison A M, Cameron J S, Grunfeld J-P, Kerr D NS, Ritz E, Winearls C G, eds. Textbook of Clinical Nephrology. 2nd Ed. London: Oxford Medical, 1998: 1854–1866.
- Katz A I, Emmaneol D S, Lindheimer M D. Thyroid hormone and kidney.Nephron. 1975;15:223–249. [PUBMED], [INFOTRIEVE], [CSA]
- Montenego J, Gonzalez O, Saracho R, Aguirre R, Gonzalez O, Martinez I. Changes in renal function in primary hypothyroidism.Am J Kidney Dis. 1996;27:195–198. [CSA]
- Berl T, Robertson G L. Pathophysiology of water metabolism. In: Brenner B M, Rector F C Jr, eds. The Kidney. 6th Ed.Philadelphia: WB Saunders, 2000:866–924.
- Alfrey A C. Thyroid and parathyroid hormones in experimental renal failure. In: Mitch W E, Brenner B M, Stein J H, eds. The Progressive Nature of Renal Disease.New York: Churchill Livingstone, 1986:37–44.
- Conger J D, Falk S A, Gillium D M. The protective mechanism of thyroidectomy in a rat model of chronic renal failure.Am J Kidney Dis. 1989;XIII:217–225. [CSA]
- Katz A I, Lindheimer M D. Renal sodium- and potessium-activated adenosine triphosphatase and sodium reabsorption in the hypothyroid rat.J Clin Invest. 1973;52:796–804. [PUBMED], [INFOTRIEVE], [CSA]
- Falk S, Buric V, Hammond W S, Conger J D. Serial glomerular and tubular dynamics in thyroidectomized rats with remnant kidneys.Am J Kidney Dis. 1991;XVII:218–227. [CSA]
- Hoffeld J T, Metzger Z VI, Oppenheim J J. Oxygen-derived metabolites as suppressors of immune responses in vitro. In: Pick E, Landy M, eds. Lymphokines.New York: Academic Press, 1981; Vol. 2:63–86.
- Wartofsky L. Has the use of antithyroid drugs for Graves' disease become obsolete?. Thyroid. 1993;3:335–344. [PUBMED], [INFOTRIEVE], [CSA]
- Bertazzoli C, Chieli T, Ferni G, Ricevui G, Solicia E. Chronic toxicity of adriamycin: a new antineoplastic antibiotic.Toxicol Appl Phamacol. 1972;21:287–301. [CSA], [CROSSREF]
- Okuda S, Oh Y, Tsaruda H, Onayama K, Fujimi S, Fujishima M. Adriamycin-induced nephropathy as a model of chronic progressive renal disease.Kidney Int. 1986;29:502–510. [PUBMED], [INFOTRIEVE], [CSA]
- Davis R G, Madsen K M, Fregly M J, Tisher C C. Kidney structure in hypothyroidism.Am J Pathol. 1983;113:41–49. [PUBMED], [INFOTRIEVE], [CSA]
- Raij L, Azar S, Kaene W. Mesangial immune injury hypertension, and progressive glomerular damage in Dahl rats.Kidney Int. 1984;26:137–143. [PUBMED], [INFOTRIEVE], [CSA]
- Risdon R A, Sloper J C, Wardener H E. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis.Lancet. 1968;ii:363–366. [CSA], [CROSSREF]
- Bennet W M, Walker R G, Kincaid-Smith P. Renal cortical interstitial volume in mesangial IgA nephropathy.Lab Invest. 1982;47:330–335. [CSA]
- Beckett G J, Henederson C J, Elwes R, Seth J, Lambie A T. Thyroid status in patients with chronic renal failure.Clin Nephrol. 1983;19:172–178. [PUBMED], [INFOTRIEVE], [CSA]
- Okuda S, Onoyama K, Fujimi S, Oh Y, Nomoto K, Omae T. Influence of hypertension on the progression of experimental autologous immune complex nephritis.J Lab Clin Med. 1983;101:461–471. [PUBMED], [INFOTRIEVE], [CSA]
- Israel Y, Videla L, Fernandez-Videla V, Bernstein J. Effects of chronic ethanol treatment and thyroxine administration on ethanol metabolism and liver oxidative capacity.J Pharmacol Exp Ther. 1975;19:565–574. [CSA]
- Katz A I, Epstein F H. Physiologic role sodium-potassium-activated adenosine triphosphatase in the trasport of cations across biologic membranes.N Engl J Med. 1968;278:253–261., [PUBMED], [INFOTRIEVE], [CSA]
- Bradley S E, Stephan F, Coelho J B, Reville P. The thyroid and the kidney.Kidney Int. 1974;6:346–365. [PUBMED], [INFOTRIEVE], [CSA]
- Yoshida Y, Fogo A, Ichikawa I. Glomerular hemodynamic changes vs. hypertrophy in experimental glomerular sclerosis.Kidney Int. 1989;35:654–660. [PUBMED], [INFOTRIEVE], [CSA]
- Walser M, Mitch W E, Collier V U. The effect of nutritional therapy on the course of chronic renal failure.Clin Nephrol. 1979;11:60–70. [CSA]
- Gimenez L F, Solez Y, Walker W G. Relation between renal calcium content and renal impairment in 246 human renal biopsies.Kidney Int. 1987;31:93–99. [PUBMED], [INFOTRIEVE], [CSA]