Abstract
Background. Radiocontrast-induced nephropathy (CIN) remains an important iatrogenic cause of acute renal failure in high-risk patients, despite the development of safer contrast media, the improvement of hydration protocols, and the introduction of additional preventive strategies. Erythropoietin (EPO) pretreatment may confer protection against acute renal failure through the induction of stress response genes. Methods. The effect of EPO has been evaluated in a rat model of CIN, induced by iothalamate, following the inhibition of nitric oxide- and prostaglandin-synthesis with indomethacin and Nω nitro-L-arginine methyl ester (L-NAME). Twenty-two male Sprague-Dawley rats were subjected to saline (CTR) or EPO injections (3000 U/kg and 600 U/kg, 24 and 2 h before the induction of CIN, respectively). Results. The decline in creatinine clearance in CTR animals from 0.38 ± 0.03 to 0.28 ± 0.03 mL/min/100 g (p < 0.005), was prevented by EPO pretreatment (from 0.34 ± 0.02 to 0.32 ± 0.03 mL/min/100 g, NS). The extent of medullary thick ascending limb- and S3-tubular damage in the outer medulla, however, was comparable in the two experimental groups. Conclusions. EPO pretreatment prevents renal dysfunction in a rat model of CIN. Further experimental and clinical studies are required to confirm these preliminary conclusions regarding a potential protective potency of EPO against CIN.
INTRODUCTION
Contrast medium-induced nephropathy (CIN) is a prominent cause of in-hospital acute renal failure, together with reduced renal perfusion, surgery, and other iatrogenic causes.Citation[[1]] The incidence of CIN with classically-admitted biochemical definition of this disorder among high-risk patients ranges between 10–25%, despite the introduction of newer low- or iso-osmolar contrast media, the improvement of hydration protocols, and the implementation of other preventive strategies, such as acetylcysteine.Citation[[2]] Renal parenchymal hypoxic injury plays an important role in the pathogenesis of CIN, with medullary oxygenation markedly declining to critically low levels following the administration of radiologic contrast agents.Citation[[3]] In intact animals, contrast-induced medullary hypoxia is not associated with renal dysfunction or overt tubular necrosis. Yet, it invokes hypoxic stress response, initiated by the regional accumulation of hypoxia-inducible factors (HIF), and subsequent up-regulation of HIF-dependent response genes,Citation[[4]] providing cellular adaptation to the hypoxic stress. By contrast, CIN develops under experimental settings whenever mechanisms designed to maintain medullary oxygen sufficiency are altered:Citation[[3]] the inhibition of prostaglandin- or NO-synthesis, mimicking clinical conditions that predispose to CIN, intensifies contrast-induced medullary hypoxic stress and ultimately leads to a decline in kidney function.Citation[[5]] Tubular HIF-1α can be consistently demonstrated under such conditions.Citation[[4]] Endothelial hypoxic stress, leading to dysfunction and damage, probably further enhances medullary hypoxia.Citation[[3]] Indeed, medullary endothelial cells express HIF-2α as well following administration of radiologic contrast agents.Citation[[4]] These nephrotoxins likely inflict an acute and severe renal hypoxic stress in high-risk patients, before the occurrence of an appropriate cellular adaptive response. Consequently, apoptotic and necrotic tubular injury appears under experimental settings within a short period and progresses over 24 h.Citation[[6]], Citation[[7]]
Erythropoietin (EPO) is one of the principal stress response genes, induced by HIF. Whereas its function in the regulation of red blood cell production has been recognized for years, a physiologic role for ubiquitously formed EPO has only recently been acknowledged. As extensively reviewed elsewhere,Citation[[8]] EPO was found to act as a pleiotropic survival and growth factor, conferring protection against hypoxia in various organ systems, including neuronal, retinal, cardiac, and hepatic tissues. Indeed, promising pilot studies in patients with stroke indicate that EPO may be used safely and effectively in humans in the prevention of ischemic tissue damage.Citation[[8]] EPO has also been reported recently to attenuate experimental acute hypoxic and toxic renal parenchymal injury.Citation[[9]] We hypothesized that a timely exogenous administration of EPO, preceding the administration of the radiologic contrast agent, might attenuate the development of CIN. This hypothesis was tested in a rat model of medullary hypoxic injury, induced by the administration of contrast medium, following the inhibition of prostaglandin- and NO-synthesis.
METHODS
Male Sprague-Dawley rats (334 ± 3 g), purchased from Harlen Co. were kept in metabolic cages for 24 h urine collections, fed on regular chaw and with free excess to water. Commercial recombinant human EPO-alpha was used (Eprex®, Janssen Cilag Co). All other chemicals were purchased from Sigma. Experiments were conducted in accord with the NIH Guide for the Care and Use of Laboratory Animals.
Experimental Design
Rats were randomized to receive EPO or saline (n = 11 per group) 24 h and 2 h prior to the CIN protocol (3000 U/kg and 600 U/kg, respectively, injected IV into the femoral vein under light ether anesthesia). Following baseline 24-h urine collection period the rats were anesthetized with ketamine (100 mg/kg IP), the femoral artery and vein were cannulated with polyethylene catheters (PE-50, Clay-Adams, Parsippany, NJ, USA) for drug administration, and baseline blood samples were obtained. All animals were subjected to the previously detailedCitation[[5]] CIN protocol, consisting of indomethacin (10 mg/kg IV), followed at 15' and at 30' with Nω nitro-L-arginine methyl ester (L-NAME, 10 mg/kg IV) and with the high-osmolar radiologic contrast agent meglumine iothalamate (Conray 60%, 6 mL/kg intra-arterial), respectively. The rats were allowed to recover in the metabolic cages for an additional 24 h-period, and then anesthetized with pentobarbital (60 mg/kg). A second set of urine and blood samples were obtained (day 1 period) and the kidneys were perfusion-fixed in vivo with glutaraldehyde for morphologic evaluation through the abdominal aorta.
Assessment of Renal Function
Blood and urine samples were analyzed for creatinine, urea, Na, and K, using Kodak dry reagent system, and corresponding creatinine clearance (ClCr), tubular sodium reabsorption (TRNa), and fractional potassium excretion (FEK) were calculated. Biochemical definition of CIN was set at a >0.25 mg/dL increase in plasma creatinine (22.1 μmol/L).
Renal Morphology
Kidney slices were postfixed in buffered 2% OsO4, dehydrated, and embedded in an Araldite-EM bed 812 mixture. Large sections were cut perpendicular to the renal capsule, containing the cortex and medulla. Sections (1 μm) were analyzed in a blinded fashion for morphologic alterations. As previously detailed,Citation[[5]] tubular necrosis was determined separately for S3 proximal tubules in the outer stripe and for medullary thick limbs (mTALs) in the outer, mid- and inner zones (A, B, and C zones, respectively) of the inner stripe of the outer medulla. The extent of damage was expressed as the percentage of necrotic tubules out of total tubules counted. Papillary necrosis was semi-quantitatively assessed using a 0–2 scale as previously outlined.Citation[[10]]
Hemodynamic Studies
In three additional anesthetized rats blood pressure was monitored for 2 h following EPO administration (300 U/kg IV), using femoral arterial lines connected to pressure transducer.
Statistics
Data are presented as the means ± SEM. Student t-test was used for within-group comparisons of functional parameters, and for between-group comparisons of morphological findings in the control and EPO-treated animals. Statistical significance was set at p = 0.05.
RESULTS
As shown in , control rats subjected to the CIN protocol developed contrast induced renal injury, with plasma creatinine rising from 52 ± 4 to 72 ± 5 μmol/L at 24 h (p < 0.001), and creatinine clearance falling from 0.38 ± 0.03 to 0.28 ± 0.03 mL/min/100 g (p < 0.005). By contrast, in rats pretreated with EPO, the rise in plasma creatinine was blunted, and the decline in creatinine clearance was prevented. CIN, defined as a rise in PCr of = 0.25 mg/dL (22.1 μmol/L) tended to be more prevalent in the CTR group (5 out of 11 rats vs. 2 out of 11 animals in the EPO group, Chi Square test p = 0.13, NS).
Table 1 Functional parameters in the two experimental groups
An increase in urine volume, perhaps indicative of defective urinary concentration, was more prominent in the control group (p < 0.01 for between-group comparison). Weight reduction by 17 g on the average was noted in both experimental groups. The rise in plasma urea was also comparable in both groups. The effect of the CIN protocol upon tubular handling of sodium and potassium were also similar in the two experimental groups.
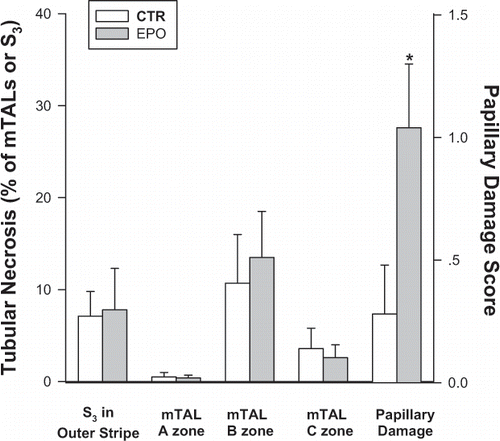
As shown in , the extent of outer medullary tubular damage, assessed 24 h after insult was also almost identical in the two experimental groups, involving about 7% of S3 segments of the proximal tubules in the outer stripe and some 12% of mTALs in the mid-inner stripe of the outer medulla. By contrast, the two groups differed by the extent of papillary necrosis, which was paradoxically more pronounced in the EPO group as compared with CTR animals (p = 0.03).
Figure 1 Morphologic assessment of tubular damage in the Control (CTR) and EPO groups. The extent of tubular necrosis among S3 segments of the proximal tubules in the outer stripe, as well as medullary thick ascending limbs (mTALs) in the outer- (A), mid- (B), and inner (C) zones of the inner stripe of the outer medulla are expressed as the percentage of damaged tubules out of all tubules counted. Papillary damage is assessed semi-quantitatively, using a 0–3 score. *p = 0.03 vs. CTR, unpaired t-test.
Systemic blood pressure remained unchanged following EPO IV injection in the ancillary hemodynamic experiments (data not shown).
DISCUSSION
CIN is a predictable iatrogenic form of acute tubular necrosis (ATN) that develops in high-risk patients, despite various protective measures. This adverse effect is associated with increased morbidity, mortality, and medical expenses, and often precludes studies with iodinated radiologic contrast agents and angiographic interventions in patients with advanced renal dysfunction and other predisposing morbidities.Citation[[2]] The development of new measures to prevent this complication is therefore critically needed. This short communication illustrates that EPO treatment, initiated 24 h before the administration of contrast, might confer protection against CIN. EPO attenuated the decline in kidney function in a rat model of CIN over 24 h, though it did not alleviate the degree of hypoxic tubular damage, noted by that time.
Tubular apoptotic or necrotic death represents the extreme outcome of contrast medium-induced hypoxia. Most often, however, medullary cells express adaptive stress response and survive. Radiocontrast-induced medullary hypoxia leads to the regional accumulation of hypoxia-inducible factors (HIF),Citation[[4]] key elements in the renal parenchymal adaptive response to hypoxic stress.Citation[[11]] Under low cellular oxygen tension, degradation of constitutively-formed HIF-α subunits is inhibited and it accumulates within the cytoplasm and binds to HIF-β subunits. The formed αβ heterodimers translocate into the nucleus and bind to nuclear “hypoxia-responsive elements,” leading to transcriptional activation of a wide set of genes, including EPO. HIF-target genes are involved in changes in cell cycle, glucose utilization, metabolism, angiogenesis, vascular tone regulation, and scavenging of free radicals.Citation[[11]] Up-regulation of these genes is to a large extent responsible for the well-known phenomenon of delayed hypoxic preconditioning; i.e., improved tolerance to repeated hypoxic stress. Indeed, HIF isoforms appear in tubular, interstitial, and vascular endothelial cells following global renal hypoxia, at the margins of infracted regions within the kidney, and in the renal medulla in response to the decline in regional ambient PO2, associated with the induction of diabetes, the inhibition of prostaglandin- or nitric oxide-synthesis, or following the administration of contrast medium.Citation[[3]], Citation[[4]]
The endocrinal role of HIF-mediated renal EPO has long been recognized in the regulation of red cell mass, and selectively involves HIF-2α expression by renal parenchymal cells. A potential physiologic autocrine or paracrine role for EPO other than the regulation of erythropoiesis has recently been documented in the kidney, as in many other tissues. Indeed, most renal cell types express EPO receptors.Citation[[12]] EPO seems to modulate renal cellular response to stress, and to attenuate apoptosis and cell necrosis. Anti-apoptotic effect of EPO was recorded in cultured mesangial and tubular cells subjected to hypoxic or toxic stress.Citation[13–15] This effect is initiated by binding of EPO to its receptor, with subsequent attenuation of apoptotic signaling and caspase activation. In addition to direct tubular cell protection, EPO probably improves regional microcirculation and oxygenation by the attenuation of endothelial dysfunction: in vitro EPO enhances endothelial cell viability and proliferation and stimulates angiogenesis.Citation[[8]] EPO may also induce endothelial e-NOS and endothelin synthesis,Citation[[8]] both important for adequate medullary perfusion,Citation[[2]], Citation[[3]] and amplifies its own effects by up-regulation of endothelial EPO receptors.Citation[[8]]
Renal protective effect of EPO has also been documented in vivo. In animals subjected to renal ischemia-reflow injury, EPO attenuates necrosis and apoptosis and enhances tubular proliferation,Citation[[16]] thus hastening functional and morphological tubular recovery.Citation[[5]], Citation[[14]], Citation[16–19] EPO also attenuates acute tubular injury induced by cis-platinum,Citation[[20]] and has been reported to abolish renal dysfunction following hypovolemic shock.Citation[[15]] In addition, EPO retards the progression of chronic tubulointerstitial disease in the 5/6 nephrectomy rat modelCitation[[21]] and prevents the progression of ischemia-mediated cyclosporine nephropathy.Citation[[22]] In the clinical practice, EPO attenuates the decline in kidney function in patients with advanced chronic renal failure,Citation[[23]] suggesting direct renal protective properties against chronic hypoxic stress, unrelated to the correction of anemia.
While EPO generation increases during acute renal ischemia under experimental settings, low EPO levels have been documented in critically ill patients, during acute renal failure,Citation[[24]] and following reduction of renal mass. This may in part reflect a paradoxical rise in renal medullary oxygenation due to diminished glomerular filtration. It is, therefore, conceivable that exogenous EPO might be beneficial, especially under such circumstances.Citation[[9]]
Our findings suggest that exogenous EPO, given in advance of the contrast study, might indeed confer hypoxic tolerance and reduce the risk of contrast induced renal injury. The lack of effect of EPO upon amelioration of morphologic damage does not necessarily contradict the functional results. Similar discrepancy has been noted in the same experimental model during the inhibition of the energy consuming DNA-reparative enzyme poly-(ADP-ribose) phosphorylase (PARPP).Citation[[25]] It may merely reflect a type 2 error (groups too small to detect a statistical significance) due to the great variability that characterizes the degree of medullary damage in our model. Thus, a very large number of animals may be required to confirm a statistically significant moderate attenuation of structural damage. A more plausible explanation for the discrepancy between functional preservation and the lack of structural protection is that the histological changes at 24 h do not represent the extent of reversible tubular damage. Hypoxic mTAL damage, characterized by swollen mitochondria and nuclear pyknosis, might be transient and reversible if hypoxic stress is attenuated,Citation[[26]] and may be entirely missed by 24 h. Moreover, apoptotic cell death, clearly defined during earlier phases of hypoxic outer medullary stress,Citation[[7]] is limited at later time periods.
We have previously documented the early appearance of cells with condensed cytoplasm in mTALs following outer medullary hypoxic injury that disappear at later stages.Citation[[6]] Interestingly, EPO-mediated attenuation of apoptosis and enhancement of cell proliferation was specifically noted in mTALs following ischemia-reflow,Citation[[16]] and renal expression of sodium transporters characterized for this nephron segment was preserved.Citation[[19]] Taken together, these works indicate that the histological changes at 24 h may by-and-large underestimate an effect of EPO in the attenuation of apoptotic or reversible hypoxic tubular changes. Possibly, EPO-mediated attenuation of renal endothelial dysfunction might also participate in the functional conservation without a marked impact upon morphology. EPO-mediated restoration of altered glomerular hemodynamics or filtration fraction are other possibilities to be considered, though EPO infusion was not shown to affect glomerular filtration rate in intact animals.Citation[[27]] Finally, a potential acute effect of intravenous EPO upon systemic hemodynamics as a cause for the preserved renal function has been excluded by the continuous monitoring of blood pressure in few additional rats administered with EPO, resembling Tojo's report in normotensive animals.Citation[[27]]
The similar rise in plasma urea in both groups probably represents comparable peri-operative dehydration, in part, perhaps, due to defective urinary concentration, as suggested by the increase in urine volume. It also rejects the possibility of an overt pre-renal component for the decline in glomerular filtration occurring selectively in the CTR group.
The basis for the statistically significant paradoxical increase in papillary damage in the EPO group is not clear and may reflect a type 2 error. In our previous studiesCitation[[10]] there has often been a dys-synchrony between morphological changes in the inner stripe and the papilla; i.e., severe papillary injury occasionally occurs with limited outer medullary alterations, and vice versa. Renal dysfunction correlates best with the extent of outer medullary hypoxic damage, rather than with papillary injury score.Citation[[10], and unpublished data] Physiological and morphological integrity of the inner medulla depends principally on direct blood flow to the papilla.Citation[[28]] This region has an extremely low ambient PO2 and has a singularly high level of HIF accumulation under hypoxic conditions.Citation[[4]] Indeed, very small changes in papillary blood flow should and does result in an infarctive type injury.Citation[[10]], Citation[[29]] In this perspective, EPO treatment possibly somehow mitigates papillary blood flow.
The dose and timing of EPO pretreatment were adopted from previous studies in rodents, where EPO was administered a day prior toCitation[[17]], Citation[[18]] or shortly beforeCitation[[14]], Citation[[15]] the induction of acute kidney injury. Noteworthy, the early EPO dose by and large exceeds doses in clinical use and may be cost-prohibited. Further studies should therefore look for the effect of smaller doses. The effect of EPO on renal parenchymal oxygenation and on the extent of apoptosis should also be explored, as well as the evaluation of its effects at additional earlier and later time points after the exposure to contrast.
It is concluded that EPO pretreatment may reduce the risk of CIN. Though our findings are modest and somewhat inconclusive, their implications might be exceptional, given the safety of EPO, its clear-cut protective properties in simpler experimental setups, and the need for effective preventive measures against contrast nephropathy. Confirmation by additional experimental studies and clinical trials is required to endorse these preliminary findings and to optimize EPO dosage and the time of its administration.
ACKNOWLEDGMENTS
This work was supported by the Harvard Medical Faculty Physicians at Beth Israel Deaconess Medical Center, Boston, MA, and by the Russell Berrie Foundation and D-Cure, Diabetes Care in Israel.
REFERENCES
- Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am. J. Kidney Dis. 2002; 39: 930–936, [INFOTRIEVE], [CSA]
- Heyman SN, Rosen S. Dye Nephropathy. Semin. Nephrol. 2003; 23: 477–485, [INFOTRIEVE], [CSA], [CROSSREF]
- Heyman SN, Rosenberger C, Rosen S. Regional alterations in renal hemodynamics and oxygenation: a role in radiocontrast nephropathy. Nephrol. Dial. Transpl. 2005; 20(Suppl 1)i6–i11, [CSA], [CROSSREF]
- Rosenberger C, Heyman SN, Rosen S, et al. Upregulation of HIF in acute renal failure – evidence for a protective transcriptional response to hypoxia. Kidney Int. 2005; 67: 531–542, [INFOTRIEVE], [CSA], [CROSSREF]
- Agmon Y, Peleg H, Greenfeld Z, et al. Nitric oxide and prostanoids protect the renal outer medulla from radiocontrast toxicity in the rat. J. Clin. Invest. 1994; 94: 1069–1075, [INFOTRIEVE], [CSA]
- Heyman SN, Brezis M, Epstein FH, et al. Early renal medullary hypoxic injury from radiocontrast and indomethacin. Kidney Int. 1991; 40: 632–642, [INFOTRIEVE], [CSA]
- Beeri R, Symon Z, Brezis M, et al. Rapid DNA fragmentation from hypoxia along the thick ascending limb of rat kidneys. Kidney Int. 1995; 47: 1806–1810, [INFOTRIEVE], [CSA]
- Jelkmann W, Wagner K. Beneficial and ominous aspects of the pleiotropic action of erythropoietin. Ann. Hematol. 2004; 83: 673–86, [INFOTRIEVE], [CSA], [CROSSREF]
- Heyman SN, Rosenberger C, Rosen S. Erythropoietin – a potential remedy for renal tubular injury?. Kidney Int. 2004; 65: 737–738, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Heyman SN, Fuchs S, Yafe R, et al. Renal microcirculation and tissue damage during acute ureteral obstruction: the effect of saline infusion, indomethacin and radiocontrast. Kidney Int. 1997; 51: 653–663, [INFOTRIEVE], [CSA]
- Maxwell P. HIF-1: an oxygen response system with special relevance to the kidney. J. Am. Soc. Nephrol. 2003; 14: 156–159, [CSA], [CROSSREF]
- Westenfelder C. Unexpected renal actions of erythropoietin. Exp. Nephrol. 2002; 10: 294–298, [INFOTRIEVE], [CSA], [CROSSREF]
- Fishbane S, Ragolia L, Palaia T, et al. Cytoprotection by Darbepoetin/Epoetin alfa in pig tubular and mouse mesangial cells. Kidney Int. 2004; 65: 452–458, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Sharples EJ, Patel N, Brown P, et al. Erythropoietin protects the kidney against the injury and dysfunction caused by ischemia-reperfusion. J. Am. Soc. Nephrol. 2004; 15: 2115–2124, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Abdelrahman M, Sharples EJ, McDonald MC, et al. Erythropoietin attenuates the tissue injury associated with hemorrhagic shock and myocardial ischemia. Shock 2004; 22: 63–69, [INFOTRIEVE], [CSA], [CROSSREF]
- Vesey DA, Cheung C, Pat B, et al. Erythropoietin protects against ischaemic acute renal injury. Nephrol. Dial. Transplant. 2004; 19: 348–355, [INFOTRIEVE], [CSA], [CROSSREF]
- Yang CW, Li C, Jung JY, et al. Preconditioning with erythropoietin protects against subsequent ischemia-reperfusion injury in the rat kidney. FASEB J. 2003; 17: 1754–1755, [INFOTRIEVE], [CSA], [CROSSREF]
- Patel NS, Sharples EJ, Cuzzocrea S, et al. Pretreatment with EPO reduces the injury and dysfunction caused by ischemia/reperfusion in the mouse kidney in vivo. Kidney Int. 2004; 66: 983–989, [INFOTRIEVE], [CSA], [CROSSREF]
- Gong H, Wang W, Kwon TH, et al. EPO and alpha-MSH prevent ischemia/reperfusion-induced down-regulation of AQPs and sodium transporters in rat kidney. Kidney Int. 2004; 66: 683–695, [INFOTRIEVE], [CSA], [CROSSREF]
- Bagnis C, Beaufils H, Jacquiaud C, et al. Erythropoietin enhances recovery after cisplatin-induced acute renal failure in the rat. Nephrol. Dial. Transplant. 2001; 16: 932–938, [INFOTRIEVE], [CSA], [CROSSREF]
- Bahlmann FH, Song R, Boehm SM, et al. Low-dose therapy with the long-acting erythropoietin analogue darbepoetin alpha persistently activates endothelial Akt and attenuates progressive organ failure. Circulation 2004; 110: 1006–1012, [INFOTRIEVE], [CSA], [CROSSREF]
- Yang CW, Li C, Lim SW, et al. Recombinant human erythropoietin treatment protects chronic cyclosporine nephropathy: anti-apoptotic effect of rHuEPO. J. Am. Soc. Nephrol 2003; 14: 627A, [CSA]
- Gouva C, Nikolopoulos P, Ioannidis JP, et al. Treating anemia early in renal failure patients slows the decline of renal function: a randomized controlled trial. Kidney Int. 2004; 66: 753–760, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Rogiers R, Zhang H, Leeman M, et al. Erythropietin response is blunted in critically ill patients. Intensive Care Med. 1997; 23: 159–163, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Darmon D, Goldfarb M, Shina A, et al. The effect of poly-(ADP-ribose) polymerase (PARP) inhibition on outer medullary hypoxic damage. Nephron. Physiol. 2003; 95: 1–9, [CSA], [CROSSREF]
- Brezis M, Kopolovic J, Rosen S. Hypoxic injury to medullary thick ascending limbs in perfused rat kidneys: reversible and irreversible phases. J. Electron. Microsc. Tech. 1988; 9: 293–298, [INFOTRIEVE], [CSA], [CROSSREF]
- Tojo A, Doumoto M, Oka K, Numabe A, Kimura K, Yagi S. Endothelin-mediated effect of erythropoietin on blood pressure and renal hemodynamics in hypertensive rats. Am. J. Physiol. 1996; 270: R744–R748, [INFOTRIEVE], [CSA]
- Zhang W, Edwards A. Oxygen transport across vasa recta in the renal medulla. Am. J. Physiol. 2002; 283: H1042–H1055, [CSA]
- Damianovich M, Ziv I, Heyman SN, Rosen S, Shina A, Kidron D, Aloya T, Grimberg H, Levin G, Reshef A, Bentolila A, Cohen A, Shirvan A. ApoSense: a novel technology for imaging of cell death in acute renal tubular necrosis. Eur. J. Nucl. Med. Mol. Im. 2006; 33: 281–291, [CSA], [CROSSREF]