Abstract
Current biomedical evidence suggests that Hypoxis hemerocallidea (Fisch. & C.A. Mey.) [Hypoxidaceae] (African Potato [AP]) corm extract may be useful in the management of type 2 diabetes mellitus. However, more recent reports have also indicated that certain herbal extracts attenuate the deterioration of kidney function in diabetes mellitus. Accordingly, this study investigated the effects of short- (acute) and long-term (chronic) administration of H. hemerocallidea corm aqueous extract (APE) on renal fluid and electrolyte handling in male Wistar rats. Acute effects of APE were investigated in separate groups of anesthetized rats challenged with a continuous jugular infusion of 0.077 M NaCl at 9 mL · h− 1. After a 3.5-h equilibration period, consecutive 30-min urine collections were made over the subsequent 4 h of 1-h control, 1.5-h treatment, and 1.5-h recovery periods for measurements of urine flow, Na+, and K+ excretion rates. To establish the effects of acute APE, the extract was added to the infusate at doses of 90, 180, or 360 μg · h− 1 in separate groups of rats during the treatment period. For chronic studies, individually caged rats were administered twice with APE (30 mg · kg− 1 PO) every third consecutive day at 09h00 and 17h00 for 5 weeks. Control rats received distilled water (3 mL · kg− 1). Urine volume and total urinary outputs of creatinine, Na+, and K+ were determined from 24-h samples. Acute infusion of APE produced a dose-dependent, significant (p < 0.01) decrease in urine flow, K+, and Na+ excretion rates. Chronic APE treatment significantly reduced urinary Na+ output between weeks 2 and 5, without affecting either urine flow or K+ excretion rates. When compared with control animals, APE significantly reduced GFR (2.54 ± 0.09 mL · min− 1 vs. 1.52 ± 0.02 mL · min− 1) and increased plasma creatinine concentration (55 ± 3 µmol · L− 1 vs. 68 ± 6 µmol · L− 1). The results from this study suggest that the H. hemerocallidea corm aqueous extract may impair kidney function.
Introduction
Plant-derived traditional medicines are gaining popularity among a wider audience because of their affordability, accessibility, and the global trend of seeking alternative methods of improving the quality of life.Citation[1] The traditional health practitioners of southern Africa have widely employed the tuberous root stock of Hypoxis hemerocallidea (Fisch. & C.A. Mey.) [Hypoxidaceae] (i.e., the corm, popularly known as the “African potato”) of the herb as a “muthi” (the South African isiZulu word for “medicine”) for an array of ailments.Citation[2] This “miracle” medicinal plant of southern Africa is a tuberous, perennial herb with long, strap-shaped leaves and yellow, star-shaped flowers.Citation[3] The African potato has been claimed to be an amazing and wonder plant medicine in the fight against various modern human disorders, including HIV/AIDS-related diseases, arthritis, hypertension, and diabetes mellitus.Citation[2], Citation[4&5] More recent biomedical evidence suggests that the corm of H. hemerocallidea corm has antidiabetic potential and may be useful in the management of adult-onset, noninsulin-dependent, type 2 diabetes mellitus.Citation[6] However, current biomedical evidence also indicates that certain herbal extracts and plant products ameliorate the deterioration of kidney function in diabetes mellitus.Citation[7&8] Therefore, we speculated that H. hemerocallidea corm extract (APE) may aggravate renal complications often associated with diabetes mellitus in humansCitation[9&10] and experimental animals.Citation[11&12] Thus, the main purpose of this study was to investigate the effects of short- (acute) and long-term (chronic) administration of H. hemerocallidea corm aqueous extract on renal fluid and electrolyte handling in male Wistar rats. Glomerular filtration rate (GFR) assessment is a fundamental parameter of evaluating renal tubular function.Citation[13&14] Therefore, this study also investigated the effects of APE on GFR.
Methods and Materials
Preparation of Plant Extract
The selection of H. hemerocallidea (Fisch. & C.A. Mey.) [Hypoxidaceae] was based on ethnomedical information from traditional healers. Corms of H. hemerocallidea were collected in April 2003 around Durban. The plant was identified and authenticated by a botanist, Professor Himansu Baijnath. Voucher specimen of the plant has been deposited in the University Herbarium (Govender 1). Freshly pulped corms were extracted with water at room temperature for 48 h. The filtrate was air-dried and milled into fine powder with the use of a commercial blender. Aqueous extracts were prepared using a modified method that has been previously described by Twaij et al. (1983) and Al-Khazraji et al. (1993).Citation[15&16]
Animals
Experiments were performed on male Wistar rats (250–300 g body weight). The rats were maintained under laboratory conditions of 12 h light/12 h dark regime at the Biomedical Resource Unit, University of KwaZulu-Natal, Westville Campus. The rats were exposed to both food (Epol-diet 4700, Epol, South Africa) and water ad libitum. Ethical clearance for this study was obtained from the University of KwaZulu-Natal's Ethics Committee.
Series 1: Acute Studies
Animal Preparation
Rats were anesthetized by an intraperitoneal injection of Trapanal (sodium 5-ethyl-[1-methyl butyl]-2-thiobarbiturate, Byk Gulden, Konstanz, Federal Republic of Germany) at a dose of 0.11 g · kg− 1 and tracheotomized to maintain clear airway entry. The right jugular vein was cannulated with polyethylene tubing (internal diameter, [id] 0.86 mm; external diameter, [od] 1.27 mm, Clay Adams, NJ, USA) to allow intravenous infusion of 0.077 M NaCl. The urinary bladder was cannulated with polyethylene tubing of the same size via an abdominal incision. The body temperature was maintained at 37 ± 1°C with a heated table.
Renal Function Studies
Control Group
Animals were placed on a continuous infusion of 0.077 M NaCl at 9 mL · h− 1 (Harvard syringe infusion pump 22, n = 6). Following an initial equilibration period of 3.5 h, eight consecutive urine collections were made into preweighed plastic vials at 30-min intervals over the subsequent 4 h for measurements of urine flow and Na+ and K+ excretion rates. The control group was designed to check the stability of renal function.
Treated Groups
The renal effects of APE extract were studied in separate groups of animals (n = 6 in each group) following a 3.5-h equilibration period. Urine samples were collected for 1 h (control period), following which, APE was added to the infusate at doses of 90, 180, or 360 μg · h− 1 for 1.5 h (treatment period) resulting in total dose of 0.45, 0.90, and 1.8 mg · kg− 1, respectively. The animals were then switched back to the infusate alone for the final 1.5 h (recovery period). In all groups, a terminal blood sample (2 mL) was collected by cardiac puncture, and plasma was separated for Na+ and K+ determination. The administration rates of the APE were chosen from previous experiences in our laboratory.Citation[6&7]
Blood Pressure Measurements
Control animals and a group of rats infused with APE at 180 μg · h− 1 during the treatment were inserted with a heparinized cannula (Portex, id 0.86 mm; od 1.27 mm) in the left common carotid artery for mean arterial blood pressure measurement. The cannula was connected to a pressure transducer (Statham MLT 0380, Ad Instruments) compatible with the PowerLab System ML410/W (Australia) to record mean arterial blood pressure at 30-min intervals. The median dose of APE was chosen because natriuretic and antidiuretic responses of the extract in acute studies were dose related.
Series 2: Chronic Studies
Male Wistar rats were divided into control and treated groups (n = 6 in each group). The animals were housed individually in separate Makrolon polycarbonate metabolic cages (Techniplats, South Africa) that were cleaned daily, and they were allowed free access to food and water. The amounts of food and water consumed were recorded daily at 09h00. The weights of the animals were measured once every week. The effects of the extract were investigated in rats orally administered APE at 30 mg · kg− 1 twice every third consecutive day for 5 weeks at 09h00 and 17h00. Control rats were administered distilled water (3 mL · kg− 1). Urine volume and total urinary outputs of Na+ and K+ were determined from 24-h samples for all groups.
Blood Pressure Measurements
Blood pressure was monitored every third consecutive day for 5 weeks at 09h00 using the noninvasive tail-cuff method with photoelectric sensors (IITC Model 31 Computerized Blood Pressure Monitor, Life Sciences, Woodland Hills, CA, USA). The unit works with IITC hardware system to measure blood pressure in conscious rats.
Hematologic and GFR Measurements
Hematologic parameters (hematocrit, hemoglobin, red blood cells, white blood cells, differential counts, and erythrocyte indices) were assessed using a coulter counter (Coulter Counter T890, Coulter Electronics, Miami Lakes, FL, USA). GFR was calculated at the end of the 5-week period using creatinine clearance. This involved 24-h urine volume, urinary creatinine, and plasma creatinine to allow calculation of creatinine clearance as a measure of GFR.
Terminal Studies
At the end of the 5-week experimental period, blood samples were collected from all groups of animals by cardiac puncture into individual precooled heparinized containers for measurements of hematologic parameters. Separated plasma was analyzed for Na+, K+, creatinine, and urea concentrations.
Analytical Methods
Measurement of Electrolytes
Urine volume was determined gravimetrically. Na+ and K+ were determined by ion activity, using the Beckman Coulter (Synchron LX20 Clinical Systems, USA). Urea and creatinine analyses were performed at the King Edward Hospital, Biochemistry Laboratory (University of KwaZulu-Natal, Durban). Creatinine estimation employed the reaction of retaining and sodium picrate to form creatinine picrate. Urea estimation employed the hydrolytic degradation of urea in the presence of urease. The assays on the Beckman coulter (Synchron LX20 Clinical Systems) used reagent kits from Beckman Coulter (Ireland, Inc.).
Data Presentation
Values are presented as means ± SE of means. Renal excretion data for acute studies are presented graphically showing 30-min collections over the 4-h postequilibration period. The total amounts of fluid voided and electrolytes excreted during the 1.5-h administration of the APE were calculated and compared with values in controls for the corresponding time. For chronic studies, calculating total weekly fluid voided and urinary amounts of electrolytes excreted also assessed renal function. GFR was evaluated by creatinine clearance test as assessed by 24-h urinary excretion rates of creatinine in relation to plasma concentration. The data were subjected to analysis of variance using a one-way design and Scheffe's multiple comparison test was used to assess any differences. A value of p < 0.05 was considered significant.
Results
Series 1: Acute Studies
Renal Effects of APE
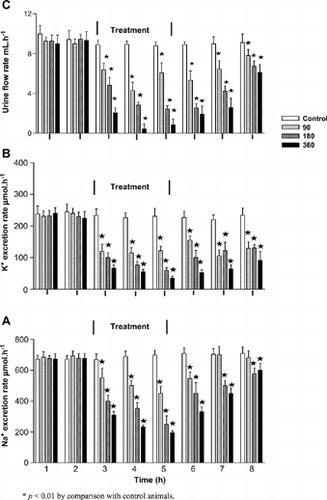
shows stable urine flow and Na+ excretion rates (ranges from 8 to 10 mL · h− 1 and 671 to 710 µmol · h− 1, respectively) in vehicle-infused control animals during the 4-h postequilibration period; values that compared with the infusion rate (9 mL · h− 1 and 693 µmol · h− 1, respectively). K+ excretion rate was also stable throughout the postequilibration period averaging, 242 µmol · h− 1. APE infusion for 1.5 h at various doses (90, 180, or 360 μg · h− 1) produced dose-dependent decreases in urine flow, K+, and Na+ excretion rates (). The decrease in urine volume and electrolyte urinary output was reflected as reduced total volume of fluid voided and amounts of K+ and Na+ excreted during the treatment period (). The reduction in fluid and electrolyte excretion rates was maintained during the posttreatment period. However, renal retention of Na+ and K+ was not reflected in the plasma collected at the end of the experimental period.
Table 1. Comparison of total amounts of urine volume, Na+, and K+ excreted during the 1.5-h treatment period in anesthetized control and rats administered Hypoxis hemerocallidea corm aqueous extract (APE) at various doses (n = 6 in all groups)
Figure 1. Comparison of urine flow (C), K+ (B), and Na+ (A) excretion rates in control rats and separate groups of anesthetized control and rats infused with Hypoxis hemerocallidea corm aqueous corm extract at 90, 180, or 360 μg · h−1 for 1.5 h. Values are presented as means for each 30-min collection; vertical bars indicate SE of means (n = 6 in each group).
Mean Arterial Blood Pressure
The mean arterial blood pressure of control animals remained stable, approximating 137 mmHg during the 4-h postequilibration period. Intravenous infusion of APE at 180 μg · h− 1 to anesthetized rats for 1.5 h induced reversible hypotensive responses. There was an initial reduction in the mean arterial pressure from a mean pretreatment value of 140 ± 7 mmHg to 110 ± 5 mmHg (n = 6) by 30 min of the infusion of APE, but thereafter, the mean arterial blood pressure gradually rose to reach 136 ± 6 mmHg (n = 6) by the last 30 min of the treatment. This blood pressure value that did not significantly differ from that recorded during pretreatment or recovery periods, and that of control animals at the corresponding time.
Series 2: Chronic Studies
Body Weight, Blood Pressure, Hematologic, Food, and Water Intake Measurements
Groups of control and treated animals progressively gained weight. They were healthy and showed no evidence of sickness at the end of the 5-week period. The total amounts of food taken, water drunk, and fluid voided per week by the two groups did not significantly differ. APE administration did not significantly modify the blood parameters measured at the end of 5 weeks. The mean systolic and diastolic blood pressure in control and treated rats were similar over the experimental period.
Renal Effects of APE
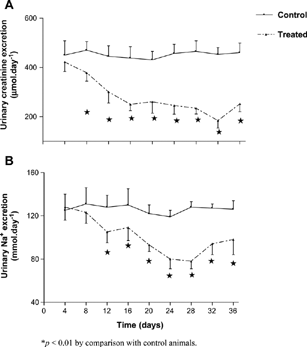
and show that APE significantly (p < 0.01) reduced the mean urinary Na+ output from the 2nd to the 5th week by comparison with control animals at the corresponding period. However, urine flow or urinary K+ excretion rates were not altered over the 5-week study period. Urinary creatinine output was significantly (p < 0.01) reduced by comparison with control rats (), but urinary urea output was not affected. A significant (p < 0.01) decrease in GFR was observed at the end of 5 weeks in treated animals the by comparison to control animals (). Renal creatinine retention was reflected by the elevated plasma creatinine concentration ().
Table 2. Plasma Na+, K+, urea, and creatinine concentrations and GFR in control and rats administered Hypoxis hemerocallidea corm aqueous extract every third consecutive day for 5 weeks (n = 6 in all groups)
Figure 2. Comparison of Na+ (B) and creatinine (A) urinary output in separate groups of control and rats orally administered Hypoxis hemerocallidea corm aqueous extract (30 mg · kg− 1) twice every third consecutive day for 5 weeks. Values are presented as daily means; vertical bars indicate SE of means (n = 6 in each group).
Discussion
The effects of short- (acute) and long-term (chronic) administration of APE on renal fluid and electrolyte handling in male Wistar rats were investigated in this study. We have been able to observe that acute infusion of APE induces dose-dependent decreases in renal fluid, Na+, and K+ excretion rates, whereas chronic administration of the extract for 5 weeks only reduced urinary Na+ excretion. We suggest that short- or long-term treatment with APE can alter kidney function. Although this study provides no evidence of active chemical compounds in APE, it is well established that hyperoxide, a glucoside, is a major constituent of Hypoxis species.Citation[18-20] Upon hydrolysis by the enzyme b-glucosidase, hyperoxide is converted to rooperol, a highly bioactive compound.Citation[20]
Interestingly, short-term intravenous infusion of APE (90, 180, or 360 μg · h− 1) to anesthetized rats for 1.5 h induced dose-dependent decreases in renal fluid, Na+, and K+ excretion rates, and could only reduce urinary Na+ output following chronic administration. Surprisingly, however, chronic APE treatment did not affect renal K+ excretion. One plausible explanation is that long-term administration of APE interferes with Na+ reabsorption in the proximal and loops of Henle to increase its delivery to the distal tubules where Na+ is reabsorbed in exchange for K+. The discrepancy observed in renal function in anesthetized and conscious preparations cannot be attributed to the different protocols used in this study because we have previously shown no significant differences in renal function in two protocols.Citation[21&22] The hormonal basis, if any, of Na+ retention in APE-treated animals remains unclear in the absence of analysis of hormones that influence renal electrolyte handling. Systemic influence of APE may be excluded as the extract elicited reversible hypotensive responses following acute infusion, and did not induce any significant changes in the mean arterial blood pressure of rats chronically treated with APE. The fall in blood pressure associated with acute treatment was within the previously reported autoregulatory range,Citation[23-25] and as such, would not be expected to affect GFR, a factor that influences renal electrolyte handling. The effect of APE on blood pressure is consistent with reports of moderate transient decreases and increases in cardiac output, stroke volume, heart rate, and vascular pressures of pure components from Hypoxis extracts.Citation[26]
A decrease in GFR as assessed by creatinine clearance and a concomitant increase in plasma creatinine concentration was exclusively observed for the group treated with APE over the 5-week period. We and several other authors have previously used creatinine clearance in rats to monitor GFR.Citation[14], Citation[27&28] It is likely that chronic APE treatment may impair kidney function as evidenced by a decrease in GFR and elevated plasma creatinine concentration, despite lack of effect on plasma urea concentration. Treatment-related increases in plasma creatinine and urea concentrations are variables used not only to indicate impairment of kidney function,Citation[29&30] but are also clinical chemistry end points to detect treatment-related toxic effects of compounds on the kidney in rats.Citation[14] Creatinine clearance estimates changes in renal function, rather than blood urea concentration, because the latter varies with dietary protein intake.Citation[31] It appears unlikely that the decrease in GFR observed in this study can be attributed to a fall in blood pressure because this was not altered by the administration of the plant extract. Presumably, APE increased tubular Na+ reabsorption, and this would be expected to elevate plasma concentration of the cation. However, despite a decrease in urinary Na+ output over the 5-week, chronic treatment of animals with APE induced a slight, but statistically nonsignificant increase of plasma Na+ concentration. We suggest that this elevation is of biological significance, indicating early stages of kidney dysfunction as indicated by reduced GFR. In summary, the findings of this study suggest that APE may impair kidney function as indicated by an increased renal fluid and electrolyte retention and reduced GFR. Further studies are needed to elucidate the possible mechanism(s) associated with this phenomenon.
References
- Brown C M. Use of alternative therapies and their impact on compliance: perceptions of community pharmacists in Texas. J Am Pharm Assoc (Wash.). 1998;38(5):603–608. [CSA]
- Dold A P, Cocks M L. The trade in medicinal plants in the Eastern Cape Province, South Africa. S Afr J Med Sci. 2002;97:589–597. [CSA]
- Van Wyk B-E, Van Oudtshoorn B, Gericke N. Medicinal Plants of South Africa. 2nd ed. Pretoria: Briza Publications, 2002: 156–157.
- Albrecht C E. Hypoxoside: a putative, non-toxic pro-drug for the possible treatment of certain malignancies, HIV-infection and inflammatory conditions, Proceedings of the 1st International IOCD-Symposium, Victoria Falls, Zimbabwe, February, 25–28, 1996.
- Bouic P JD, Estebeth S, Liebenberg R W, Albretcht C E, Pegel K, Van Jaarsveld P P. β-Sitosterol and β-sitosterol glucose stimulate the human immunomodulatory vitamin combination. Int J Immunopharmacol. 1996;18:693–700. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Mahomed I M, Ojewole J AO. Hypoglycaemic effect of Hypoxis hemerocallidea corm (‘African potato’) aqueous extract in rats. Methods Find Exp Clin Pharmacol. 2003;25(8):617–623. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Grover J K, Vats V, Rathi S S, Dawar R. Traditional Indian anti-diabetic plants attenuate progression of renal damage in streptozotocin-induced diabetic mice. J Ethnopharmacol. 2001;76(3):233–238. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Kim Y Y, Kang K M, Chung S H. Long-term administration of Sopungsungiwon (SP) prevents diabetic nephropathy in Zucker diabetic fatty rats. Arch Pharm Res. 2002;25(6):917–922. [PUBMED], [INFOTRIEVE], [CSA]
- Joss N, Paterson K R, Deighan C J, Simpson K, Boulton-Jones J M. Diabetic nephropathy: how effective is treatment in clinical practice?. QJM. 2002;95(1):41–49. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Krishnan R, Izatt S, Bargman J M, Oreopoulos D. Prevalence and determinants of erectile dysfunction in patients on peritoneal dialysis. Int Urol Nephrol. 2003;35(4):553–556. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Bank N, Aynedjian H S. Progressive increases in luminal glucose stimulate proximal sodium absorption in normal and diabetic rats. J Clin Invest. 1990;86(1):309–316. [PUBMED], [INFOTRIEVE], [CSA]
- Bwititi P, Musabayane C T, Nhachi C F.B. The effects of Opuntia megacantha (prickly pear) on blood glucose and kidney function in streptozotocin (STZ) diabetic rats. J Ethnopharmacol. 2000;69:247–252. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Bursztyn M, Ben-Ishay D, Mekler J, Raz I. Insulin-induced renal dysfunction in regular Sabra rats. Clin Exp Pharmacol. 1995;22(1):S32–S33. [CSA]
- Travlos G S, Morris R W, Elwell M R, Duke A, Rosenblum S, Thompson M B. Frequency and relationships of clinical chemistry and liver and kidney histopathology findings in 13-week toxicity studies in rats. Toxicology. 1996;107(1):17–29. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Twaij H A, Kery A, Al-Khazraji N K. Some pharmacological, toxicological and phytochemical investigations on Centaurea phyllocephala. J Ethnopharmacol. 1983;9(2–3):299–314. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Al-Khazraji S M, al-Shamaony L A, Twaij H A. Hypoglycaemic effect of Artemisia herba alba. Effect of different parts and influence of the solvent on hypoglycaemic activity. J Ethnopharmacol. 1993;40(3):163–166. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Ojewole J AO. Antiinflammatory properties of Hypoxis hemerocallidea corm (African potato) extracts in rats. Methods Find Exp Clin Pharmacol. 2002;24(10):685–687. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Marini-Bettolo G B, Patamia M, Nicoletti M, Galeffi C, Messana I. Hypoxoside—a new glycoside of uncommon structure from Hypoxis obtusa Busch. Tetrahedron. 1982;38:1683–1687. [CSA], [CROSSREF]
- Drewes S E, Hall A J, Learmonth R A, Upfold U J. Isolation of hypoxoside from Hypoxis rooperi and synthesis of (E)-1,5 bis(3′,4′-dimethoxyphenyl)pent-4-en-1-yne. Phytochemistry. 1984;23:1313–1316. [CSA], [CROSSREF]
- Drewes S E, Khan F. The African potato (Hypoxis hemerocallidea): a chemical-historical perspective. S Afr J Sci. 2004;100:425–430. [CSA]
- Balment R J, Brimble M J, Forsling M L, Musabayane C T. Natriuretic response of the rat to plasma concentrations of arginine vasopressin within the physiological range. J Physiol. 1984;252:517–526. [CSA]
- Brimble M J, Forsling M L, Musabayane C T. Natriuretic action of arginine vasopressin in the conscious unrestrained rat. Acta Endocrinol (Copenh). 1988;119:386–390. [CSA]
- Beierwaltes W H, Sigmon D H, Carretero O A. Endothelium modulates renal blood flow but not autoregulation. Am J Physiol. 1992;262(6 Pt 2):F943–F949. [PUBMED], [INFOTRIEVE], [CSA]
- Mattson D L, Lu S, Roman R J, Cowley A W, Jr. Relationship between renal perfusion pressure and blood flow in different regions of the kidney. Am J Physiol. 1993;264(3 Pt 2):R578–R583. [PUBMED], [INFOTRIEVE], [CSA]
- Moosavi S MS, Johns E J. Effect of renal perfusion pressure reduction and nerve stimulation on PRA and renal renin and angiotensinogen mRNA in the anesthetized rat. J Physiol. 1997;520:261–269. [CSA], [CROSSREF]
- Coetzee J F, Kruger P B, Albrecht C F, Jahed N, Van Jaarsveld P P. Pharmacokinetic behaviour and cardiovascular effects of intravenously administered hypoxoside and rooperol. Arzneim Forsch. 1996;46:997–1000. [CSA]
- Girchev R, Markova P, Mikhov D, Natcheff N. Renal excretory function in conscious Long Evans and vasopressin deficient (Brattleboro) rats after endothelin-A receptor inhibition. Acta Physiol Pharmacol Bulg. 1998;23(3–4):73–77. [PUBMED], [INFOTRIEVE], [CSA]
- Bertuzzi M L, Bensi N, Mayer N, Niebylski A, Armario A, Gauna H F. Renal mechanisms involved in stress-induced antinatriuresis and antidiuresis in rats. Arch Physiol Biochem. 2003;111(3):259–264. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Hwang D F, Lai Y S, Chiang M T. Toxic effects of grass carp, snake and chicken bile juices in rats. Toxicol Lett. 1997;85(2):85–92. [CSA], [CROSSREF]
- Braunlich H, Marx F, Fleck C, Stein G. Kidney function in rats after 5/6 nephrectomy (5/6 NX): effort of treatment with vitamin E. Exp Toxicol Pathol. 1997;49(1–2):135–139. [PUBMED], [INFOTRIEVE], [CSA]
- Landewe R BM, Vergouwen M SC, Goeithe H S, van Rijthoven W AM, Breedveld C F, Dijkmans B AC. Antimalarial drug induced decrease in creatinine clearance. J Rheumatol. 1995;22(1):34–37. [PUBMED], [INFOTRIEVE], [CSA]