Abstract
This study was designed to assess the principal markers of thrombogenicity and biocompatibility during continuous venovenous hemodiafiltration (CVVHDF) using regional citrate anticoagulation (RCA). In a prospective study, 11 procedures with a polysulfone membrane were performed in nine critically ill patients with acute renal failure and impaired hemostasis. Blood samples were taken before and during CVVHDF at diafilter outlet—before calcium-induced reversal of the effect of citrate—at 15, 60, 360, and 1440 minutes. In four patients, 10 CVVHDF sessions were performed with systemic heparin anticoagulation (HA) using a polyacrylonitrile membrane. During RCA, blood thrombocyte count, plasma thrombin-antithrombin III complexes, beta-thromboglobulin, and von Willebrand factor levels did not differ significantly from baseline. Plasma D dimer levels rose significantly at 360 minutes; however, the difference between diafilter inlet and outlet levels was nonsignificant. There was a significant increase in plasma C5a concentrations and a decline in blood leukocyte count in the early phase of CVVHDF. Just as in RCA, no increase in plasma thrombogenicity indices was observed during HA. However, clotting times in blood entering patients' circulation were significantly prolonged. Plasma C5a concentrations increased significantly at the beginning of CVVHDF. RCA can effectively inhibit the thrombogenic effect of the extracorporeal circuit in CVVHDF. The effect of HA may be similar, however, at the expense of systemic anticoagulation and risk of bleeding. RCA, performed in a way that overcomes thrombogenicity, does not completely eliminate complement activation and/or transient leukopenia during CVVHDF.
INTRODUCTION
Methods of continuous renal replacement therapy (CRRT) are effective in blood purification in the critically ill patient. Some authors, at least in some cases, prefer CRRT to intermittent extracorporeal procedures.Citation[1] The most frequent problems associated with CRRT are bleeding and thrombotic complications.Citation[2] There are several reasons for this. Patients may have a hemostasis defect due to renal failure, sepsis or SIRS, or presence of liver dysfunction, or as a result of pharmacotherapy. Some patients have recently had an injury, surgical procedure, and invasive diagnostic procedure. The administration of unfractionated heparin, an anticoagulant used most frequently to overcome thrombogenicity of CRRT systems,Citation[3] may be associated with an inadequately high risk for bleeding. The identification of effective, yet safer, anticoagulation in CRRT poses a challenge even more exciting than that associated with intermittent blood purification methods. Protracted contact with the thrombogenic extracorporeal circuit and the need for protracted anticoagulation are considered the main drawbacks of CRRT.Citation[3]
Regional anticoagulation with heparin, whose effect is neutralized by protamine, is difficult to perform and, after heparin release from its complex with protamine, is associated with a risk for bleeding. An additional concern is protamine accumulation in acute renal failure. This technique is no longer recommended by the Consensus Conference on CRRT.Citation[1] The body of experience with low-molecular-weight heparins in CRRT is substantially smaller than with intermittent methods. The advantages of low-molecular-weight heparins over unfractionated heparin in CRRT are controversial.Citation[4] Use of prostacyclin as an antithrombotic in CRRT may be beneficial under certain circumstancesCitation[5]; however, it has failed to achieve widespread acceptance mainly because of problems related to hypotension and because of its high costs. The possibility to perform a procedure completely without anticoagulants and with regular extracorporeal circuit rinsing with saline—an approach used occasionally with success with intermittent methods—is limited with CRRT by the lower blood flow rate and long procedure times. Hirudin or other direct thrombin inhibitors can possibly be employed in sporadic cases.Citation[6] In the presence of an increased risk for bleeding, these agents should better be avoided.Citation[1] Nafamostat mesylate, just as in the case of intermittent procedures sometimes used in Japan,Citation[7] does not by far meet the criteria of an optimal antithrombotic for CRRT, if only because of the absence of an antidote or potential anaphylactic reactions.
Regional citrate anticoagulation (RCA) seems to be a reasonable alternative to heparin or other antithrombotics in bleeding patients or those at high risk of bleeding. The safety and efficacy of RCA are usually assessed using clinical criteria. The parameters assessed include bleeding and clot formation in the extracorporeal circuit with visual detection of blood clots, efficacy of procedures, and survival times of dialyzers/filters. In laboratory terms, the efficacy or safety of anticoagulation in CRRT was routinely assessed using clotting times or, possibly, ionized calcium levels.Citation[8–11] However, both clinical judgment and clotting times are not sensitive enough to furnish evidence of hemostasis activation caused by contact of blood with the thrombogenic surface. As a result, one may fail to notice changes predisposing both to blood clotting in the extracorporeal circuit and in the patients' circulation and to the development of bleeding complications while consuming thrombocytes and coagulation factors. Judging by recent studies, evaluation of anticoagulation efficacy by ionized calcium levels at dialyzer filter outlet is even less reliable than clotting times.Citation[12]
Although CRRT is a long- and well-established therapeutic method, the number of studies assessing thrombogenicity, hemostasis activation, and effect of anticoagulants in detail, is small.Citation[13–16] We are unaware of any such studies evaluating CRRT with RCA. This is surprising because it is just RCA that patients with impaired hemostasis are indicated for. These facts taken together made us conduct this study in an effort to assess thrombogenicity and hemostasis activation during RCA using sensitive and specific methods.
Citrate and heparin do not only affect hemostasis and thrombogenicity. They may interfere with other reactions occurring during interaction of blood with an artificial surface.Citation[17–20] It was for this reason that the behavior of two basic markers of biocompatibility (i.e., C5a as a marker of complement activation by an alternative pathway and blood leukocyte count) were evaluated in our study.
GROUPS AND METHODS
In our study, nine critically ill patients (APACHE II 24.4 ± 1.6, arithmetic mean ± SEM) with acute renal failure, aged 53.3 ± 4.6 years, had 11 continuous venovenous hemodiafiltration (CVVHDF) sessions using RCA. Another four critically ill patients with acute renal failure with similar APACHE II (27.0 ± 1.3, p = NS) and of similar age (67 ± 8.6 years, p = NS) underwent 10 CVVHDF procedures using systemic anticoagulation with unfractionated heparin. The decision whether RCA- or heparin-based CVVHDF would be performed was made by each patient's attending physician, depending on the clinical status of the former. Patients with active bleeding were scheduled for RCA, whereas those without bleeding were scheduled for heparin anticoagulation. Patients were treated at a medical ICU or a department of anesthesiology and resuscitation (both medical and surgical patients) of a university hospital. The study was approved by a local ethics committee. Patients were free of arterial or venous thrombosis and were not being treated by heparin or warfarin. A control group was made up by 11 healthy individuals aged 59.8 ± 4.4 years. Selected laboratory parameters, as determined immediately before the start of the 11 CVVHDF procedures with citrate, 10 procedures with heparin, and during a single investigation of the control group of healthy individuals, are shown in .
Table 1 Selected laboratory characteristics of patients immediately before CVVHDF with regional citrate anticoagulation (RCA), with systemic heparin anticoagulation (HA), and of healthy controls (HC)
CVVHDF procedures in RCA patients were performed using an Ultraflux AV600S hemodiafilter (Fresenius Medical Care, St. Wendel, Germany) featuring a high-flux polysulfone membrane with an area of 1.4 m2. All procedures with systemic heparinization were performed using a M100 hemodiafilter of a Prisma SET (Hospal Industrie, Meyzieu, France) fitted with a high-flux AN69 polyacrylonitrile membrane with an area of 0.9 m2. Prior to CVVHDF, the extracorporeal circuit was rinsed with 3 L of saline with unfractionated heparin at a concentration of 2000 IU/L at a rate 100 mL/min. This was a single-pass rinse. The rinsing solution was not infused into the patient's circulation. Venovenous access was obtained using a double-lumen 12F Arrow catheter (Arrow International, Inc., Reading, PA, USA). Hygiea (Kimal, Middlesex, United Kingdom) and Prisma (Hospal Dasco S.p.A., Medolla, Italy) monitors were used.
The blood flow rate during CVVHDF procedures was set at 150 mL/min. During RCA, substitution was achieved using a bicarbonate solution (bicarbonate concentration 18 mmol/L, sodium concentration 131 mmol/L, calcium concentration 0 mmol/L, chloride concentration 117 mmol/L) administered in postdilution mode at a rate of 1000 mL/h. A zero fluid balance was maintained in the first hour of the procedure. In the ensuing course, the degree of filtrate substitution depended on each patient's clinical state. The dialysis solution was of the same composition as the substitution solution. The dialysis solution was of room temperature and its flow rate was set at 2 L/h. For procedures with heparin-based anticoagulation, the substitution and dialysis solution was Medisol-K3 (Medites Pharma s.r.o., Roznov pod Radhostem, Czech Republic, sodium concentration 140 mmol/L, calcium concentration 1.75 mmol/L, magnesium concentration 1.00 mmol/L, chloride concentration 108.5 mmol/L, lactate concentration 40.0 mmol/L).
Study procedures were scheduled for 24 h. However, some patients had to undergo diagnostic or therapeutic procedures requiring earlier completion of CVVHDF procedures. As a result, CVVHDF procedures with RCA lasted 17.2 ± 2.2 h and those with heparin anticoagulation 19.4 ± 2.4 (p = NS) h.
No blood or blood products were given to patients during procedures.
During RCA, 2.2% trisodium citrate as ACD-A solution (Baxter Health Care, Deerfield, IL, USA) at an initial rate of 400 mL/h was infused into the CVVHDF extracorporeal circuit before the diafilter. Behind the diafilter, the effect of citrate was neutralized by infusion of 10% calcium gluconicum (Calcium Biotika, Hoechst-Biotika spol. s r. o., Martin, Slovakia) administered at an initial rate of 15 mL/h. Using a central venous catheter, patients were receiving 20% magnesium solution (Magnesium sulfuricum Biotika, Hoechst-Biotika spol. s r. o.) at a rate of 3 mL/h. Citrate and calcium dosing was adjusted by activated clotting time values (ACT; Hemochron 401, International Technidyne Corporation Limited, Edison, NJ, USA, with P214 test tubes). Every effort was made to maintain ACT in patients' systemic circulation unchanged and postdiafilter ACT levels at least 15% higher than the baseline value. During the procedure, the average citrate infusion rate was between 356.0 ± 62.7 and 389.6 ± 57.9 mL/h. The infusion rate of calcium solution varied between 13.4 ± 1.5 and 15.6 ± 2.7 mL/h.
Five minutes prior to being connected to the extracorporeal circuit, patients treated with CVVHDF using systemic heparinization received an intravenous bolus of unfractionated heparin from bovine lungs of the same lot (Heparin, Zentiva, Prague, Czech Republic) at a dose of 30 IU/kg body weight (bw). Upon connection, heparin infusion into the arterial line of the set was initiated at a rate 10 IU/kg bw/h. Converted to the bw of patients in the study, the bolus equaled 2700 ± 81.7 IU and continuous infusion 993.5 ± 82.5 IU/h. The dosing of heparin used was determined empirically.
The assessed laboratory parameters of thrombogenicity and biocompatibility included the thrombin-antithrombin III complexes (Enzygnost TAT micro, Behring Diagnostics GmbH, Marburg, Germany), beta-thromboglobulin (Asserachrom Beta-thromboglobulin, Diagnostica Stago, Asnieres-sur-Seine, France), D-dimers (TintElize D-dimer, Biopool International, Umeå, Sweden), activated partial thromboplastin times (aPTT) determined using a kefalin-kaolin reagent (EXBIO Olomouc s.r.o., Olomouc, Czech Republic), complement C5a component (Enzygnost C5a micro, Behring Diagnostics GmbH), and blood leukocyte and thrombocyte counts (SE 9000 autoanalyzer, Sysmex, Kobe, Japan). With RCA, the plasma levels of von Willebrand factors were also measured (IMUBIND vWF ELISA kit, American Diagnostica, Inc., Greenwich, CT, USA).
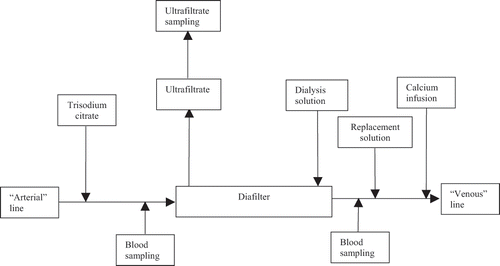
Thrombogenicity and biocompatibility markers were assessed prior to the start of procedures and anticoagulant administration in blood withdrawn from an arterial catheter in the arm. During CVVHDF, blood for investigations was obtained at diafilter outlet (with RCA still before calcium and substitution solution infusion) and at diafilter inlet (with RCA after citrate infusion). During RCA, ACT was additionally determined in the systemic arterial blood of patients. Complement C5a component, thrombin-antithrombin III complexes, beta-thromboglobulin, and D‐dimer were also analyzed in the filtrate (). For clinical purposes, other substances were determined in systemic blood samples obtained before and after CVVHDF. Only results of ionized calcium and bicarbonate measurement in patients with RCA are to be published.
Figure 1. Schematic representation of sampling sites during CVVHDF with regional citrate anticoagulation.
During CVVHDF with RCA, 23.8 ± 2.2 l of ultrafiltrate and, during CVVHDF with heparinization, 20.3 ± 2.0 (p = NS) l of ultrafiltrate were removed. Although the difference did not reach statistical significance, the levels of substances and cell counts as measured during CVVHDF were adjusted using hematocrit to exclude an effect of hemoconcentration or hemodilution. Statistical analysis was performed using SigmaStat for Windows 2.03 software (SPSS, Inc., Chicago, IL, USA). Depending on the result of the normality test and the equal variance test, either one-way ANOVA or the Kruskal-Wallis test was used to compare values measured in patients before CVVHDF with RCA, in those before CVVHDF with systemic heparinization, and in healthy individuals. If the previous methods demonstrated a significant difference, then ANOVA was complemented with the Tukey test and the Kruskal-Wallis test with Dunn's method. Preprocedural values were compared with those obtained during CVVHDF using the paired t test or Wilcoxon's test, again depending on the result of the normality test and the equal variance test. To compare changes in the thrombogenicity markers and in C5a occurring during RCA-based CVVHDF and heparin-based RCA, areas under the curve were calculated. The unpaired t test was used for their comparison.
RESULTS
Prior to RCA-based CVVHDF, patients showed impaired hemostasis with significant decreases in blood thrombocyte count and increases in the plasma levels of beta-thromboglobulin, thrombin-antithrombin III complexes, and D-dimer. Another marker assessed later during the course of CVVHDF, blood leukocyte count, showed a significant increase ().
With RCA, ACT served to bedside assessment of anticoagulation and its adjustment. Values measured in patients' systemic blood during CVVHDF did not differ significantly from those obtained at time 0 prior to the start of the procedure. The implication is that citrate had no anticoagulant effect outside the extracorporeal circuit. Values at diafilter outlet before calcium infusion were significantly prolonged at all collecting intervals, except for the end of the procedure. Effective anticoagulation in the extracorporeal circuit is also documented by aPTT, as determined after citrate infusion before the diafilter and before calcium infusion behind the diafilter. Measurement at 24 h at the end of the procedure is an exception ().
Table 2 ACT and aPTT during CVVHDF using regional citrate anticoagulation
Changes in thrombogenicity markers, plasma C5a levels, and blood leukocyte counts during RCA-based CVVHDF are summarized in . Blood thrombocyte counts, plasma levels of beta-thromboglobulin, thrombin-antithrombin III complexes, and von Willebrand factor did not differ significantly from baseline values in any collecting period. Compared with preprocedural levels, the plasma levels of D-dimer were significantly higher after 1 and 6 h of CVVHDF. There was a temporary significant decrease in blood leukocyte count at 15 min into the procedure. The plasma levels of the complement C5a component were significantly elevated at 15 and 60 min.
Table 3 Parameters of thrombogenicity, WBC count, and C5a during CVVHDF using regional citrate anticoagulation
Serum ionized calcium levels prior to and after CVVHDF with RCA did not change significantly (0.79 ± 0.08 mmol/L vs. 0.84 ± 0.10 mmol/L, p = 0.34). Bicarbonate levels likewise did not show significant changes (22.26 ± 1.45 mmol/L vs. 21.26 ± 1.57, p = 0.66).
Before connection to CVVHDF with heparin anticoagulation, just as with RCA-based CVVHDF, blood thrombocyte count was significantly decreased, while the plasma levels of beta-thromboglobulin and D-dimer were significantly increased as was blood leukocyte count compared with baseline. However, the decrease in blood thrombocyte count did not reach the magnitude seen with RCA anticoagulation, and TAT III levels did not differ from those of healthy individuals ().
In heparin-based CVVHDF, aPTT values at diafilter inlet were significantly prolonged at all collecting periods compared with preprocedural values. Significantly prolonged aPTT was also measured in diafilter outlet blood returning to the patients' circulation ().
Table 4 aPTT during CVVHDF using systemic heparin anticoagulation
In CVVHDF with heparin anticoagulation, no significant decrease in thrombocyte count or increase in plasma concentrations of other thrombogenicity markers indicating hemostasis activation was demonstrated. Statistically significant changes in CVVHDF with heparin anticoagulation included a fall in the plasma levels of beta-thromboglobulin at 60 min, D-dimer at 360 and 1440 min, and, finally, an increase in plasma C5a levels 15 min into the procedure ().
Table 5 Parameters of thrombogenicity, WBC count, and C5a during CVVHDF using systemic heparin anticoagulation
The substances being assessed were not detectable in ultrafiltrate, or, if present, their levels must have been lower by several orders compared with the plasma.
Areas under the curve were used to compare the plasma levels of markers of thrombogenicity and/or biocompatibility and aPTT between procedures with RCA and heparin anticoagulation. A significant difference was only seen in the areas under the curve for aPTT. The difference in the areas under the curve for beta thromboglobulin, thrombin-antithrombin III complexes, D-dimer, or C5a did not reach statistical significance ().
Table 6 AUCs for some biocompatibility parameters during CVVHDF with regional citrate anticoagulation (RCA) and systemic heparin anticoagulation (HA)
DISCUSSION
During RCA, citrate administration at the start of the extracorporeal circuit binds calcium necessary at several levels of the process of blood coagulation. As a result, the blood running through the extracorporeal circuit used in blood purification methods shows a reduced ability to coagulate. Calcium infusion at the end of the extracorporeal circuit eliminates the effect of citrate, whose complexes with calcium are also partly removed from the blood. Ideally, the blood returning to the patient's circulation is one with a fully restored capacity to coagulate. Compared with heparinization, RCA is technically more demanding, laborintensive, and associated with a risk for acute metabolic complications. Therefore, special monitors are being developed to simplify RCA while warranting patient's safety.Citation[21] When developing such monitors, utmost attention is given primarily to maintaining appropriate ionized calcium levels because its fluctuations may even result in fatal arrhythmias.Citation[22]
Clinical experience shows this technique of anticoagulation can indeed work locally in the extracorporeal circuit and, in terms of bleeding complications, is a safer alternative to systemic heparinization.Citation[23],Citation[24] Using clinical criteria (visible clots, extracorporeal circuit pressures, dialyzer/filter survival), most studies also demonstrate that clotting within the extracorporeal circuit is equal to or less intense than that seen with other techniques of anticoagulation.Citation[11],Citation[23],Citation[25],Citation[26] In contrast, some studies have reported shorter dialyzer/filter survival rates with RCA compared with heparinization and, consequently, higher rates of coagulation.Citation[27] A comparison and explanation of the differences is made impossible by the fact the studies were not designed to exactly assess thrombogenicity, hemostasis activation, and the degree of overcoming these phenomena by antithrombotics administration.
Thrombogenicity, changes in hemostasis, whether predisposing to thrombosis or bleeding, can only be diagnosed using laboratory methods. Considering the importance of hemostasis impairment, thrombogenicity, and complications related to antithrombotics use in CRRT-treated patients, it is surprising that the previous three factors have to date been only minimally assessed with exact laboratory methods. If any such data are available, they were actually derived from studies with heparin anticoagulation.Citation[13–16] In fact, the only laboratory parameter used to assess hemostasis with RCA in CRRT has to date been clotting times. These tests show reliably whether anticoagulation is not extremely effective with the attending risk of bleeding. However, these tests are inadequately, if at all, sensitive to provide evidence of hemostasis activation on contact of blood with an artificial thrombogenic surface. At the same time, hemostasis activation due to artificial surface thrombogenicity is potentially associated with serious undesirable effects. It is associated with the risk for thrombosis in the extracorporeal circuit and in the patients' circulation. Hemostasis activation may result in the induction or enhancement of consumption coagulopathy with subsequent bleeding. Our study furnished some of the lacking information on hemostasis activation during RCA in CRRT.
The examinations prior to CVVHDF with RCA have shown that patients undergoing therapy have comprehensive hemostasis impairment. Pre-CVVHDF examinations have suggested thrombocyte activation (a decrease in thrombocyte count associated with a rise in the plasma levels of beta-thromboglobulin), coagulation activation (increase in plasma levels of thrombin-antithrombin III complexes), and fibrinolysis activation (increase in D‐dimer levels). The plasma levels of beta-thromboglobulin increase as renal function decreases. However, beta-thromboglobulin levels in our group of patients did not correlate with creatinine, making the role played by thrombocyte activation on their increase self-evident (n = 11, Pearson's correlation coefficient r = −0.20; p = 0.56).
Judging by clotting times, citrate anticoagulation was truly regional and confined to the extracorporeal circuit. The procedure was not associated with a decrease in thrombocyte count or a rise in beta-thromboglobulin suggesting further platelet activation, and there was no rise in thrombin-antithrombin III complex levels suggesting coagulation activation, or in von Willebrand factor levels documenting stimulation of the endothelium and/or thrombocytes. The behavior of the previous markers was not biased by their removal into the diafiltrate because none of the markers were shown to be present in the diafiltrate in detectable amounts. The only significant change in the course of CVVHDF with RCA would be a transient rise in plasma D-dimer levels. No simple explanation for the rise has been suggested. The rise is not due to blood flow through the dialfilter as diafilter inlet and outlet D-dimer levels did not differ significantly at any of the collecting periods (data not shown). In the absence of other hemostasis changes during CVVHDF, we think the increase in D‐dimers should not be perceived as an adverse effect of the procedure.
Prior to CVVHDF with systemic heparin anticoagulation, patients also showed impaired hemostasis. However, it was less marked compared with patients due to receive CVVHDF with RCA. Patients undergoing CVVHDF with heparin anticoagulation did not show increased levels of thrombin-antithrombin III complexes, a sensitive marker of coagulation activation. Thrombocytopenia was also absent; however, thrombocyte activation was indicated by high levels of beta-thromboglobulin. The levels of beta-thromboglobulin, just as with RCA, did not correlate with serum creatinine (n = 10; r = 0.16; p = 0.655) and cannot be explained by decreased renal function.
The levels of aPTT with heparin anticoagulation suggested effective anticoagulation, although one set too high. Heparin doses were chosen empirically and, in the absence of clinical problems with bleeding of patients and clotting in the extracorporeal circuit, the levels were no further adjusted at bedside. Under these conditions, no manifestations of thrombocyte, coagulation, or fibrinolysis activation were observed. In their earlier study using heparinization, Stefanidis et al. were also able to prevent coagulation activation during CRRT; however, thrombocyte activation was enhanced.Citation[15] Another study reported elevated levels of thrombin-antithrombin III complexes and, hence, increased coagulation system activation despite heparin administration.Citation[16] It is possible that we prevented hemostasis activation by “overshooting” heparinization. This is, however, merely speculation as neither of the two previous studies with evidence of coagulation or thrombocyte activation provided a clear definition of the level of heparinization. A role in the significantly reduced plasma levels of beta-thromboglobulin during CVVHDF with heparin, seen in our study, may be played by beta-thromboglobulin adsorption to the polyacrylonitrile membrane.Citation[28] The decrease in D-dimer was just another major alteration. This cannot be explained by D-dimer elimination during the procedure because its diafiltrate levels were lower by orders compared with its plasma levels, and no significant difference between inlet and outlet D-dimer levels was seen. An association of this change with CVVHDF is problematic.
We sought to compare thrombogenicity and hemostasis activation in CVVHDF with either RCA or systemic heparinization. The comparison is fraught with some methodologic problems, which have to be discussed. Selection of patients for RCA or heparinization was not made at random, giving preference to the clinical criterion. Bleeding patients were treated with RCA, a method considered to be safer. Laboratory investigations in these patients subsequently revealed more appreciable preprocedural hemostasis activation. Despite this, hemostasis was no longer activated during procedures with RCA just as with heparinization. This finding highlights the appropriateness of RCA in the critically ill patient. Furthermore, it should be noted that procedures with RCA were undertaken with polysulfone membranes, whereas polyacrylonitrile membranes were used for procedures involving heparin anticoagulation. The question is whether both membrane materials differ in their thrombogenicity. This issue was addressed by some studies involving intermittent procedures and using sensitive and specific tests. The results were inconsistent.Citation[29],Citation[30] A study was also designed to determine, under conditions of CRRT, whether the hemofilter using the polysulfone or polyacrylonitrile membrane clots earlier.Citation[31] No significant difference was found. Hence, no clear-cut evidence of a marked difference in the thrombogenicity of both materials is currently available. The area of the polysulfone membrane employed for CVVHDF with RCA was bigger than that of the polyacrylonitrile membrane used for heparin anticoagulation. A bigger area clearly entails more interaction with blood and, consequently, more thrombogenic action. Although the membrane area with RCA was bigger than with heparin anticoagulation, the values of thrombogenicity markers (except for aPTT) were comparable. This is an additional finding supporting the use of RCA. Finally, the levels of anticoagulation were different with RCA and heparin anticoagulation. However, a completely opposite perspective is also conceivable. Both RCA and heparin anticoagulation were associated with comparable coagulation activation (comparable area under the curve for TATIII), thrombocyte activation (beta-thromboglobulin), and fibrinolysis activation (D-dimer). However, hemostasis activation was only comparable due to the fact that aPTT with heparin anticoagulation was substantially much longer than the aPTT with RCA. In addition, the effect of heparin had not been reversed before returning blood to the patient, whereas citrate action was regional only. Van de Wetering et al. showed aPTT values as low as 45 to 55 s are associated with a triple risk for bleeding.Citation[32] In our patients receiving heparin anticoagulation, aPTT were much higher.
There have been reports about a beneficial effect of citrate anticoagulation in intermittent procedures on manifestations of bio(in)compatibility such as complement activation, leukopenia, or cytokine production.Citation[33],Citation[34] The beneficial effect on complement activation and transient leukopenia was observed when using a Cuprophan dialysis membrane (i.e., a potent complement activator).Citation[33],Citation[35] When a polymethylmethacrylate membrane, which is a weaker complement activator, was tested in the same study, the beneficial effect of citrate was not demonstrated.Citation[33] When using yet another membrane, an even weaker complement activator compared with Cuprophan (i.e., the cellulose acetate membrane) no difference in the effects of citrate versus heparin anticoagulation on complement activation was likewise seen.Citation[19] The complexity of the problem is attested to by the fact that a study has been published whereby complement activation during intermittent hemodialysis with RCA was higher as against heparinization with polysulfone membrane (i.e., a relatively weak complement activator).Citation[34]
In our study, postdiafilter plasma C5a levels were significantly increased at the beginning of heparin anticoagulation-based procedures. The procedures were not associated with a decrease in blood leukocyte counts. Both findings are consistent with those reported when using the AN69 membrane for intermittent procedures with heparinizationCitation[36] and with the ability of the AN69 to adsorb complement activation products.Citation[37] A mild, yet significant, decrease in leukocyte count seen at the beginning of CVVHDF with RCA and polysulfone membrane is well known from intermittent procedures with heparin. It is typical for interaction of blood with the polysulfone membrane.Citation[38] The same applies to the transient and mild increase in C5a levels at diafilter outlet. It follows from this that citrate, at least at concentrations sufficient for effective coagulation, does not have the capacity to completely prevent complement activation and transient leukopenia during CVVHDF with a membrane, which is a weak complement activator.
The main message of this study is that RCA can be guided to overcome, in the presence of unaltered clotting times in patients' blood, the thrombogenicity of the man-made surface and avoid hemostasis activation on the extracorporeal circuit. This finding was made in critically ill patients with severely impaired hemostasis—in terms of its activation—before the start of CVVHDF. Hemostasis activation was successfully prevented throughout the long course of the continuous procedure. Moreover, CVVHDF procedures were performed in postdilution mode, which is associated with higher risks in terms of clotting compared with the predilution arrangement.Citation[39] A similar effect in CVVHDF with systemic heparinization can only be achieved at the expense of increased risk of bleeding. Results support the notion of RCA safety in terms of both bleeding and thrombotic complications.
Another finding made from the study is that, during RCA, blood leukocyte count and complement C5a component behave the same as reported with intermittent procedures with heparin. In this particular case, the determining factor seems to be the membrane, not the technique, of anticoagulation.
ACKNOWLEDGMENTS
The study was supported by Research Projects No. 111400002 and No. MSM 0021620819 “Replacement of and support to some vital organs”.
REFERENCES
- Kellum JA, Mehta RL, Angus DC, Palevsky P, Ronco C. The first international consensus conference on continuous renal replacement therapy. Kidney Int 2002; 62: 1855–1863, [PUBMED], [INFOTRIEVE]
- Ronco C, Zanella M, Brendolan A. Management of severe acute renal failure in critically ill patients: an international survey in 345 centres. Nephrol Dial Transplant 2001; 16: 230–237, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Manns M, Sigler MH, Teehan BP. Continuous renal replacement therapies: an update. Am J Kidney Dis 1998; 2: 185–207
- Reeves JH, Graan M. Randomized controlled trial enoxaparin versus heparin in continuous renal replacement therapy. Blood Purif 2003; 21: 183–207, [CROSSREF]
- Davenport A, Will EJ, Davison AM. Comparison of the use of standard heparin and prostacyclin anticoagulation in spontaneous and pump-driven extracorporeal circuits in patients with combined acute renal and hepatic failure. Nephron 1994; 66(4)431–437, [PUBMED], [INFOTRIEVE]
- Schneider T, Heuer B, Deller A, Boesken WH. Continuous hemofiltration with r-hirudin (lepirudin) as anticoagulant in a patient with heparin induced thrombocytopenia (HIT II). Wien Klin Wochenschr 2000; 112(12)552–555, [PUBMED], [INFOTRIEVE]
- Ohtake Y, Hirasawa H, Sugai T, et al. Nafamostat mesylate as anticoagulant in continuous hemofiltration and continuous hemodiafiltration. Contrib Nephrol 1991; 93: 215–217, [PUBMED], [INFOTRIEVE]
- Cointault O, Kamar N, Bories P, et al. Regional citrate anticoagulation in continuous venovenous hemodiafiltration using commercial solutions. Nephrol Dial Transplant 2004; 19: 171–178, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Tolwani AJ, Campbell RC, Schenk MB, Allon M, Warnock DG. Simplified citrate anticoagulation for continuous renal replacement therapy. Kidney Int 2001; 60: 370–374, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Swartz R, Pasko D, O'Toole J, Starmann B. Improving the delivery of continuous renal replacement therapy using regional citrate anticoagulation. Clin Nephrol 2004; 61(2)134–143, [PUBMED], [INFOTRIEVE]
- Kutsogiannis JD, Mayers I, Nin Chin WD, Gibney N. Regional citrate anticoagulatinon in continuous venovenous hemodiafiltration. Am J Kidney Dis 2000; 35: 802–811, [PUBMED], [INFOTRIEVE]
- Zemanová P, Opatrný K, Jr, Polanská K, et al. Is it appropriate to tune regional citrate anticoagulation during hemodialysis by ionized calcium levels?. Blood Purif 2005; 23: 407
- Opatrný K, Jr, Polanská K, Kroužecký A, Vít L, Novák I, Kasal E. The effect of heparin rinse on the biocompatibility of continuous veno-venous hemodiafiltration. Int J Artif Organs 2002; 25(6)520–528
- Salmon J, Cardigan R, Mackie I, Cohen SL, Machin S, Singer M. Continuous venovenous hemofiltration using polyacrylonitrile filters does not activate contact system and intrinsic coagulation pathways. Intensive Care Med 1997; 23: 38–43, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Stefanidis I, Hägel J, Kierdorf H, Maurin N. Influencing hemostasis during continuous venovenous hemofiltration after acute renal failure: comparison with intermittent hemodialysis. Contrib Nephrol 1995; 116: 140–144, [PUBMED], [INFOTRIEVE]
- Cardigan RA, McGloin H, Mackie IJ, Machin J, Singer M. Activation of the tissue factor pathway occurs during continuous venovenous hemofiltration. Kidney Int 1999; 55: 1568–1574, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Cheung AK, Faezi-Jenkin B, Leypoldt JK. Effect of thrombosis on complement activation and neutrophil degranulation during in vitro hemodialysis. J Am Soc Nephrol 1994; 5(1)110–115, [PUBMED], [INFOTRIEVE]
- Tayama E, Hayashida N, Akasu K, et al. Biocompatibility of heparin-coated extracorporeal bypass circuits: new heparin bonded bioline system. Int J Artif Organs 2000; 24: 618–623, [CROSSREF]
- Bos JC, Grooteman MPC, van Houte AJ, Schoorl M, van Limbeek J, Nubé MJ. Low polymorphonuclear cell degranulation during citrate anticoagulation: a comparison between citrate and heparin dialysis. Nephrol Dial Transplant 1997; 12: 1387–1393, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Plötz FB, van Oeveren W, Hultquist KA, Miller C, Bartlett RH, Wildevuur CRH. A heparin-coated circuit reduces complement activation and the release of leukocyte inflammatory mediators during extracorporeal circulation in a rabbit. Int J Artif Organs 1992; 16: 366–370
- Schrefl A, Kellner K-H, Hartmann J, Strobl W, Falkenhagen D. A novel monitor for anticoagulation with citrate. Int J Artif Organs 2001; 24: 535
- Charney DI, Salmond R. Cardiac arrest after hypertonic citrate anticoagulation for chronic hemodialysis. ASAIO Trans 1990; 36: M217–M219, [PUBMED], [INFOTRIEVE]
- Mehta RL, McDonald BR, Aguilar MM, Ward DM. Regional citrate anticoagulation for continuous arteriovenous hemodialysis in critically ill patients. Kidney Int 1990; 38: 976–981, [PUBMED], [INFOTRIEVE]
- Monchi M, Berghmans D, Ledoux D, Canivet JL, Dubois B, Damas P. Citrate vs heparin for anticoagulation in continuous venovenous hemofiltration: a prospective randomized study. Intensive Care Med 2004; 30(2)260–265, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Kutsogiannis DJ, Gibney RTN, Stollery D, Gao J. Regional citrate versus systemic heparin anticoagulation for continuous renal replacement in critically ill patients. Kidney Int 2005; 67: 2361–2367, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Tobe SW, Aujla P, Walele AA, et al. A novel regional citrate anticoagulation protocol for CRRT using only commercially available solutions. J Crit Care 2003; 18: 121–129, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Gabutti L, Marone C, Colucci G, Duchini F, Schönholzer C. Citrate anticoagulation in continuous venovenous hemodiafiltration: a metabolic challenge. Intensive Care Med 2002; 28: 1419–1425, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Opatrný K, Jr, Vít L, Opatrná S, et al. Hemocompatibility in hemodialysis and erythropoietin therapy. Artif Organs 1995; 19(8)814–820
- Frank RD, Weber J, Dresbach H, Thelen H, Weiss C, Floege J. Role of contact system activation in hemodialyzer-induced thrombogenicity. Kidney Int 2001; 60: 1972–1981, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Ivanovich P, Hammerschmidt DE, Quintanilla A, et al. Behaviour of platelets and beta-thromboglobulin. Nephrol Dial Transplant 1993; 8(suppl 2)15–19, [PUBMED], [INFOTRIEVE]
- Silvester W, Honoré P, Sieffert E, et al. Interleukins 6 and 8, tumour necrosis factor alpha and complement D clearance by polyacrylonitrile and polysulphone membranes during hemofiltration in critically ill patients. Presented at: Continuous Hemofiltration in Intensive Care Meeting, Paris, 1996
- van de Wetering J, Westendorp RG, van der Hoeven JG, Stolk B, Feuth JD, Chang PC. Heparin use in continuous renal replacement procedures: the struggle between filter coagulation and patient hemorrhage. J Am Soc Nephrol 1996; 7: 145–150, [PUBMED], [INFOTRIEVE]
- Bohler J, Schollmeyer P, Dressel B, Dobos G, Horl WH. Reduction of granulocyte activation during hemodialysis with regional citrate anticoagulation: dissociation of complement activation and neutropenia from neutrophil degranulation. J Am Soc Nephrol 1996; 7: 234–241, [PUBMED], [INFOTRIEVE]
- Gabutti L, Ferrari N, Mombelli G, Keller F, Marone C. The favourable effect of regional citrate anticoagulation on interleukin-1beta release is dissociated from both coagulation and complement activation. J Nephrol 2004; 17: 819–825, [PUBMED], [INFOTRIEVE]
- Wiegmann TB, McDougall ML, Diederich DA. Dialysis leukopenia, hypoxemia, and anaphylatoxin formation: effect of membrane, bath, and citrate anticoagulation. Am J Kidney Dis 1988; 11: 418–424, [PUBMED], [INFOTRIEVE]
- Wegmuller E, Montandon A, Nydegger U, Descoeudres C. Biocompatibility of different hemodialysis membranes: activation of complement and leukopenia. Int J Artif Organs 1986; 9: 85–92, [PUBMED], [INFOTRIEVE]
- Cheung AK, Parker CJ, Wilcox LA, Janatova J. Activation of complement by hemodialysis membranes: polyacrylonitrile binds more C3a than cuprophan. Kidney Int 1990; 37: 1055–1059, [PUBMED], [INFOTRIEVE]
- Hoenich NA, Woffindin C, Brennan A, Cox PJ, Matthews JN, Goldfinch M. A comparison of three brands of polysulfone membranes. J Am Soc Nephrol 1996; 7: 871–876, [PUBMED], [INFOTRIEVE]
- Davenport A. The coagulation system in the critically ill patient with acute renal failure and the effect of an extracorporeal circuit. Am J Kidney Dis 1997; 30(5, suppl. 4)S20–S27, [PUBMED], [INFOTRIEVE]