Abstract
Intravenous immunoglobulin preparations are being used for an increasing number of indications. To minimize adverse reactions, sugar additives such as sucrose, maltose, and glycine are added to some preparations to serve as stabilizing agents. Intravenous immunoglobulin infusion induces acute renal failure (ARF) via a mechanism of osmotic nephrosis. Most reported cases are related to the use of sucrose-based intravenous immunoglobulin. Herein, we describe a patient with lupus nephritis treated with an immunoglobulin preparation containing maltose who developed ARF with histologic changes characterized by vacuolization and swelling of renal proximal tubular cells. Our case draws nephrologists' attention to the potential of maltose-based immunoglobulin in producing renal failure. Awareness and exercising caution in high-risk groups is elementary to the prevention of this condition.
INTRODUCTION
Intravenous immunoglobulin (IVIG) is widely used in the treatment of immunodeficient and autoimmune hematologic, neurologic, rheumatologic, cutaneous, and renal disorders. IVIG is usually well tolerated with adverse events occurring less than 5% of the time. These complications are generally believed to be allergic reactions consisting of vasomotor symptoms, including fever, headache, myalgia, chills, nausea, vomiting, and chest tightness. Severe adverse events have been reported that include hypersensitivity, anaphylaxis, thrombotic events, and aseptic meningitis. The formation of IgG aggregates during manufacture or storage of IVIG are believed to cause these reactions. To prevent aggregate formation, purified IgG is stabilized with various carbohydrates, such as sucrose, maltose, glucose, glycine, or sorbitol or albumin. The addition of sugar moieties to IVIG formulations has reduced the frequency and severity of these adverse effects but may increase the frequency of acute renal failure (ARF). IVIG-induced acute renal injury is an uncommon side effect and has been mostly related to the administration of sucrose-based IVIG. We report a case of osmotic nephropathy-induced ARF following the use of a maltose-based IVIG in a patient with lupus nephritis. The potential implications of this observation have also been discussed.
CASE
A 43-year-old female with systemic lupus erythematosus (SLE) presented with fatigue and edema. She was diagnosed with SLE in 1997, when she had developed polyarthritis, fever, and malar erythema with positive autoimmune serology. There was symptomatic improvement with the prescribed dose of steroids. Past medical history was significant for recent-onset hypertension and diabetes.
Physical examination on admission revealed a blood pressure of 140/90 mmHg and pedal edema. Laboratory examination revealed an erythrocyte sedimentation rate of 101 mm/h, C-reactive protein 17.5 mg/L, LDH 665 U/L, Coombs-positive hemolytic anemia, thrombocytopenia, and leucopenia. Renal investigation showed a nephritic-nephrotic syndrome with proteinuria 2 g/24 h, urinalysis with red cells and casts, creatinine clearance 30 mL/min, and serum creatinine 2.9 mg/dL. Autoimmune marker studies revealed positive antinuclear factor with hypocomplementemia, but the anti-dsDNA antibody test result was negative. Virologic and bacteriologic tests were unremarkable.
A percutaneous kidney biopsy performed at this stage revealed diffuse proliferative glomerulonephritis with segmental accentuation and capillary wall thickening consistent with WHO class IV lupus nephritis. She was treated with intravenous pulses of cyclophosphamide (750 mg/m2) and oral prednisolone (1 mg/kg body weight/day).
Two days after the first pulse of cyclophosphamide, she developed altered sensorium and seizures. After ruling out metabolic and infective causes by appropriate investigations, a presumptive diagnosis of central nervous system (CNS) lupus was made. Treatment was started with maltose-based intravenous immunoglobulin G (IVI Glob EX, VHB Life Sciences Inc., Mumbai, India) 25 g/day, for 5 consecutive days. The infusion rate of IVIG did not exceed the manufacturer's recommended infusion rate of 2 mg Ig/kg/min.
From the second day of IVIG administration, a gradual reduction of daily urine output was observed simultaneously with an increase of blood urea and creatinine levels, which by the fifth day of treatment reached 240 mg/dL and 7.3 mg/dL, respectively. On the fifth day of treatment, she became oliguric with no allergic symptoms or evidence of hemodynamic derangements during the 5 days of IVIG administration. She did not receive any IV contrast for radiologic studies during evaluation of seizures nor was she on any nephrotoxic drugs. At the start of the IVIG infusion, the blood glucose level was 164 mg/dL.
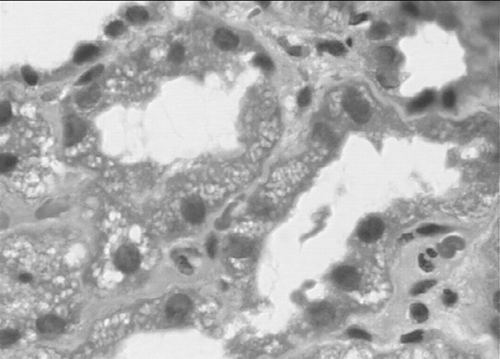
She was initiated on hemodialysis, and after adequate dialysis, was submitted to a renal biopsy to ascertain the etiology of renal dysfunction. Light microscopy showed swelling and vacuolization of the epithelial cells of the proximal tubules with preservation of the brush border (). After four sessions of daily dialysis, a gradual increase of the urine output was observed, together with a decrease of blood urea and creatinine levels, which after 14 days were at pre-IVIG levels.
DISCUSSION
Osmotic nephropathy is a distinct pathological entity that may result in ARF following intravenous administration of high doses of hyperosmolar substances, such as mannitol, sucrose, IVIG, dextran, starch, and inulin.
A number of cases of intravenous immunoglobulin-induced renal impairment have been reported in the literature,Citation[1–3] and in almost all these reports, sucrose-containing products were implicated. IVIG contains a large amount of pooled human immunoglobulin that requires stabilization by a sugar or protein moiety to prevent it from aggregating. Sucrose was first introduced as an osmotic diuretic and had been one of the most commonly used stabilizing agents in IVIG preparations. However, the use of sucrose has been demonstrated to cause sucrose nephropathy and ARF, and its clinical use is now limited.
It is postulated that the proximal tubular cells take up filtered sucrose via pinocytosis.Citation[4] The pinocytic vesicles coalesce to form vacuoles and then fuse with lysosomes to form phagolysomes. Because renal cells do not have disaccharidase, sucrose cannot be metabolized and accumulates in the cytoplasm, creating an osmotic gradient across the membrane. Water enters the proximal tubular cells through AQP1 water channel on the apical and possibly the basolateral membrane, leading to cell swelling and subsequent narrowing and occlusion of tubule lumen. Similar renal injury has been observed with other nonmetabolizable macromolecules, such as mannitol, dextran, and radiocontrast infusion. Therefore, the term osmotic nephrosis has also been used to describe ARF associated with these osmotically active molecules.
In addition to the osmotic injury, renal artery vasoconstriction and ischemic renal damage are also implicated as possible mechanisms leading to ARF, albeit to a lesser extent.Citation[4] Intravenous immune globulin preparations containing maltose, glycine, or dextrose as a stabilizing agent are apparently better tolerated because renal tubular cells can metabolize these molecules.
The renal biopsy documents osmotic nephropathy unequivocally revealing acute tubule necrosis, as well as marked vacuolization and edema of the proximal tubules, with preservation of the brush borders without evidence of immunoglobulin deposition.Citation[5] This degree of proximal tubular cell swelling and vacuolization has also been seen in biopsy specimens from patients with severe hypokalemia, contrast-induced nephropathy, and cyclosporine toxicity, as well as with the intravenous administrations of carbohydrates such as glucose, mannitol, dextran, and sucrose.Citation[6] Complementing the diagnosis is the presence of multivacuolated foamy tubular cells on urine cytology.Citation[7]
In an effort to reduce the risk of ARF, the following precautions should be taken when considering administration of IVIG products: (1) ensure patients are not volume depleted prior to the initiation of the infusion of IVIG. (2) Exercise particular caution in the administration of IVIG products in patients at increased risk for developing ARF, which includes patients with any degree of preexisting renal insufficiency, diabetes mellitus, age older than 65, volume depletion, sepsis, paraproteinemia, or concomitant nephrotoxic drugs. (3) For patients at increased risk, physicians should carefully weigh the potential benefits of administering sucrose-containing IVIG products against the risks of causing renal damage. (4) Reduction in dose, concentration, and/or rate of administration in patients at risk for ARF has been proposed to reduce the risk. Recommended doses should not be exceeded, and the concentration and infusion rate selected should be the minimum levels practicable.
Diagnosis requires a high index of suspicion with regular monitoring of urine output, renal function, and osmolar gap.Citation[8] Management is essentially supportive with discontinuation of the IVIG and restoration of extracellular fluid volume. Recovery may take as long as 14 days, and hemodialysis may be required in the interim.Citation[7],Citation[8]
Our patient underwent hemodialysis, and her renal functions have improved. The temporal sequence of the ARF, was highly suggestive of an osmotic renal injury resulting from the high maltose content of the IVIG, and the renal biopsy substantiated the diagnosis.
Theoretically, the new generation IVIG preparations, which use maltose, glucose, or glycine as the stabilizing agents instead of sucrose, should not lead to osmotic injury as the tubular brush border enzymes metabolize them. For example, maltases in the brush border of the proximal convoluted tubules can rapidly break down maltose into glucose, which is then reabsorbed. This case lends credence to the argument that any preparation of IVIG can cause renal failure.Citation[9] However, the incidence and intensity of the renal impairment may vary with different preparations. Perhaps, IVIG itself is tubulotoxic, and the putative stabilizing agents might contribute to the renal injury, with varying degrees of propensity.
An alternate explanation could be that toxicity results when tubular load is higher than metabolic capacity of the brush border. This could occur in hyperglycemia, where the breakdown of maltose to glucose is greatly reduced,Citation[10] where there are excessive plasma concentrations due to rapid IVIG infusions, or where there are diminished maltase activity secondary to tubular damage associated with the underlying disease (i.e., lupus nephropathy in the presented case).
Up to now, only occasional reports of osmotic renal damage due to maltose containing IVIG exist.Citation[11] However, this is perhaps the first case where the diagnosis is substantiated unequivocally by renal biopsy.
Our case draws nephrologists' attention to the potential of maltose-based IVIG in producing renal failure. Awareness and exercising caution in a high-risk group is elementary to the prevention of this condition. With increasing use of IVIG in renal disorders, intravenous immunoglobulin is indeed a double-edge sword.
REFERENCES
- Cayco AV, Perazella MA, Hayslett JP. Renal insufficiency after intravenous immune globulin therapy: a report of two cases and an analysis of the literature. J Am Soc Nephrol 1997; 8(11)1788–1794, [PUBMED], [INFOTRIEVE]
- Hansen-Schmidt S, Silomon J, Keller F. Osmotic nephrosis due to high-dose immunoglobulin therapy containing sucrose (but not with glycine) in a patient with immunoglobulin A nephritis. Am J Kidney Dis 1996; 28: 451–453, [PUBMED], [INFOTRIEVE]
- Ahsan N, Palmer BF, Wheeler D, Greenlee RG, Jr, Toto RD. Intravenous immunoglobulin-induced osmotic nephrosis. Arch Intern Med 1994; 154: 1985–1987, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Schwartz SL, Johnson CB. Pinocytosis as the cause of sucrose nephrosis. Nephron 1971; 8: 246–254, [PUBMED], [INFOTRIEVE]
- Monserrat AJ, Gotelli C, Garay R. Sequential histochemical features in experimental osmotic nephrosis. Virchows Arch B Cell Pathol 1969; 3: 365–376, [PUBMED], [INFOTRIEVE]
- Cantu TG, Hoehn-Saric EW, Burgess KM, Racusen L, Scheel PJ. Acute renal failure associated with immunoglobulin therapy. Am J Kidney Dis 1995; 25: 228–234, [PUBMED], [INFOTRIEVE]
- Khalil M, Shin HJ, Tan A, DuBose TD, Jr, Ordonez N, Katz RL. Macrophage like vacuolated renal tubular cells in the urine of a male with osmotic nephrosis associated with intravenous immunoglobulin therapy. A case report. Acta Cytol 2000; 44: 86–90, [PUBMED], [INFOTRIEVE]
- Visweswaran P, Massin EK, Dubose TD, Jr. Mannitol-induced acute renal failure. J Am Soc Nephrol 1997; 8: 1028–1033, [PUBMED], [INFOTRIEVE]
- Levy JB, Pusey CD. Nephrotoxicity of intravenous immunoglobulin. Q J Med 2000; 93: 751–755
- Silbernagl S. The role of brush border enzymes in tubular reabsorption of disaccharides: a microperfusion study in rat kidney. Pflugers Arch 1977; 371: 141–145, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Centers for Disease Control and Prevention (CDC). Renal insufficiency and failure associated with immune globulin intravenous therapy—United States, 1985–1998. MMWR Morb Mortal Wkly Rep 1999; 48: 518–521