Abstract
Rhabdomyolysis-induced myoglobinuric acute renal failure (ARF) accounts for about 10% to 40% of all cases of ARF. Reactive oxygen intermediates have been demonstrated to play an etiologic role in myoglobinuric renal failure. This study was designed to investigate the effect of resveratrol, a polyphenolic phytoalexin in glycerol-induced ARF in rats. Seven groups of rats were employed in this study, group I served as control; group II was given 50% glycerol (8mL/kg, intramuscularly); groups III, IV, and V were given glycerol plus resveratrol (2mg/kg, 5mg/kg, and 10mg/kg p.o. route, respectively) 60 min prior to the glycerol injection; group VI received L-NAME (10mg/kg, i.p.) along with glycerol and resveratrol (5 mg/kg), group VII animals received L-NAME (10 mg/kg) 30 min prior to glycerol administration. Renal injury was assessed by measuring plasma creatinine, blood urea nitrogen, creatinine, and urea clearance. The oxidative stress was measured by renal malondialdehyde levels and reduced glutathione levels, and by enzymatic activity of catalase, glutathione reductase, and superoxide dismutase. Tissue and urine nitrite levels were measured as an index of total nitric oxide levels. Glycerol treatment resulted in a marked decrease in tissue and urine nitric oxide levels, renal oxidative stress, and significantly deranged the renal functions along with deterioration of renal morphology. Pretreatment of animals with resveratrol (5 and 10 mg/kg) 60 min prior to glycerol injection markedly attenuated the fall in nitric oxide levels, renal dysfunction, morphologic alterations, reduced elevated thiobarbituric acid reacting substances, and restored the depleted renal antioxidant enzymes. This protection afforded by resveratrol was significantly reversed by cotreatment of L-NAME along with resveratrol, clearly indicating that resveratrol exerts its protective effect through nitric oxide release along with the antioxidative effect in glycerol-induced ARF.
INTRODUCTION
Rhabdomyolysis is a clinical syndrome in which injury to the skeletal muscle results in leakage of intracellular contents from myocytes into the circulation.Citation[1] The muscle injury can result from a variety of causes such as intrinsic muscle dysfunction (including trauma, burns, intrinsic muscle disease, and excessive physical exertion), metabolic disorders, hypoxia, drugs, toxins, infections, temperature extremes, and idiopathic disorders.Citation[2] Complications associated with rhabdomyolysis include disseminated intravascular coagulation, hyperkalemia and other metabolic imbalances, acute renal failure (ARF), and acute cardiomyopathy.
In general, about 10% to 40% of cases with rhabdomyolysis develop ARF, and it accounts for 2% to 15% of all cases of ARF.Citation[3] Following rhabdomyolysis, inordinate amounts of myoglobin are released into the systemic circulation, and its prompt discharge into the renal tubules sets the stage for the initiation of renal injury process.Citation[4] The intramuscular administration of hypertonic glycerol induces myolysis and hemolysis, and affords a faithful and widely used model of heme protein-induced renal injury. Intrarenal hemodynamic changes were considered as important pathogenic events in the early phase after glycerol administration.Citation[5],Citation[6] The endothelium damage may be one of the important consequences of ischemia. The endothelium releases several agents that regulate the underlying vascular smooth muscle tone. Nitric oxide (NO) plays an important role in regulating renal hemodynamics and functions.Citation[7] A great deal of evidence has suggested that NO is generated not only in renal vascular endothelium, but also in other renal cells such as mesangium, macula densa, and tubular cells,Citation[8] thereby suggesting that endogenous NO plays an important role in the regulation of renal blood flow, renal perfusion pressure, renal vascular tone, renal tubular reabsorption, and glomerular filtration rate. This molecule is very unstable with a half-life of very few seconds. NO has been shown to affect mesangial and juxtaglomerular cells, glomerular function and renal hemodynamics profoundly.Citation[9] L-arginine has been shown to cause renal vasodilation associated with an increase in urinary cGMP. The administration of nitric oxide synthase (NOS) inhibitors (L-arginine antagonist) increases renal vascular resistance, decreases renal blood flow, and reduces urinary cGMP excretion, reflecting a continuous basal secretion of NO.Citation[10]
Resveratrol (trans 3,5,4′-trihydroxy stilbene), a naturally occurring phenolic compound abundantly available in grape skins and in wines, has been found to protect the heart from ischemic-reperfusion injury.Citation[14],Citation[15] Resveratrol is a polyphenol phytolexin (trans 3,5,4′-trihydroxy stilbene) that possesses diverse biochemical and physiological actions, which includes estrogenic, antiplatelet, and anti-inflammatory properties.Citation[16],Citation[17] Recently, resveratrol was found to protect kidney, heart, and brain from ischemic-reperfusion injury.Citation[11],Citation[12],Citation[15],Citation[16] In kidney cells, resveratrol was found to exert its protective action through upregulation of NO.Citation[16]
This work was undertaken to study the possible involvement of NO in the development of glycerol-induced ARF and to study the mechanism underlying the protection afforded by resveratrol in glycerol-induced ARF in rats. To aid this latter objective, the effect of coadministration of L-NAME (a nonselective inhibitor of NOS) with resveratrol was studied.
METHODS
Animals
Male Wistar rats (150–200g) bred in the central animal house of Panjab University (Chandigarh, India) were used. The animals were housed under standard conditions of light and dark cycle with free access to food (Hindustan Lever Products, Kolkata, India) and water. The experimental protocols were approved by the institutional ethical committee of Panjab University.
Drugs
Glycerol was purchased from Ranbaxy Laboratories (Mohali, India). Resveratrol and L-NAME were purchased from Sigma (St. Louis, MO, USA). Resveratrol was suspended in 0.25% sodium carboxy methyl cellulose (CMC), whereas L-NAME was dissolved in distilled water.
Study Design
Seven groups were employed in this study, each consisting of five to seven animals. The animals were allowed free access to food but deprived of drinking water for 24 h before glycerol injection. Group I (C) was comprised of a control group that received an equivalent volume of saline for glycerol. Group II (G) animals received an intramuscular injection of 8 mL/kg hypertonic glycerol as a divided dose into the hind limbs. Groups III, IV, and V animals received resveratrol (2 mg/kg, 5 mg/kg, and 10 mg/kg p.o. route, respectively) 60 min prior to the glycerol injection. Group VI animals received L-NAME (10 mg/kg, i.p.) 60 min prior to and resveratrol (5 mg/kg) 30 min prior to glycerol administration. Group VII animals received L‐NAME (10 mg/kg, i.p.) 30 min prior to glycerol administration. The animals were placed in individual metabolic cages after the glycerol injection for 24 h for urine collection. All animals were sacrificed with a high dose of anesthesia 24 h after the glycerol injection, and the blood was collected in heparinized centrifuge tubes through the abdominal aorta. Freshly isolated serum was used for the assessment of renal function tests. Both kidneys were harvested through a midline incision; the left kidney was deep frozen until further enzymatic analysis, whereas the right kidney was stored in 10% formalin for the histologic sectioning.
Assessment of Renal Function
Serum samples were assayed for blood urea nitrogen (BUN) and serum creatinine by using standard diagnostic kits (Span Diagnostics, Gujarat, India).
Tissue and Urine Nitrate and Nitrite Measurements
Nitric oxide production in renal tissue and urine was determined by standard Total Nitric Oxide Assay kit (Assay Design, Inc., USA). Nitrate was reduced to nitrite by 3-h incubation with nitrate reductase in the presence of nicotinamide adenine dinucleotide 3-phosphate. Nitrite was converted into a deep purple azo compound by the addition of griess reagent. Total nitrite/nitrate concentration was calculated by using standard of sodium nitrate. Results were expressed as μmol/L.
Postmitochondrial Supernatant Preparation
After sacrificing the animals, their kidneys were quickly removed, perfused immediately with ice-cold normal saline, and homogenized in chilled potassium chloride (1.17%) using a Potter Elvehjem homogenizer. The homogenate was differentially centrifuged to obtain postmitochondrial supernatant (PMS), which was used for further enzymatic analysis.
Estimation of Lipid Peroxidation
The malondialdehyde (MDA) content, a measure of lipid peroxidation, was assayed in the form of thiobarbituric acid reacting substances (TBARS).Citation[17] In brief, the reaction mixture consisted of 0.2 mL of 8.1% sodium lauryl sulphate and 1.5 mL of 20% acetic acid solution adjusted to pH 3.5 with sodium hydroxide, and 1.5 mL of 0.8% aqueous solution of thiobarbituric acid was added to 0.2 mL of 10%(w/v) of PMS. The mixture was brought up to 4.0 mL with distilled water and heated at 95°C for 60 min. After cooling with tap water, 1.0 mL distilled water and 5.0 mL of the mixture of n-butanol & pyridine (15:1 v/v) was added and centrifuged. The organic layer was taken out, and its absorbance was measured at 532 nm. TBARS were quantified using an extinction coefficient of 1.56 × 105 M−1/cm−1 and expressed as nmol of TBARS per mg protein. Tissue protein was estimated using Biuret method of protein assay and the renal MDA content expressed as nanomoles of malondialdehyde per milligram of protein.Citation[18]
Estimation of Antioxidant Enzymes
The antioxidant enzymes were estimated by the well-established procedures already published elsewhere.Citation[19] The reduced glutathione (GSH) was measured by the method of Jollow et al.Citation[20] and the yellow color developed by the reduction of Ellman's reagent by –SH groups of GSH was read at 412 nm. The catalase (CAT) activity was assayed by the method of Claiborne,Citation[21] and the rate of decomposition of H2O2 was followed at 240 nm. The superoxide dismutase (SOD) activity was assessed by the method of Kono.Citation[22] The nitro blue tetrazolium reduction by superoxide anion to blue formazan was followed at 560 nm.
Renal Histology
The right kidney was isolated immediately after sacrificing the animal and washed with ice-cold saline. It was then fixed in a 10% neutral buffered formalin solution, embedded in paraffin and used for histopathological examination. Five-micrometer (μm) thick sections were cut, deparaffinized, hydrated, and stained with hematoxylin and eosin. The renal sections were examined in blind fashion for hemorrhagic and hyaline casts, tubular necrosis and apical blebbing in all treatments. A minimum of 10 fields for each kidney slide were examined and assigned for severity of changes using scores on a scale of none (−), mild (+), moderate (++), and severe (+++) damage.
STATISTICAL ANALYSIS
Values are expressed as mean±SEM. One way analysis of variance (ANOVA) followed by Dunnett's test was applied to calculate the statistical significance between various groups. A value of p<0.05 was considered to be statistically significant.
RESULTS
Effect of Resveratrol, L-NAME on Glycerol-Induced Renal Dysfunction
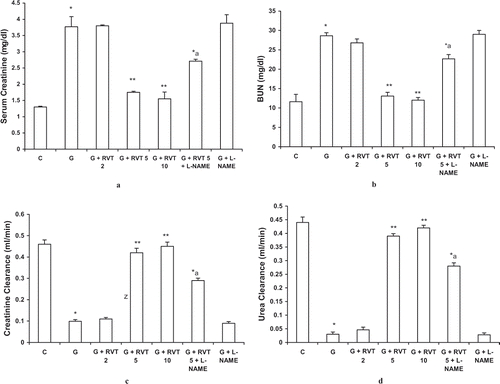
Intramuscular injection of 8 mL/kg of hypertonic glycerol produced a marked derangement in the renal function and lead to a significant increase in the level of serum creatinine, BUN, and a severe fall in the clearance values of urea and creatinine. Pretreatment of animals with resveratrol (5, 10 mg/kg) significantly attenuated the impairment of renal function caused by glycerol administration. A dose of 2 mg/kg resveratrol did not modify the effect of glycerol on these parameters measured (see Results sections). L‐NAME alone had no effect on the impairment of renal function caused by glycerol but partially reversed the protective effect of resveratrol ().
Figure 1. Effect of resveratrol (2, 5, and 10 mg/kg) and L-NAME (10 mg/kg) on serum creatinine (a), blood urea nitrogen (BUN) (b), creatinine clearance (c), and urea clearance (d) in glycerol-treated rats. The values are expressed as mean ± SEM. *p < 0.05 as compared with the control group; **p < 0.05 as compared with the glycerol-treated group; a p < 0.05 as compared with RVT 5 + G-treated group (one-way ANOVA followed by Dunnett's test).
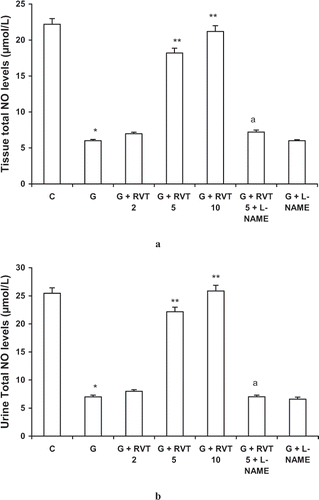
Effect of Resveratrol, L-NAME on Total Nitric Oxide Contents in Glycerol-Treated Animals
Renal tissue and urine NO, as measured by total nitric oxide levels, was significantly lower in glycerol treated group as compared to control animals. These levels were significantly improved by treatment with resveratrol (5, 10 mg/kg); however, animals treated with L-NAME and RVT along with L-NAME had total nitric oxide contents similar to glycerol treated animals ( and ).
Figure 2. Effect of resveratrol (2, 5, and 10 mg/kg) and L-NAME (10 mg/kg) on tissue (a) and urine (b) total nitric oxide levels in glycerol-treated rats. The values are expressed as mean ± SEM. *p < 0.05 as compared with the control group; **p < 0.05 as compared with the glycerol-treated group; a p < 0.05 as compared to RVT 5 + G-treated group (one-way ANOVA followed by Dunnett's test).
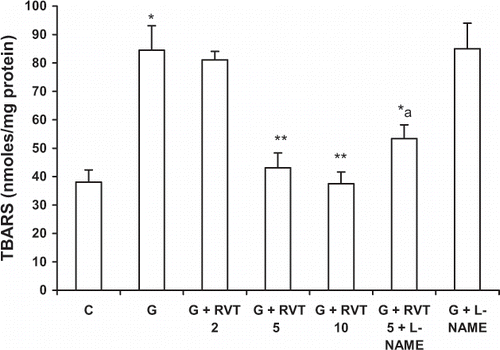
Effect of Resveratrol, L-NAME on Glycerol-Induced Lipid Peroxidation
TBARS levels were increased significantly by glycerol treatment as compared with the control group. Pretreatment with resveratrol (5, 10 mg/kg) produced a significant reduction in TBARS in glycerol-treated rats, while L-NAME+resveratrol-treated animals had high TBARS levels as compared with animals treated with resveratrol alone ().
Figure 3. Effect of resveratrol (2, 5, and 10 mg/kg) and L‐NAME (10 mg/kg) on glycerol-induced lipid peroxidation (MDA). The values are expressed as mean ± SEM. *p < 0.05 as compared with the control group; **p < 0.05 as compared with the glycerol-treated group; a p < 0.05 as compared with RVT 5 + G-treated group (one-way ANOVA followed by Dunnett's test).
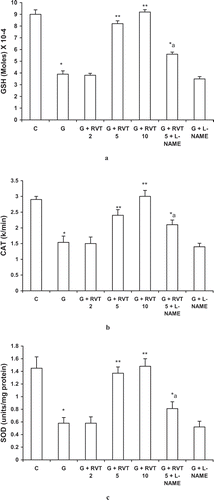
Effect of Resveratrol, L-NAME on Glycerol-Induced Changes in the Antioxidant Pool
Treatment with glycerol significantly decreased the enzymatic activity of reduced GSH, CAT, and SOD. This reduction was significantly improved by pretreatment with resveratrol (5, 10 mg/kg). This protection against decrease in GSH, CAT, and SOD afforded by resveratrol was partially reversed by L-NAME. However, L-NAME did not modify the beneficial effect of resveratrol on glycerol-induced decrease in CAT ().
Figure 4. Effect of Effect of resveratrol (2, 5, and 10 mg/kg) and L-NAME (10 mg/kg) on reduced glutathione (GSH) (a), catalase (b), and superoxide dismutase (SOD) (c) in glycerol-treated rats. The values are expressed as mean ± SEM. *p < 0.05 as compared with the control group; **p < 0.05 as compared with the glycerol-treated group; a p < 0.05 as compared with RVT 5 + G-treated group (one-way ANOVA followed by Dunnett's test).
Effect of Resveratrol, L-NAME on Glycerol-Induced Changes on Renal Morphology
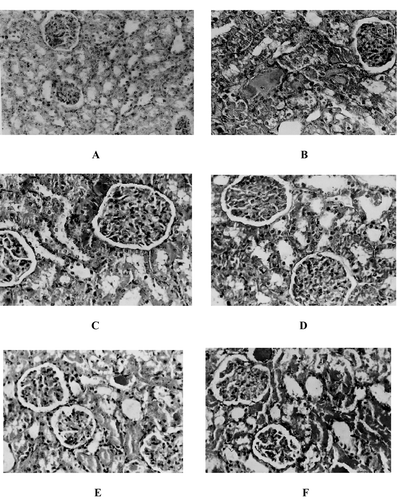
The histopathological changes were graded and summarized in . The control group did not show any morphologic changes. In contrast, the kidneys of rats treated with glycerol showed marked histologic changes in the cortex and outer medulla. The renal sections showed severe epical blebbing, hyaline casts, tubular necrosis, and hemorrhagic casts. Treatment with resveratrol (2 mg/kg) did not show any significant morphologic protection; however, with resveratrol (5, 10 mg/kg), the kidney sections preserved the normal morphology of the kidney. The animals treated with L-NAME showed similar morphology as that of glycerol-treated animals ().
Table 1. Effect of resveratrol (RVT) (2, 5, and 10 mg/kg) and L-NAME (10 mg/kg) pretreatment on morphologic changes as assessed by histopathological examination of kidney in glycerol-treated rats
Figure 5. Hematoxylin and eosin-stained sections of rat kidneys. (A) Normal kidney section. (B) Kidney section of glycerol-treated rat showing severe hemorrhagic, hyaline casts, and apical blebbing. (C) Kidney section of resveratrol (5 mg/kg, p.o.) + glycerol-treated rat showing mild casts and blebbing. (D) Kidney section of resveratrol (10 mg/kg, p.o.) + glycerol-treated rat showing a near-normal morphology. (E) Kidney section of resveratrol (5 mg/kg, p.o.) and L-NAME (10 mg/kg) + glycerol-treated rat showing cast formation, epical blebbing, and tubular necrosis. (F) Kidney section of L-NAME (10 mg/kg) + glycerol-treated rat showing morphologic damage similar to glycerol-treated rats.
DISCUSSION
In this study, the treatment of animals with glycerol, led to a significant derangement of renal function, reduced the tissue and urine total NO contents, significantly reduced the enzyme activities of GSH, SOD, and CAT, and produced morphologic alterations. The animals treated with resveratrol 5 and 10 mg/kg, significantly reversed all these parameters. The pretreatment of animals with L-NAME, a nonselective NOS blocker, abolished the protective effect of resveratrol in glycerol-treated animals.
Since its description by Bywaters and Beall,Citation[23] the relationship between rhabdomyolysis and ARF has drawn physician attention. Clinical experience in disaster situations shows that predisposing intravascular volume depletion with or without metabolic acidosis appears to be essential for the development of ARF.Citation[24] However, a direct casual relationship between muscle injury and ARF has been suggested from an accumulation of cases with nontraumatic rhabdomyolysis, in which clinically apparent circulatory disturbance is minimal.Citation[25]
The intramuscular administration of hypertonic glycerol induces myolysis and hemolysis, and affords a faithful and widely used model of heme protein-induced renal injury. The heme protein-induced renal injury represents the integrated effects of three major pathophysiological mechanisms: renal vasoconstriction, direct cytotoxicity, and cast formation.Citation[26],Citation[27] The pigments (hemoglobin and myoglobin) themselves are unlikely to induce ARF, but their presence within the systemic circulation during periods of acidosis, dehydration, shock, or other conditions associated with reduced renal perfusion may lead to both direct toxic and hemodynamic abnormalities resulting in ARF.Citation[27]
Despite these insights, the specific mediator(s) of proximal tubular necrosis during myohemoglobinuric ARF have been difficult to define. In large part, this is due to the multifactorial nature of the insult, which has necessitated whole animal experiments to test specific hypotheses. One of the most important and earliest events occurring after glycerol injection is a reduction of renal blood flow (RBF).Citation[5],Citation[28] It has been demonstrated that animals treated with glycerol undergo a decrease in NO production. This decrease may play a role in the decreased RBF observed in these animals because NO is a potent vasodilator.Citation[29] After glycerol injection, an important release of myoglobin and hemoglobin into the circulation occur. The structure of these pigments includes heme group, which shows strong affinity for NO. Thus, the binding of NO to heme group would lead to a decrease in NO activity and also to a decrease in NO oxidation to nitrite. Nevertheless, this approach has also produced the view that heme-iron-driven hydroxyl radical (·OH) generation is a critical mediator of the evolving tubular damage. This conclusion is supported by the following pieces of information: (1) iron chelation (Deferoxamine) therapy partially mitigates the extent of tubular necrosis and filtration failure;Citation[19],Citation[30] (2) •OH scavengersCitation[31],Citation[32] and glutathioneCitation[33] can exert protective effects; (3) lipid peroxidation, a biochemical hallmark of oxidative stress, has been reported in the aftermath of heme protein nephrotoxicityCitation[30]; and (4) induction or suppression of heme oxygenase (HO• the enzyme that degrades heme porphyrin) has been shown to decrease or increase the severity of myohemoglobinuric (glycerol) ARF, respectively.Citation[34]
In this present study, all animals were dehydrated for 24 h. Prior dehydration allows the full expression of renal injury, particularly the cast formation, compared with the nondehydrated model.Citation[32] The animals lost an average of 5% to 8% of their body weight in the period of dehydration, during which time their food intake was one-third less than that in the nondehydrated state. Moreover, oliguria or anuria developed only in the rats, which were dehydrated prior to the glycerol injection. The urinary volume of dehydrated control animals was one-half of the urinary volume in nondehydrated animals (data not shown). Furthermore, micropuncture studies have shown that the glomerular filtration rate of dehydrated controls falls significantly as compared with nondehydrated controls.Citation[35]
Recent studies have shown that, like with many other polyphenols, resveratrol (trans 3,5,4′-trihydroxy stilbene, a polyphenol in grapes and present in wines) is responsible for the cardiovascular benefits associated with moderate wine consumption.Citation[36] During the last decade, it has been shown that resveratrol modulates lipid and lipoprotein metabolism,Citation[37],Citation[38] inhibits platelet activation/aggregationCitation[13] and the activity of some protein kinases,Citation[39] and has strong antioxidant activity.Citation[40],Citation[41] Resveratrol is an antioxidant more powerful than vitamin E in preventing LDL oxidationCitation[40] and is considered as a possible therapeutic agent in treating acute scenarios in I/R injury. Resveratrol also has anticancer, estrogenic, and vasorelaxing activity.Citation[42] It has recently been found that resveratrol exerts a cardioprotective action as a result of its antioxidant activityCitation[12] and the upregulation of nitric oxide.Citation[43],Citation[44] In kidney cells, resveratrol was found to exert its protective action through upregulation of NO.Citation[16]
In this study, intramuscular injection of glycerol lead to markedly high levels of serum creatinine and BUN and reduced the creatinine and urea clearance. This glycerol-induced renal dysfunction was associated with a marked decrease in tissue and urine total nitric oxide contents, increased renal lipid peroxidation, and severely depleted the pools of antioxidant enzymes as evident from reduced levels of GSH, CAT, GR, and SOD enzymes. Moreover, the histologic pattern of glycerol-treated rats showed characteristic hemorrhagic and hyaline cast deposits, tubular necrosis, and apical blebbing. Pretreatment of animals with resveratrol (5, 10, but not 2 mg/kg) significantly improved most of these alterations observed with glycerol injection. However, the protection afforded by the resveratrol was significantly attenuated by prior treatment with L-NAME, suggesting that resveratrol exerted its protective effect at least partly through release of NO. The pretreatment of animals with L-NAME significantly abolished the protective effect of RVT on tissue and urine NO levels. However, RVT has also been shown to possess strong activity. In case of less abolishing effect of L-NAME on functional and morphologic parameters, the strong antioxidative property of RVT might have played a part in L-NAME+RVT 5+G-treated group.
In summary, NO seems to play an important role in glycerol-induced ARF. The protection afforded by resveratrol in rats with glycerol-induced ARF suggests that the protection afforded by resveratrol is mediated by release of NO and through its antioxidative property.
ACKNOWLEDGMENTS
The Senior Research Fellowship of the Council of Scientific and Industrial Research, New Delhi, is gratefully acknowledged.
REFERENCES
- Curry SC, Chang D, Connor D. Drug- and toxin-induced rhabdomyolysis. Ann Emerg Med 1989; 18: 1068–1084, [PUBMED], [INFOTRIEVE]
- Salluzzo RF. Rhabdomyolysis. Emergency Medicine: Concepts and Clinical Practice, AL Rosenet. Mosby, St. Louis, MO 1992
- Beetham R. Biochemical investigation of suspected rhabdomyolysis. Ann Clin Biochem 2000; 37: 581–587, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Better OS, Stain JH. Early management of shock and prophylaxis of acute renal failure in traumatic rhabdomyolysis. N Engl J Med 1990; 322: 825–827, [PUBMED], [INFOTRIEVE]
- Ayer G, Grandchamp A, Wyler T, Truniger B. Intrarenal hemodynamics in glycerol-induced myohemoglobinuric acute renal failure in the rat. Circ Res 1971; 29: 128–135, [PUBMED], [INFOTRIEVE]
- Hsu CH, Kurtz TW, Waldinger TP. Cardiac output and renal blood flow in glycerol-induced acute renal failure in the rat. Circ Res 1977; 40: 178–182, [PUBMED], [INFOTRIEVE]
- Ruilope LM, Lahera V, Rodicio JL, Romero JC. Participation of nitric oxide in the regulation of renal function: possible role in the genesis of arterial hypertension. J Hypertens 1994; 12: 625–631, [PUBMED], [INFOTRIEVE]
- Mundel P, Bachmann S, Bader M, et al. Expression of nitric oxide synthase in kidney macula densa cells. Kidney Int 1992; 42: 1017–1019, [PUBMED], [INFOTRIEVE]
- Baylis C, Harton P, Engels K. Endothelial derived relaxing factor controls renal hemodynamics in the normal rat kidney. J Am Soc Nephrol 1990; 1: 875–881, [PUBMED], [INFOTRIEVE]
- King AJ, Troy JL, Anderson S, Neuringer JR, Gunning M, Brenner BM. Nitric oxide: a potential mediator of amino acid-induced renal hyperemia and hyperfiltration. J Am Soc Nephrol 1991; 1: 1271–1277, [PUBMED], [INFOTRIEVE]
- Das DK, Sato M, Ray PS, et al. Cardioprotection of red wine: role of polyphenolic antioxidants. Drugs Exp Clin Res 1999; 25: 115–120, [PUBMED], [INFOTRIEVE]
- Ray PS, Maulik G, Cordis GA, Bertelli AA, Bertelli A, Das DK. The red wine antioxidant resveratrol protects isolated rat hearts from ischemia reperfusion injury. Free Rad Biol Med 1999; 27: 160–169, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Bertelli AA, Giovannini L, Bernini W, et al. Antiplatelet activity of cis-resveratrol. Drugs Exp Clin Res 1996; 22: 61–63, [PUBMED], [INFOTRIEVE]
- Ferrero ME, Bertelli AA, Pellegatta F, Fulgenzi A, Corsi MM, Bertelli A. Phytoalexin resveratrol (3–4′-5-trihydroxystilbene) modulates granulocyte and monocyte endothelial adhesion. Transplant Proc 1998; 30: 4191–4193, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Bastianetto S, Zheng WH, Quirion R. Neuroprotective abilities of resveratrol and other red wine constituents against nitric oxide-related toxicity in cultured hippocampal neurons. Br J Pharmacol 2000; 131: 711–720, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Giovannini L, Migliori M, Longoni BM, et al. Resveratrol, a polyphenol found in wine, reduces ischemia reperfusion injury in rat kidneys. J Cardiovasc Pharmacol 2001; 37: 262–270, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Ann Biochem 1979; 95: 351–357, [CROSSREF]
- Varley H. The plasma proteins. Practical Clinical Biochemistry, H Varley. CBS, Delhi 1989; 236–238
- Chander V, Singh D, Chopra K. Attenuation of glycerol-induced acute renal failure in rats by trimetazidine and deferoxamine. Pharmacology 2003; 67: 41–48, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Jollow DJ, Mitchell LR, Zampaglione N, Gillete JR. Bromobenze induced liver necrosis: protective role of glutathione and evidence for 3,4-bromobenzeneoxide as the hepatotoxic intermediate. Pharmacology 1974; 11: 151–156, [PUBMED], [INFOTRIEVE]
- Claiborne A. CRC Handbook of Methods for Oxygen Radical Reaserch, RA Greenwald. CRC Press, Boca Raton, Fla 1985; 283–287
- Kono Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys 1978; 186: 189–195, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Bywaters RG, Beall D. Crush injuries with impairment of renal function. Br Med J 1941; 1: 427–432
- Ron D, Taitelman U, Michaelson M, Bar-Joseph G, Bursztein S, Better OS. Prevention of acute renal failure in traumatic rhabdomyolysis. Arch Int Med 1984; 144: 277–280, [CROSSREF]
- Grossman RA, Hamilton RW, Morse BM, Penn AS, Goldberg M. Nontraumatic rhabdomyolysis and acute renal failure. N Engl J Med 1974; 291: 807–811, [PUBMED], [INFOTRIEVE]
- Zager RA. Rhabdomyolysis and myohemoglobinuric acute renal failure. Kidney Int 1996; 49: 314–326, [PUBMED], [INFOTRIEVE]
- Acute Renal Failure, A Dubrow, W Flamenbaum, BM Brenner, JM Lazarus. Churchill Livingstone, New York, NY 1988
- Venkatachalan MA, Rennke HG, Sandstorm DJ. The vascular basis for acute renal failure in the rat. Preglomerular and postglomerular vasoconstriction. Circ Res 1976; 38: 267–269
- Valdivielso JM, Lopez-Novoa JM, Eleno N, Perez Barriocanal F. Role of glomerular nitric oxide in glycerol-induced acute renal failure. Can J Physiol Pharmacol 2000; 78: 476–482, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Paller MS. Hemoglobin- and myoglobin-induced acute renal failure in rats: role of iron in nephrotoxicity. Am J Physiol 1988; 255: F539–F544, [PUBMED], [INFOTRIEVE]
- Shah SV, Walker PD. Evidence suggesting a role for hydroxyl radical in glycerol-induced acute renal failure. Am J Physiol 1988; 255: F438–F443, [PUBMED], [INFOTRIEVE]
- Zager RA. Combined mannitol and deferoxamine therapy for myohemoglobinuric renal injury and oxidant tubular stress. Mechanisms and therapeutic implications. J Clin Invest 1992; 90: 711–716, [PUBMED], [INFOTRIEVE]
- Abull-Ezz SR, Walker PD, Shah SV. Role of glutathione in an animal model of myoglobinuric acute renal failure. Proc Nat Acad Sci USA 1991; 88: 9833–9837
- Nath KA, Balla G, Vercellotti GM, et al. Induction of heme oxygenase is a rapid protective response in rhabdomyolysis in the rat. J Clin Invest 1992; 90: 267–270, [PUBMED], [INFOTRIEVE]
- Oken DE, Arce ML, Wilson DR. Glycerol-induced hemoglobinuric acute renal failure in the rat. I. Micropuncture study of the development of oliguria. J Clin Invest 1966; 45: 724–735, [PUBMED], [INFOTRIEVE]
- Constant J. Alcohol, ischemic heart disease, and the French paradox. Clin Cardiol 1997; 20: 420–424, [PUBMED], [INFOTRIEVE]
- Kimura Y, Okuda H, Arichi S. Effects of stilbenes on arachidonate metabolism in leukocytes. Biochim Biophys Acta 1985; 834: 275–278, [PUBMED], [INFOTRIEVE]
- Ragazzi E, Froldi G, Fassina G. Resveratrol activity on guinea pig isolated trachea from normal and albumin-sensitized animals. Pharmacol Res Comm 1988; 20: 79–82
- Jayatilake GS, Jayasuriya H, Lee ES, et al. Kinase inhibitors from Polygonum cuspidatum. J Nat Prod 1993; 56: 1805–1810, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Frankel EN, Waterhouse AL, Kinsella JE. Inhibition of human LDL oxidation by resveratrol. Lancet 1993; 341: 1103–1104, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Rotondo S, Rajtar G, Manarini S, et al. Effect of trans-resveratrol, a natural polyphenolic compound, on human polymorphonuclear leukocyte function. Br J Pharmacol 1998; 123: 1691–1699, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Fremont L. Biological effects of resveratrol. Life Sci 2000; 66: 663–673, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Bradamante S, Barenghi L, Piccinini F, et al. Resveratrol provides late-phase cardioprotection by means of a nitric oxide- and adenosine-mediated mechanism. Eur J Pharmacol 2003; 465: 115–123, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Hung LM, Su MJ, Chen JK. Resveratrol protects myocardial ischemia-reperfusion injury through both no-dependent and no-independent mechanisms. Free Rad Biol Med 2004; 36: 774–781, [PUBMED], [INFOTRIEVE], [CROSSREF]