Abstract
Hypercalcemia can result from excessive bone resorption, renal calcium retention, excessive intestinal calcium absorption, or a combination of these conditions. Hypercalcemia may also provoke acute renal failure (ARF) or hypertension, or aggravate the tubular necrosis that is frequently found in cases of ARF. The association of ARF and hypercalcemia was studied retrospectively in eight patients based in the data in their charts. Data are expressed as median and percentile (25th; 75th). Our results show that ARF associated with hypercalcemia was related with comorbidity in all cases (cancer, multiple myeloma, hyperparathyroidism, sarcoidosis, vitamin D intoxication, and leprosy). Maximum median serum creatinine levels were 3.3 mg/dL (2.7, 3.8 mg/dL) before treatment and 1.1 mg/dL (0.9, 1.3 mg/dL) after treatment. Maximum total median serum calcium was 15.9 mg/dL (13.5, 19.8 mg/dL) before treatment and 9.1 mg/dL (8.4, 9.7 mg/dL) after treatment. Maximum median ionized serum calcium was 2.1 mmol/L (1.8, 2.2 mmol/L) before treatment and 1.1 mmol/L (1.0, 1.2 mmol/L) after treatment. Different kinds of treatment induced a rapid fall in serum calcium concentration. All patients were treated with hydration and diuretics, and three patients also received calcitonin. Serum creatinine concentration always fell simultaneously with the decrease in serum calcium in all cases. All patients progressed with nonoliguric renal failure. In conclusion, in ARF, patients are frequently hypocalcemic. Usually, the presence of hypercalcemia associated with ARF is indicative of the presence of comorbidity, as observed in all eight patients studied here. There was an improvement of renal function in all cases as serum calcium levels decreased.
INTRODUCTION
Calcium ion is an essential regulator of many body processes, including muscle contraction, many secretory mechanisms, and neuronal excitation. Plasma calcium levels are regulated by the action of parathyroid hormone (PTH) on bone reabsorption and renal calcium excretion, with dietary calcium being provided through the action of 1.25-dihydroxycholecalciferol on the intestine. Hypercalcemia can result from excessive bone resorption, renal retention of calcium, excessive intestinal absorption, or a combination of these conditions.[Citation[1]]
In acute renal failure (ARF), patients are frequently hypocalcemic, showing a decrease in two of the three major calcium fractions (ionized calcium and total plasma calcium). Hypercalcemia may reflect the presence of other morbidities. Hypercalcemia may also provoke hypertension, intensify renal vascular contraction, and aggravate the tubular necrosis frequently found in cases of ARF.[Citation[2],Citation[3]]
The list of causes of hypercalcemia is long, the most common being primary hyperparathyroidism and cancer (with and without metastases). Other causes of hypercalcemia are sarcoidosis, tuberculosis and other granulomatous disorders, milk-alkali syndrome, vitamin D and vitamin A intoxication, immobilization, medications, and the recovery phase of rhabdomyolysis.[Citation[1],Citation[4–7]]
This study evaluates eight patients with ARF associated with hypercalcemia and the relation of the latter to causes and outcome after treatment.
METHODS
From January 2002 to June 2004, eight patients (five men and three women) with hypercalcemia and renal failure were studied retrospectively based on the data in their charts at the University Hospital of the Faculty of Medicine of Ribeirão Preto, University of São Paulo. The presence of ARF was defined by an increase above 2.0 mg/dL of previously normal creatinine levels. Serum creatinine concentration was measured by Jaffé‘s reaction (Dimension RXL, DADE Behring, Newark, DE, USA). Total serum calcium concentration (normal range 8.4–10.4 mg/dL) was measured by colorimetric method (Dimension RXL), and ionized serum calcium (normal range 1.12–1.32 mmol/L) by selective electrode (RapidLab 860, East Walpole, MA, USA). Serum PTH (normal range 10–69 pg/mL) was measured by chemiluminescence immunoassay (Immulite DPC, USA). Serum 1.25 dihydroxycholecalciferol (1.25 DHCC; normal level ranges from 15 to 60 pg/mL) was measured by chromatography and radioreceptor assay, and parathyroid hormone-related protein (normal value lower than 1.35 pmol/L) was measured by immunoradiometric assay, both at the Nichols Institute (San Juan Capistrano, CA, USA). Data are expressed as median and percentile (25th; 75th).
RESULTS
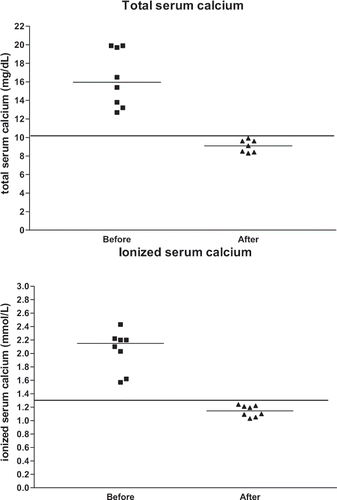
shows the clinical data of all eight patients (age range: 28–85 years, median: 61 years, 41.5, 71.0), and shows the serum creatinine levels and serum calcium levels of the same patients. and illustrate the serum creatinine levels and serum calcium levels, respectively, before and after treatment of hypercalcemia. Maximum median serum creatinine levels were 3.3 mg/dL (2.7, 3.8 mg/dL) before treatment and 1.1 mg/dL (0.9, 1.3 mg/dL) after treatment. Maximum median total serum calcium was 15.9 mg/dL (13.5, 19.8 mg/dL) before treatment and 9.1 mg/dL (8.4, 9.7 mg/dL) after treatment. Maximum median ionized serum calcium was 2.1 mmol/L (1.8, 2.2 mmol/L) before treatment and 1.1 mmol/L (1.0, 1.2 mmol/L) after treatment.
Table 1 Age, gender, diagnosis and treatment of eight patients with hypercalcemia and renal failure
Table 2 Maximum creatinine and ionized and total calcium values before and after treatment
Figure 1. Serum creatinine levels before and after treatment of hypercalcemia. The horizontal lines represent the medians. The line drawn for creatinine levels represents the upper limit of normal serum creatinine levels (1.3 mg/dL).
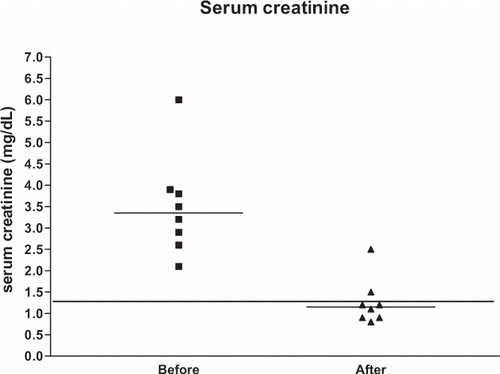
Figure 2. Total serum calcium levels (top) and ionized serum calcium levels (bottom) before and after treatment of hypercalcemia. The horizontal lines represent the medians. The lines drawn for total and ionized serum calcium represent the upper limit of normal laboratory levels (10.1 mg/dL and 1.32 mmol/L, respectively).
One of the two patients with multiple myeloma (patient 1) presented with anorexia and vomiting, and died 10 days after diagnosis and 7 days after the beginning of chemotherapy. The second patient (patient 2) had mental confusion at hospital admission, was also submitted to chemotherapy, and died 1 year later from the disease. In both cases, renal function as measured by serum creatinine improved with decreasing serum calcium levels. In the first patient, creatinine had decreased to 2.5 mg/dL when the patient died.
One of the two patients with cancer (patient 3) had a non-Hodgkin's lymphoma, and the other (patient 4) had a mediastinal mass due to an undifferentiated tumor. Both patients were submitted to chemotherapy and died because of infectious complications. ARF as expressed by increased creatinine levels improved with decreasing serum calcium levels.
One patient with hyperparathyroidism (patient 5) had a PTH level of 935 pg/mL, and was admitted to the hospital with edema, dyspnea, and atrial fibrillation. Renal function improved with decreasing serum calcium after treatment with hydration and diuretics. The patient died 30 days later because of infectious complications, and the diagnosis of hyperparathyroidism was confirmed at autopsy.
Patient 6 came to the outpatient clinic presenting with weakness, anorexia, myalgia, progressive renal failure, proteinuria of 1.5 g/24 h, hypercalcemia, and increased creatinine levels. A renal biopsy led to a diagnosis of sarcoidosis. Hypercalcemia and renal failure responded well to corticosteroid treatment (prednisone 1 mg/kg/day).
One patient had vitamin D intoxication (patient 7). She had been taking this medication for the past 2 years due to osteoporosis. PTH level was 4.3 pg/mL. Renal function and calcium serum levels simultaneously improved after vitamin D was discontinued.
One patient (patient 8) came to the hospital with a history of nephrolithiasis of 1 year duration. He presented epigastric pain, hypercalcemia, and renal failure. The laboratory findings showed PTH of 5.3 pg/mL, 1.25 DHCC of 46.0 pg/mL, and PTH-related protein lower than 0.7 pmol/L. The patient was submitted to a lymphonode biopsy that revealed atypical mycobacteriosis. This finding and a skin biopsy led to the diagnosis of leprosy, lepromatous type.
All patients progressed with nonoliguric ARF, and the median time for renal function recovery was 10.5 days (9.0, 11.5).
DISCUSSION
In the cases reported in this investigation, hypercalcemia associated with cancer was more prevalent, as observed in one case of non-Hodgkin's lymphoma, one case of a poorly differentiated tumor, and two cases of multiple myeloma. In the other four cases, hypercalcemia was associated with hyperparathyroidism, with vitamin D intoxication, with sarcoidosis, and with leprosy (one case each).
The most common causes of hypercalcemia described in the literature are malignant tumors, primary hyperparathyroidism, immobilization, ingestion of vitamin A or D, thiazide diuretics, granulomatous diseases, chronic renal failure, milk-alkali syndrome, Addison's disease, familial hypocalciuric hypercalcemia, and intrinsic bone disease (Paget's disease).[Citation[7]] The most common cause of hypercalcemia in hospitalized patients is cancer,[Citation[8]] especially pulmonary squamous cell carcinoma and hematologic tumors such as Hodgkin's disease, B and T cell lymphomas, and bone metastases.[Citation[1],Citation[7–10]] In the two patients with hypercalcemia associated with cancer, ARF improved after a reduction in serum calcium levels. The isolation of secreted PTH-related protein from several tumor types explains many, but not all, cases or humoral hypercalcemia of malignancy.[Citation[7]] Tumors that have been regularly associated with the production of PTH-related protein are bronchogenic non-small cell carcinoma, breast cancer, squamous cell carcinoma of the esophagus, and T- or B-cell lymphoma. Hematologic cancers produce PTH-related protein less frequently.[Citation[7]]
Hypercalcemia frequently occurs in association with multiple myeloma.[Citation[1],Citation[6],Citation[11]] Renal damage appears to be more likely in those with light chain and IgD myeloma.[Citation[6]] A study on 56 patients with myeloma and severe renal failure[Citation[6]] identified a potential precipitant of renal failure in 43% of the cases, usually hypercalcemia or a nonsteroidal anti-inflammatory agent. Survival in myeloma is principally determined by response to chemotherapy and acquisition of a stable plateau phase.[Citation[6]] In these two cases, renal function improved with decreasing serum calcium levels. One patient died before his serum creatinine levels could reach normal levels.
Primary hyperparathyroidism is the most common cause of hypercalcemia in the community.[Citation[1]] It is caused by a single adenoma in about 85% of cases, with hyperplasia of all four parathyroids in 10% to 15% and with carcinoma in less than 2%.[Citation[1]] Sometimes, hypercalcemia can cause decrease of glomerular filtration in these patients, provoking ARF, which is reversible when calcemia is normalized.[Citation[1],Citation[4]] In the case of hyperparathyroidism described here, glomerular filtration improved, as shown by a decrease in serum creatinine with decreasing calcemia.
In patients with otherwise unexplained hypercalcemia, renal impairment in conjunction with suppressed plasma PTH levels should raise the possibility of self- medication with antacid preparations containing calcium and alkali, the so-called and now outdated term “milk-alkali syndrome”.[Citation[10],Citation[12],Citation[13]] Vitamin D toxicity is an uncommon but well-recognized cause of hypercalcemia and also shows suppressed plasma PTH levels. In these cases, the hypercalcemia is mediated by increased bone reabsorption, and bisphosphonates have a role in its management.[Citation[14]] Sometimes, renal failure and toxic acute tubular necrosis may be seen after the use of a highly potent bisphosphonate zoledronate employed in the treatment of hypercalcemia of malignancy.[Citation[15]] In the case described here, there was a complete reversal of the calcemia and renal function alterations after discontinuation of vitamin D treatment.
Sarcoidosis has been associated with a wide spectrum of renal manifestations, including disordered calcium metabolism, nephrocalcinosis, nephrolithiasis, granulomatous interstitial nephritis, and glomerulonephritis.[Citation[16],Citation[17]] Hypercalcemia occurs in up to 10% to 15% of patients, causing abnormal glomerular or tubular function, nephrocalcinosis or nephrolithiasis in 10% of cases.[Citation[17]] Macrophages within sarcoid granulomas contain the enzyme 1-alpha hydroxylase that is responsible for activating vitamin D to form excess levels of calcitriol, which subsequently cause hypercalcemia.[Citation[16],Citation[17]] The capacity of macrophages to synthesize calcitriol is not unique to sarcoidosis but has been shown to occur in other granulomatous disorders associated with hypercalcemia, including tuberculosis, leprosy, plasma cell granuloma, berylliosis, certain fungal infections, Wegener's granulomatosis, lymphoma, and extensive foreign body formation.[Citation[9],Citation[16]] Glucocorticoids are the mainstay of therapy for more severe hypercalcemia, blocking calcitriol synthesis by directly inhibiting macrophage 1 alpha-hydroxylase activity and by suppressing the immune activation of macrophages.[Citation[16],Citation[17]] In the case described here, ARF improved with decreasing serum calcium levels after corticosteroid treatment.
Leprosy is another of the granulomatous diseases recognized to be associated with hypercalcemia,[Citation[18–20]] but there are few reports of such cases, probably because serum calcium may not be routinely assessed in patients with leprosy.[Citation[20]] Serum PTH concentrations are suppressed and abnormal calcitriol levels may play a role in the pathogenesis of hypercalcemia in some patients with leprosy.[Citation[20]] In the case described in this investigation, PTH was suppressed and calcitriol levels were normal. There was improvement of the calcium and renal function alterations after treatment with calcitonin, general measures, and specific treatment for leprosy.
The clinical features of hypercalcemia are the same regardless of the etiology. Hypercalcemia can present with symptoms observed in many organ systems: dehydration, nausea, anorexia, vomiting, abdominal pain, and mental confusion, as presented by some of our patients.[Citation[1]] Renal insufficiency in the context of hypercalcemia can arise via several mechanisms such as prerenal involvement, direct alterations of intravascular tone, and glomerular permeability.[Citation[2],Citation[20]] An increase in renal vascular resistance with a reduction in renal blood flow appears to result from direct vasoconstrictive effects of excess calcium ion on arteriolar smooth muscle.[Citation[2]] As a consequence, the following renal effects of hypercalcemia may occur: arteriolar vasoconstriction, reduction in the glomerular ultrafiltration coefficient (Kf), reduction in tubular sodium reabsorption, nephrogenic diabetes insipidus, prerenal azotemia, acute tubular necrosis, nephrocalcinosis, and tubulointerstititial fibrosis.[Citation[2],Citation[4],Citation[21]]
A fall in the concentration of total serum calcium is noted in patients with ARF.[Citation[22],Citation[23]] Skeletal resistance to the calcemic action of PTH, hyperphosphatemia, and reduced availability of the active metabolite of vitamin D, 1.25 DHCC, may underlie the hypocalcemia of the oliguric phase.[Citation[22],Citation[23]] Hypercalcemia can be found in the diuretic phase in some cases of acute tubular necrosis, especially in oliguric cases associated with rhabdomyolysis.[Citation[23–25]] Patients with rhabdomyolysis and ARF are hypocalcemic during the oliguric phase, and more than 30% of them develop hypercalcemia during the diuretic phase.[Citation[23],Citation[24]] The elevation in serum levels of 1.25 DHCC plays an important role in the genesis of this type of hypercalcemia,[Citation[23]] although other authors[Citation[25]] have stated that there is no evidence of derangement of the PTH/1.25-(OH)2-vitamin D axis in calcium metabolism in rhabdomyolysis. According to these authors, the most likely explanation for hypercalcemia during the diuretic phase is the physicochemical dissolution of calcium and phosphate deposited in the injured muscles during the oliguric phase.
Usually, the presence of hypercalcemia associated with ARF in the initial phase is indicative of the presence of comorbidity, as observed in this investigation and in the literature.
In general, hydration with isotonic saline should be used to repair the volume deficit and the relative water deficit, and to aid the reduction of serum calcium levels. The 0.9% saline solution takes advantage of the linkage between sodium transport in the proximal tubule and ascending limb of Henle.[Citation[1]] Furosemide is frequently used with sodium chloride infusions for the treatment of hypercalcemia. The objective is to block calcium reabsorption by the ascending limb of Henle and directly increase calciuresis.[Citation[2]] However, forced saline diuresis may not be safe and effective enough, particularly in patients with advanced renal failure. In these cases, calcium-free hemodialysis is indicated when the presence of severe renal failure prevents the administration of large volumes of intravenous fluids to hypercalcemic patients. Single 2- to 3-h treatment with a calcium-free dialysate is safe provided that predialysis plasma calcium is high.[Citation[26]] Hydration with saline and the use of diuretics were employed in all of our eight cases to decrease serum calcium levels. In most circumstances, hypercalcemia is due to excessive bone resorption and calcitonin; diphosphonate or mithramycin is usually also required. Calcitonin is an important therapeutic approach to acute hypercalcemia. Like the bisphosphonates, calcitonin inhibits osteoclast bone resorption and also facilitates urinary calcium excretion. The major advantage of calcitonin is its rapidity of action with maximum reduction in the serum calcium within 12 to 24 h. The major disadvantage of calcitonin is that is not a potent agent.[Citation[27],Citation[28]] Combination therapy with calcitonin and etidronate or pamidronate appears to lead to a rapid fall in the serum calcium. The sustained reduction over several days is undoubtedly due to the bisphosphonate.[Citation[27]] Mithramycin used to be one of the first-line drugs for the therapy of hypercalcemia. Like the other anticalcemic agents, the reduction in the serum calcium is usually not sustained. It has been replaced as a first-line drug by the bisphosphonates, with or without calcitonin, because of mithramycin's potential to cause a number of disturbing side effects (e.g., hepatotoxicity, nephrotoxicity). This drug is now held in reserve for particularly difficult and unusual situations.[Citation[27]]
In summary, we presented eight patients with ARF associated with hypercalcemia and detected the presence of comorbidity in all cases. All patients were treated with hydration and diuretics, and three of them also received calcitonin. There was an improvement of renal function in all cases as serum calcium levels decreased.
ACKNOWLEDGMENTS
This study was partially funded by Fundação de Assistência ao Ensino, Pesquisa e Assistência (FAEPA) of the University Hospital, Faculty of Medicine of Ribeirão Preto, University of São Paulo.
REFERENCES
- Martin TJ, Grill V. Hypercalcaemia. Clin Endocrinol (Oxf) 1995; 42: 535–538
- Mahnensmith RL. Hypercalcemia, hypernatremia, and reversible renal insufficiency. Am J Kidney Dis 1992; 19: 604–608, [PUBMED], [INFOTRIEVE]
- Burdmann E, Yu L. Metabolic and electrolyte disturbances: secondary manifestations. Acute Renal Failure, WF Finn, BA Molitoris. WB Saunders, Philadelphia, PA 2001; 169–191
- Lins LE. Reversible renal failure caused by hypercalcemia. A retrospective study. Acta Med Scand 1978; 203: 309–314, [PUBMED], [INFOTRIEVE]
- Kreisberg RA. Clinical problem-solving. Stopping short of certainty. N Engl J Med 1994; 331: 42–45, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Irish AB, Winearls CG, Littlewood T. Presentation and survival of patients with severe renal failure and myeloma. QJM 1997; 90: 773–780, [PUBMED], [INFOTRIEVE]
- Erban JK, Tang Z. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 38-2002. A 54-year-old man with hypercalcemia, renal dysfunction, and an enlarged liver. N Engl J Med 2002; 347: 1952–1960, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Frolich A. Prevalence of hypercalcemia in normal and in hospital populations. Dan Med Bull 1998; 45: 436–439, [PUBMED], [INFOTRIEVE]
- Lam KK, Kuo CY. Bone marrow examinations as final clue to diagnosis of hypercalcemia: report of two cases. Ren Fail 1999; 21: 101–105, [PUBMED], [INFOTRIEVE]
- Sulkin T, Krentz AJ. Iatrogenic recurrent severe hypercalcemia and renal impairment. Postgrad Med J 2000; 76: 802D, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Reilly BM, Clarke P, Nikolinakos P. Clinical problem-solving Easy to see but hard to find. N Engl J Med 2003; 348: 59–64, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Abreo K, Adlakha A, Kilpatrick S, Flanagan R, Webb R, Shakamuri S. The milk-alkali syndrome. A reversible form of acute renal failure. Arch Intern Med 1993; 153: 1005–1010, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Gunda S, Kwan JT, Sampson S. Hypercalcemia and acute renal failure. Nephrol Dial Transplant 2001; 16: 425–426, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Selby PL, Davies M, Marks JS, Mawer EB. Vitamin D intoxication causes hypercalcemia by increased bone resorption which responds to pamidronate. Clin Endocrinol (Oxf) 1995; 43: 531–536
- Markowitz GS, Fine PL, Stack JI, et al. Toxic acute tubular necrosis following treatment with zoledronate (Zometa). Kidney Int 2003; 64: 281–289, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Casella FJ, Allon M. The kidney in sarcoidosis. J Am Soc Nephrol 1993; 3: 1555–1562, [PUBMED], [INFOTRIEVE]
- Jayawardene SA, Pattison JM. An elderly man with confusion, hypercalcemia and acute renal failure—an important diagnosis not to miss. Nephrol Dial Transplant 2000; 15: 1468–1470, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Ryzen E, Singer FR. Hypercalcemia in leprosy. Arch Intern Med 1985; 145: 1305–1306, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Hoffman VN, Korzeniowski OM. Leprosy, hypercalcemia, and elevated serum calcitriol levels. Ann Intern Med 1986; 105: 890–891, [PUBMED], [INFOTRIEVE]
- Ryzen E, Rea TH, Singer FR. Hypercalcemia and abnormal 1,25-dihydroxyvitamin D concentrations in leprosy. Am J Med 1988; 84: 325–329, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Levi M, Ellis MA, Berl T. Control of renal hemodynamics and glomerular filtration rate in chronic hypercalcemia. Role of prostaglandins, renin-angiotensin system, and calcium. J Clin Invest 1983; 71: 1624–1632, [PUBMED], [INFOTRIEVE]
- Massry SG, Arieff AI, Coburn JW, Palmieri G, Kleeman CR. Divalent ion metabolism in patients with acute renal failure: studies on the mechanism of hypocalcemia. Kidney Int 1974; 5: 437–445, [PUBMED], [INFOTRIEVE]
- Akmal M, Bishop JE, Telfer N, Norman AW, Massry SG. Hypocalcemia and hypercalcemia in patients with rhabdomyolysis with and without acute renal failure. J Clin Endocrinol Metab 1986; 63: 137–142, [PUBMED], [INFOTRIEVE]
- Marques MM, Kimachi T, Coimbra T. Acute renal failure: disorders of calcium regulation. AMB Rev Assoc Med Bras 1976; 22: 374–377, [PUBMED], [INFOTRIEVE]
- Shrestha SM, Berry JL, Davies M, Ballardie FW. Biphasic hypercalcemia in severe rhabdomyolysis: serial analysis of PTH and vitamin D metabolites. A case report and literature review. Am J Kidney Dis 2004; 43: e31–e35, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Koo WS, Jeon DS, Ahn SJ, Kim YS, Yoon YS, Bang BK. Calcium-free hemodialysis for the management of hypercalcemia. Nephron 1996; 72: 424–428, [PUBMED], [INFOTRIEVE]
- Bilezikian JP. Clinical review 51: management of hypercalcemia. J Clin Endocrinol Metab 1993; 77: 1445–1449, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Fatemi S, Singer FR, Rude RK. Effect of salmon calcitonin and etidronate on hypercalcemia of malignancy. Calcif Tissue Int 1992; 50: 107–109, [PUBMED], [INFOTRIEVE], [CROSSREF]
