Abstract
Objective. The aim of this study was to evaluate the importance of glomerular expression of von Willebrand factor (vWF) in human renal allografts. Methods. We investigated graft biopsies from 72 renal transplant recipients, 40 with acute rejection (AR) and 32 with chronic allograft nephropathy (CAN). All biopsy specimens were immunostained with vWF and CD68 and graded using 3-tiered scales. The follow-up biopsies of patients with AR were reevaluated for development of glomerular sclerosis. Results. A significant difference was found between type 1 and type 2 AR with regard to glomerular vWF expression (P < 0.01). None of the patients with type 1 AR showed mesangial vWF expression, but 36.4% of patients with type 2 AR showed segmental mesangial vWF expression. In follow-up biopsies, 18 of 40 patients developed significant glomerular sclerosis, and patients with mesangial vWF expression (grade 3 GvWF) showed glomerular sclerosis earlier than did others (P < 0.01). In addition, the outcome for grafts that showed grade 3 glomerular vWF was significantly worse than was the outcome noted for grafts that showed grade 1 or grade 2 glomerular vWF (P < 0.001). Half of the biopsy specimens in the CAN group showed global mesangial vWF expression. Glomerular macrophage infiltration was correlated with degree of glomerular vWF expression both in the AR and in the CAN groups (P < 0.05, P = 0.001, respectively). Conclusion. We hypothesized that the increasing amount of glomerular vWF may be used as a marker of acute vascular rejection and may help for the evaluation of renal allograft biopsies without sufficient arteries. In addition, it can also be a marker for development of early glomerular sclerosis and may help us determine which patients are in need of further treatment.
INTRODUCTION
Endothelial cells are involved in many physiological and pathological processes, including graft rejection. Vascular endothelial cells in renal allografts play a part in most events in allograft rejection, including the initial recruitment of cells into the graft, expression of MHC class I and II molecules (which provide alloantigen-dependent signals and activation responses via the direct pathway of allorecognition), induction of angiogenesis through secretion of growth factors, and thus, initiation of early interstitial fibrosis.Citation[1],Citation[2]
Expression of adhesion molecules on endothelial cells in renal allografts and secretion of cytokines by these cells are well-documented in the literature. However, little is known about other, non-immunologic substances that are produced by endothelial cells, such as von Willebrand factor (vWF). This factor is a multimeric plasma glycoprotein secreted by endothelial cells and platelets that plays key roles in platelet adhesion, thrombus formation, and coagulation.Citation[3] In the endothelial cell, vWF is stored in a rod-shaped organelle called the Weibel-Palade body.Citation[4] vWF can be found in platelet alpha-granules, plasma, and subendothelium.Citation[3] Mature vWF comprises a series of multimers that range in molecular weight from 500 kDa to over 10,000 kDa, each one with a complex structure. Investigation has shown that stimulation of endothelial cells leads to rapid release of this multimeric form.Citation[5] These multimers are most effective in promoting platelet adhesion and aggregation.
Deposition of vWF has been demonstrated in the glomerular mesangium of various chronic glomerulonephritidesCitation[6] and in primate renal allografts with CAN.Citation[7] However, the significance of intraglomerular vWF deposition in human renal allografts has not been investigated. The aim of this study was to evaluate the importance of intraglomerular vWF expression in human renal allografts that exhibit deteriorated function after renal transplantation.
MATERIAL AND METHODS
Patient Selection
Seventy-two patients, 18 year of age or older (44 men and 28 women; mean age, 32.5 ± 1.3 years; age range, 18–59 years) who received their first renal allograft at Baskent University were enrolled in this series. The renal grafts came from cadaveric donors in 16 patients and from living-related donors in 56 cases. Patients were carefully matched for risk factors that would predispose them to glomerular sclerosis. Patients with cyclosporine toxicity, CMV infection, and other infections were excluded from the study.
A diagnosis of acute CsA toxicity was made on the basis of clinical (patients with a rapid return of renal function after reduction of CsA dose) and laboratory (CsA blood levels) criteria, including histopathology (i.e., presence of isometric vacuolization on proximal tubules, giant mitochondria, or thrombotic microangiopathy) of the biopsy specimens.
Triple-drug immunosuppressive protocols were used in all cases, specifically corticosteroids, calcineurin inhibitors (cyclosporine or FK506), and azathioprine or mycophenolate mofetil. In each case, methylprednisolone (1 mg/kg/d) was initiated 3 days before transplantation and was tapered by 10 mg/d until a dose of 20 mg/d was reached. This dosage was continued until the end of the third post-transplantation month and was then reduced to 10 mg/d. This maintenance dose was continued for 6 months.
During follow-up, the criteria for performing a renal allograft biopsy were proteinuria, elevated creatinine, suspected rejection, suspected infection, delayed graft function, and poor response to anti-rejection therapy. Specimens were obtained using a biopsy gun with an 18‐gauge needle. In each case, two core specimens were used for light microscopy and one was used for immunofluorescence analysis. Two biopsy cores were fixed in formalin and embedded in paraffin.
For light microscopy assessment, several sections (3 μm thick) were obtained from each paraffin-embedded block, and separate slides were stained with hematoxylin-eosin, periodic acid-Schiff, Congo red, and Masson's trichrome. One pathologist with no knowledge of the results of previous scoring or of clinical data or outcome scored the biopsies according to the Banff classification.Citation[8] One of the participants used these scores to select the biopsies in such a way that there was a nearly equal distribution of scores for the various types of lesions. Biopsy specimens from the first 40 patients with an episode of AR were chosen, and biopsy specimens that showed tubulointerstitial scarring, necrosis, or evidence of tubular injury were excluded. In addition, biopsy specimens of 32 patients with CAN were chosen. All biopsies met Banff classification criteria for renal allograft biopsies. The mean follow-up of the selected 72 patients was 38 ± 3.2 months (range, 26–128 months).
Treatment of the initial AR episode was a 3-day course of 500 mg methylprednisolone. Lack of response to two such courses of pulse-steroid treatment was defined as steroid-resistant AR, and OKT3 treatment was initiated. This therapy was scheduled for 14 days (5 mg/d). During OKT3 therapy, calcineurin inhibitors were discontinued, and CD3 levels were monitored daily.
After AR treatment, patients were monitored for recurrent rejection episodes and graft outcome. Each patient with an AR episode had follow-up biopsies. Patients with an insufficient number of follow-up biopsies (at least two, taken yearly) were excluded from the study. Follow-up biopsy specimens of all patients with AR were reevaluated for development of significant glomerular sclerosis. “Significant glomerular sclerosis” was diagnosed if the glomeruli with global sclerosis were 35% or more of the total glomeruli. (No significant functional deterioration was seen in our cases with glomerulosclerosis lower than 35%. Because of this reason the cut-off point of glomerulosclerosis was chosen as 35%.)
We evaluated recipients' renal function using serial creatinine level measurements. Reversal of rejection was defined as a return of the patient's serum creatinine concentration to at least 20% of the baseline serum creatinine concentration. Graft loss was considered to have occurred when the patient's glomerular filtration rate was under 25 mL/minute.
Immunohistochemistry
Immunostaining was performed on sections (3 μm thick) cut from the previously mentioned archived cores. Renal tissue specimens of five autopsied patients (age < 50 years) with normal histologic profiles were used as controls. None of the controls died as the result of renal and/or cardiac disease.
For glomerular deposition of vWF, each biopsy specimen was immunostained with vWF (Dako, Denmark) antibody using the streptavidin-biotin peroxidase method on formalin-fixed, paraffin-embedded specimens.
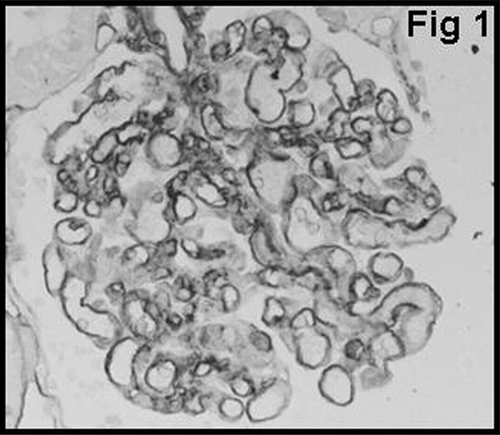
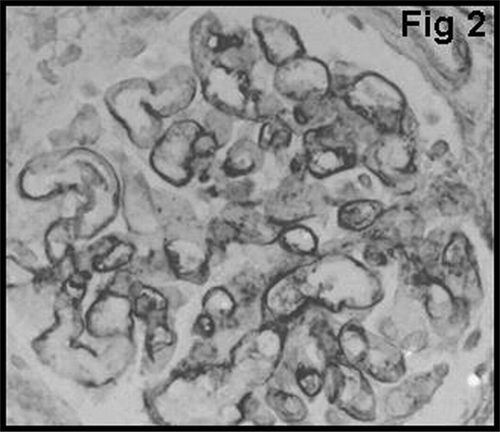
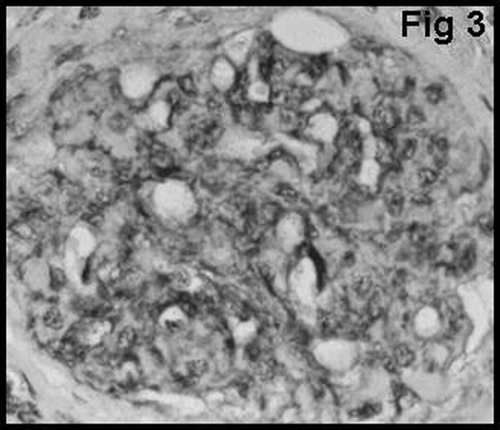
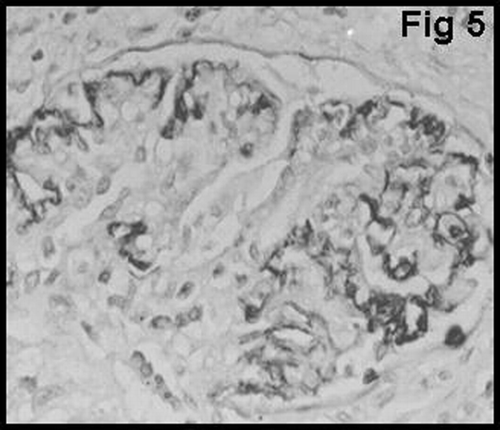
Glomerular staining for vWF was graded using 3-tiered scales to signify positivity in the glomerular endothelium, subendothelium, and mesangium. The scale for vWF was as follows: GvWF 1: staining in the glomerular endothelium (); GvWF 2: staining in the glomerular subendothelium (); and GvWF 3: staining in the mesangial cells and mesangium ().
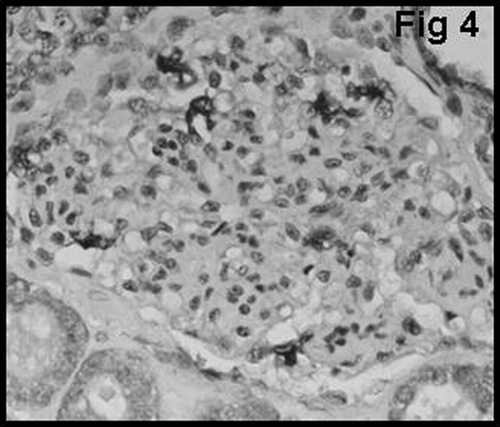
The proportion of macrophages in the glomerulus was examined using the monoclonal antibody CD68 (KPI clone, Dako, Denmark). CD68 positive macrophages in the glomerulus were graded as follows: GM0 = no glomerular macrophage; GM1 = 1 to 3 macrophages per glomerulus; and GM2 = more than 3 macrophages per glomerulus ().
Statistics
Statistical analyses were performed using SPSS software (Statistical Package for the Social Sciences, version 9.05, SSPS Inc., Chicago, IL, USA). For quantitative variables, values are given as means±SE. Mean values were compared using the Student t test for normally distributed variables. The Kruskal-Wallis test was used to compare variables that were not normally distributed. Fischer's exact test and Chi-square testing were used to compare categorical data. Values for P less than 0.05 were accepted as statistically significant.
RESULTS
Of 72 patients, 40 showed AR and 32 showed CAN. Of the 40 patients demonstrating AR, 18 showed type 1 AR and 22 showed type 2 AR.
Immunohistochemical staining of vWF in specimens from control patients (n = 5) showed vWF expression in the endothelium of blood vessels and glomerular capillary loops. None of the control patients' specimens showed mesangial and/or subendothelial staining in the glomeruli.
shows that there was a significant difference between type 1 and type 2 AR with regard to glomerular vWF expression (P < 0.01). None of the patients with type 1 cellular AR showed mesangial vWF (GvWF 3) expression. Only 8 of 40 patients showed mesangial vWF expression, and all of them had type 2 vascular AR. In most of the glomeruli in patients with acute vascular rejection, distribution of mesangial vWF expression was found to be segmented (). Glomerular macrophage infiltration showed a strong correlation with the degree of glomerular vWF expression in patients with AR (P < 0.05) ().
Table 1 Correlation of glomerular vWF (GvWF) expression with the type of AR
Table 2 Relationship of glomerular vWF (GvWF) expression and glomerular macrophage (GM) infiltration in patients with AR
Histological control biopsies after the anti-rejection treatment and follow-up biopsies of all patients with AR were available. In follow-up biopsies, 18 of 40 patients developed significant glomerular sclerosis. The mean time from initial biopsy study to development of glomerular sclerosis was 27.4 ± 6.2 months. A positive correlation between the degree of glomerular vWF expression and development of glomerular sclerosis on follow-up biopsies was found in patients with AR (P < 0.01) ().
Table 3 Influence of glomerular vWF (GvWF) expression on the development of glomerular sclerosis (GS) in follow-up biopsies in patients with AR
Development of glomerular sclerosis occurred earlier in seven cases (87.5%) with grade 3 glomerular vWF expression (15.4 ± 1.2 months) when compared with the other three cases (20%) with grade 1 (31.6 ± 5.1 months) and eight cases (47.1%) with grade 2 (23.4 ± 3.7 months) glomerular vWF expression (P < 0.01). The intervals for these three groups were significantly different (P < 0.05).
During follow-up, 16 of 40 patients with AR lost their graft at an average of 14.9 ± 1.6 months (range, 5–30 months). The remaining 14 patients with AR showed functional deterioration, and 10 patients showed stable renal functions in the months following (mean, 32.7 ± 6.6 months). Worse graft outcomes were found with increasing amounts of glomerular vWF expression (). All patients with grade 3 glomerular vWF expression had graft loss, while only 26% of the patients with grade 1 and 23% of the patients with grade 2 glomerular vWF expression had graft loss. Nearly half the patients with grade 1 glomerular vWF expression showed stable renal function, while only 17% of the patients with grade 2 glomerular vWF expression showed stable renal function. In addition, functional deterioration was determined to be 26% and 59% in patients with grade 1 and grade 2 glomerular vWF expression, respectively.
Table 4 Relationship between glomerular vWF (GvWF) expression and graft outcome in patients with AR
Of 32 patients with CAN, 8 showed grade 1, 14 showed grade 2, and 10 showed grade 3 CAN. shows that there was a significant difference between the grades of CAN with regard to glomerular vWF expression (P < 0.05). Half (50%) of the patients with CAN showed markedly increased expression of vWF in the mesangium. The distribution of mesangial vWF expression was global in these patients.
Table 5 Correlation of glomerular vWF (GvWF) expression with grade of CAN
Glomerular macrophage infiltration showed a strong correlation with the degree of glomerular vWF expression in patients with CAN (P = 0.001) ().
Table 6 Relationship of glomerular vWF (GvWF) expression and glomerular macrophage (GM) infiltration in patients with CAN
In addition, a significant difference was found between patients with AR and CAN with regard to glomerular vWF expression (P = 0.001) ().
Table 7 Expression of glomerular vWF expression in patients with AR and CAN
DISCUSSION
Endothelial cells are the only cell type of donor origin that come in contact with circulating recipient immune cells, and they play an integral role in allograft rejection. Several studies have reported the immune mechanisms involved in endothelial cells, but almost nothing is known about non-immune mechanisms. Von Willebrand factor is a non-immunological substance produced by endothelial cells, and the impact of this substance on human renal allografts has not been previously reported. It has been previously reported, however, that renal allografts removed from rhesus monkeys that showed chronic rejection were associated with high levels of glomerular mesangial vWF deposition, whereas kidneys removed owing to AR showed low-to-minimal staining for vWF in the glomeruli, and the vWF staining was confined largely to the endothelium.Citation[7]
In the current study, glomerular vWF expression was confined largely to the endothelium and subendothelium in patients with AR. In contrast with previous studies, some of our patients with AR showed segmental mesangial and subendothelial vWF expression. Only patients with vascular AR (type 2) showed mesangial vWF expression, and none of the patients with cellular acute rejection (type 1) showed mesangial vWF deposition.
In agreement with the other study, the subendothelial connective tissue of the glomerular capillaries and the mesangium stained strongly for vWF in our patients with CAN. Of 32 patients with CAN, 16 showed (50%) strong global mesangial staining, whereas only 20% of the patients with AR showed segmental mesangial staining.
Endothelial activation and injury are very significant in the process of acute vascular rejection. It has been reported that the secretion of vWF most likely increases with activation of the endothelial cells and with the immunological injury of endothelial cells.Citation[3–5] Supporting this, we found that our cases with vascular rejection showed increased mesangial and subendothelial vWF expression, while cases with cellular rejection did not show mesangial vWF expression. Thus, we suggested that the increasing amount of glomerular vWF may be used as a marker of immunologically mediated acute vascular injury and may help for the evaluation of renal allograft biopsies without sufficient arteries. But, this suggestion will not be true for other than cases with acute vascular rejection because we noticed that most of our cases with CAN have mesangial vWF deposition and we cannot use increased glomerular vWF expression as a marker for acute vascular injury in these cases. Lagoo and coworkersCitation[7] reported that the reason of increased mesangial vWF deposition in cases with CAN is not only due to endothelial activity, but also due to the exudation of vWF from plasma from subsequent leaky endothelium. This hypothesis would explain why mesangial deposition is increased in cases with CAN. As the rejection process progresses to CAN, alteration in the structure of the endothelium becomes pronounced,Citation[9],Citation[10] and therefore, leakage from the endothelium increases. Thus, increased secretion from activated endothelial cells and increased exudation of vWF from plasma may explain the strong and distinctive mesangial vWF deposition in most patients with CAN.
Repeated episodes of endothelial activation and injury follow secretion of platelet-derived growth factor (PDGF), monocyte chemo attractant protein 1 (MCP-1), transforming growth factor beta (TGF-β), and chemokines that favor further entry of macrophages into the glomeruli.Citation[1],Citation[11] In our previous study, we showed that intraglomerular macrophage accumulation was increased both in patients with AR and CAN and was related with poor graft outcome.Citation[12] Similarly, in the present study, we found a significant increase in intraglomerular macrophages in both patients with AR and with CAN. Intraglomerular macrophages, with their ability to produce tumor necrosis factor alpha (TNF-α), TGF-β, macrophage-derived growth factor (MDGF), PDGF, and MCP-1 increase expression of adhesion molecules, induce proliferation of fibroblasts, promote endothelial activation, and therefore, increase secretion of vWF in the glomeruli.Citation[2],Citation[11] Our results confirm the suggestion that a significant relationship exists between intraglomerular macrophage infiltration and intraglomerular vWF deposition. In addition, both the activated endothelial cells and macrophages in the glomeruli will induce the secretion of growth factors, such as PDGF, TGF-β, and MDGF, which promote mesangial matrix accumulation.Citation[2],Citation[13],Citation[14] This theory was supported by our findings that patients with AR, who showed subendothelial and mesangial vWF staining, developed significant glomerular sclerosis in subsequent months. Thus, increased intraglomerular vWF expression and intraglomerular macrophage accumulation may help us determine which patients are in need of treatment to prevent early development of glomerulosclerosis, because sclerosis of glomeruli may be preventable with certain therapeutic options.
Recent studies indicate that aside from its anticoagulant action, heparin has immunobiological activities that inhibit cell trafficking to the site of an antigen. Additionally, heparin inhibits smooth muscle cell proliferation and decreases synthesis of extracellular matrix proteins.Citation[15],Citation[16] These non-anticoagulant therapeutic possibilities of heparin and glycosaminoglycans have been used in renal allograft recipients and in patients with diabetes mellitus. It has been shown in both groups that low–molecular-weight heparin has potential therapeutic value for preventing proteinuria, glomerulosclerosis, and CAN.Citation[16–18] These renoprotective properties of heparins also have been reported in experimental models of proliferative glomerulopathies and glomerulosclerosis.Citation[19],Citation[20]
In conclusion, we hypothesized that the increasing amount of glomerular vWF may be used as a marker of acute vascular rejection and may help for the evaluation of renal allograft biopsies without sufficient arteries. In addition, it can also be a marker for development of early glomerular sclerosis in these cases and may help us determine which patients are in need of treatment to prevent early development of glomerulosclerosis.
REFERENCES
- Denton MD, Davis SF, Baum MA, Melter M, Reinders ME, Exeni A, Samsonov DV, Fang J, Ganz P, Briscoe DM. The role of the graft endothelium in transplant rejection: evidence that endothelial activation may serve as a clinical marker for the development of chronic rejection. Pediatr. Transplant 2000; 4: 252–260, [INFOTRIEVE], [CSA]
- Ozdemir BH, Ozdemir FN, Gungen Y, Haberal M. Role of macrophages and lymphocytes in the induction of neovascularization in renal allograft rejection. Am. J. Kidney Dis 2002; 39: 347–353, [INFOTRIEVE], [CSA]
- Meyer D, Girma JP. von Willebrand factor: structure and function. Thromb. Hemost 1993; 70: 99–104, [CSA]
- Wagner DD, Olmsted JB, Marder VJ. Immunolocalization of von Willebrand protein in Wiebel-Palade bodies of human endothelial cells. J. Cell Biol 1982; 95: 355–360, [INFOTRIEVE], [CSA], [CROSSREF]
- Sporn LA, Marder VJ, Wagner DD. Inducible secretion of large, biologically potent von Willebrand factor multimers. Cell 1986; 46: 185–190, [INFOTRIEVE], [CSA], [CROSSREF]
- Sato M, Koshikawa S, Arakawa M. Glomerular deposition of antihemophilic factor antigen in various renal diseases. Clin. Nephrol 1981; 15: 80–86, [INFOTRIEVE], [CSA]
- Lagoo AS, Buckley PJ, Burchell LJ, Peters D, Fechner JH, Tsuchida M, Dong Y, Hong X, Brunner KG, Oberley TD, Hamawy MM, Knechtle SJ. Increased glomerular deposits of von Willebrand factor in chronic, but not acute, rejection of primate renal allografts. Transplantation 2000; 70: 877–886, [INFOTRIEVE], [CSA], [CROSSREF]
- Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Yamaguchi Y, et al. The Banff 97 working classification of renal allografts pathology. Kidney Int 1999; 55: 713–723, [INFOTRIEVE], [CSA], [CROSSREF]
- Monga G, Mazzucco G, Novara R, Reale L. Intertubular capillary changes in kidney allografts: an ultrastructural study in patients with transplant glomerulopathy. Ultrastruct. Pathol 1990; 14: 201–209, [INFOTRIEVE], [CSA]
- Kiyici H, Demirhan B, Özdemir BH, Aktaş G, Karabay G, Haberal M. Significance of peritubular capillary basement membrane multilamellation in diagnosis of chronic allograft nephropathy. Transplant Proc 2003; 35: 2643–2644, [INFOTRIEVE], [CSA], [CROSSREF]
- Schlondorff D, Nelson PJ, Luckow B, Banas B. Chemokines and renal disease. Kidney Int 1997; 51: 610–621, [INFOTRIEVE], [CSA]
- Özdemir BH, Demirhan B, Gungen Y. The presence and prognostic importance of glomerular macrophage infiltration in renal allografts. Nephron 2002; 90: 442–446, [CSA], [CROSSREF]
- Pichler R, Giachelli C, Young B, Alpers CE, Couser WG, Johnson RJ. The pathogenesis of tubulointerstitial disease associated with glomerulonephritis: the glomerular cytokine theory. Miner. Electrolyte Metab 1995; 21: 317–327, [INFOTRIEVE], [CSA]
- Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C. Macrophages and angiogenesis. J. Leuk. Biol 1994; 55: 410–422, [CSA]
- Floege J, Eng E, Young BA, Couser WG, Johnson RJ. Heparin suppresses mesangial cell proliferation and matrix expansion in experimental mesangioproliferative glomerulonephritis. Kidney Int 1993; 43: 369–380, [INFOTRIEVE], [CSA]
- Gradowska L, Lao M, Morzycka-Michalik M, Gorski A. Low dose heparin: efficacious treatment for chronic renal allograft rejection. Arch. Immunol. Ther. Exp. (Warz) 1993; 41: 133–135, [CSA]
- Krzossok S, Birck R, Koeppel H, Schnulle P, Waldherr R, van der Wounde FJ, Braun C. Treatment of proteinuria with low-molecular weight heparin after renal transplantation. Transplant Int 2004; 17: 468–472, [CSA]
- van der Pijl JW, van der Woude FJ, Geelhoed-Dujvestijn PH, Fröhlich M, van der Meer FJ, Lemkes HH, van Es LA. Danaparoid sodium lowers proteinuria in diabetic nephropathy. J. Am. Soc. Nephrol 1997; 8: 456–462, [INFOTRIEVE], [CSA]
- Ceol M, Vianello D, Schleicher E, Anglani F, Barbanti M, Bonfante L, Bertaglia G, Graziotto R, D'Angelo A, Del Prete D, Gambaro G. Heparin reduces glomerular infiltration and TGF-β protein expression by macrophages in puromycin glomerulosclerosis. J. Nephrol 2003; 16: 210–218, [INFOTRIEVE], [CSA]
- Braun C, Schultz M, Fang L, Schaub M, Back WE, Herr D, Laux V, Rohmeiss P, Schnuelle P, van der Woude FJ. Treatment of chronic renal allograft rejection in rats with a low-molecular weight heparin (Reviparin). Transplantation 2001; 72: 209–215, [INFOTRIEVE], [CSA], [CROSSREF]