Abstract
Introduction. Recently, the identification of the SEN virus as a possible etiological agent of parental transmission hepatitis led to the study of the prevalence of such pathogen agents, particularly SENV-H, in our population. This paper compares the rate prevalence in high-risk subjects, such as dialysis patients, and low-risk subjects, such as blood donors. Material and Methods. The study was carried out on SEN virus DNA extracted from serum of dialysis patients and blood donors, and the presence of viral genomes was performed by the nested PCR method. Results. The results showed a higher prevalence in male blood donors, supporting the hypothesis of an epidemiological role for sexual and also parental transmission, as is clearly demonstrated by the high prevalence in dialysis patients. The result reduced the importance of the possible etiological role of the SEN virus due to the high percentage of positivity in healthy population, and it induces one to consider poorly significant the pathogenicity of such viral agents. Conclusion. For this instance, the authors, in agreement with the phylogenically related TT virus, described SEN viruses as absolutely not pathogens and considered them as “simple guests.”
INTRODUCTION
In 1999, while completing research for the identification of causative agents of non-A-E hepatitis, a new family of viruses was found and designated as SENV.Citation[1],Citation[2] This research showed that SENV is a single-stranded circular DNA virus (ssDNA) with 3800 non-enveloped nucleotides,. Nine genotypes were identified with letters of the alphabet from A to I and belong to the family of Circonviridae as TTV.Citation[3] In spite of several studies, the pathogenetic role has not yet been clarified,Citation[4–13] and there are no epidemiological data for the evaluation of the diffusion around the world.
Until now, epidemiological studies registered prevalence from 1.8% to 28.6% for the blood donors in Italy, USA, Germany, and Japan; from 12.8% to 38% for dialysis patients in Japan and Germany; from 15% to 22% for open population in Japan; and 36% for a closed population in Canada.Citation[6],Citation[8–11],Citation[14–17] Studies from the literature showed that the main routes of transmission are parental, sexual, and vertical,Citation[10],Citation[15],Citation[18],Citation[19] and also supposed that the virus could be ubiquitous. Moreover, a study from Umemura et al.Citation[10] showed that the more involved genotypes in post-transfusion infection are D and especially H, and the possible route of transmission is parental.
According to literature data, it is unclear if SENV D and SENV H could be responsible for hepatitis non-A-E with parental and post-transfusional transmission.Citation[20–22] Therefore, considering the shortage of data from literature regarding the epidemiological and pathogenetically role of SENV D and H, the present authors completed a study enrolling a population of healthy blood donors and hemodialysis patients from three different dialysis units. Finally, considering the prevalence of SENV D and SENV H in blood donors and dialysis patients (corresponding to low and high risk of parental exposure), the authors tried to verify the importance of this route of transmission in the diffusion of the viruses.
MATERIAL AND METHODS
Samples
Serum samples for the determination of viral DNA were studied from 187 subjects (as follows), and informed consent has been obtained:
99 serum samples from typical blood donors were collected in the blood bank of Piemonte Hospital in Messina, Italy
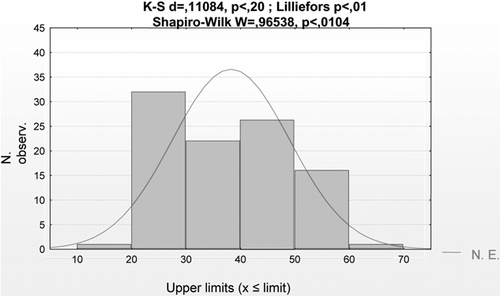
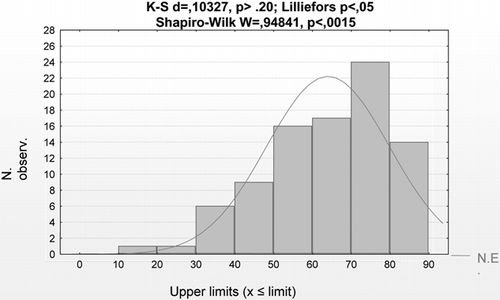
88 serum samples from dialysis patients, collected in three different dialysis units (two from the University Hospital of Messina, Italy, and one from Papardo Hospital). Relative to the age of the patients in the two groups, blood donors and dialysis patients, it is clear that the first group is more homogeneous and younger, while distribution for age in uremic patients is asymmetric, as is evident in and .
It has to be noted that in the three dialysis units, HBV- and HCV-positive patients complete dialysis with dedicated machines for each category of patients (HBV and HCV machines), according to the law's normative.
Methods
After ethical committee approval, the presence of SENV DNA D and H were determined by Nested-PC.Citation[6],Citation[14] The DNA virus was extracted through 200 (l of serum with the QIAmp DNA Blood Mini Kit (Quiagen, Valencia, California, USA), according to the manufacturer's instructions. For maximizing the sensitivity of the diagnostic test, the final extracted was eluted in 50 (l of the elution buffer (10mM TRISHCl, 0.5mM EDTA, pH 9.0). The extracted DNA was quantified by Bio Mate spectrophotometer (Analytical Control) at 260 nm, using the arbitrary ratio 1 OD = 50 mg/L. Further reading at 280 nm allowed an evaluation of the purity of the sample. After quantitation, each sample was frozen at –20°C until the analyses.
Nested Polymerase Chain Reaction
For the determination of the two SENV, the sequences to be amplified were chosen in the more preserved ORF1 region. The nested PCR was performed using the following primers manually exported from the published sequence of the NCBI GenBank data base (SENV-H accession number AY206683; SENV-D accession number AB059352):
External primers for SENV H and D:
F = 5′-CCYAARCTMTTTGAAGACMA-3′
R = 5′-ADKGGRTTRTADGTRVHRTC-3′
Internal sense primer for SENV H:
F = 5′-GGTGCCCCTWGTYAGTTGGCGGTT-3′
Internal sense primer for SENV D:
F = 5′-gTAACTTTgCggTTCAACTgCC-3′
Internal reverse primer for SENV H and D:
R = 5′-CCTCGGTTKSAAAKGTYTGATAGT-3′
Considering the high capacity of mutation for SENV-H and D, the oligonucleotide primers of the first amplification, external primers, were designed with a moderate degree of degeneration to allow hybridization for the viral population with heterogeneous sequences. The selected targets of 230 bp for SENV-H and 231 bp for SENV-D were more conservative and allowed the detection of the presence of the mutated viral population.
The reactions were carried out in 50 μl of a solution 160 mM (NH4)2SO4, 670mM Tris-HCl (pH 8.8), 0.1% Tween 20, 3 mM MgCl2, 200 μM of each dNTP, 0.2 μM of each primer, 2.5 mM dimethylsulphosside (DMSO) containing 10 μl of extracted DNA, and 2.5 U Taq DNA polymerase (EuroTaq, Euroclone, UK). PCRs were running with a programmable thermocycler (TC9600 Applied Biosystems) for 40 and 35 cycles, respectively, for the first and the second amplification (after an initial denaturation step at 95°C for 1 min). Each cycle was 95°C for 15 s, 56°C for 20 s, 70°C for 25 s and a final extension at 70°C for 10 min. The PCR products were separated by electrophoresis on 3% agarose gel (Gelly For HR, Proligo, France), and the specific fragments were identified by ethidium bromide fluorochromatization and a 50 bp ladder (Stratagene, La Jolla, California, USA).
Nucleotide Sequence Analysis
The specificity of the amplified products and similarity of the sequences of 230 bp and 231 bp to the SENV H and D reported on the Gene Bank data base have been verified through nucleotide sequence analysis.
Each PCR product was isolated from gel through an electroelution method using a set of dialysis tubes. The fragments extracted were directly sequenced with the primers for the PCR assay and labeled with the ABI PRIMS BigDye terminators cycle sequencing ready reaction kit (vers. 1.1, Applied Biosystems Inc., Foster City, California, USA) according to the followed instructions: about 5 ng of DNA was sequenced in 10 μl of reaction mixture containing 1.7 pmol of primer, 1.0 μl of master mix, and 1.5 μl of 5x sequencing buffer. Sequences were performed on both DNA strands. The labeled fragments of sequence were separated by capillary electrophoresis on 310 ABI PRISM Sequencer Analyzer, with an uncoated capillary, 47 cm in length and 30 μm in internal diameter. Sequenced products were diluted 1:5 in DDW before loading. Polynucleotide fragments were loaded electrokinetically with 2.2 KV for 30 s. The electrophoresis were carried out at 15.0 KV and 8 μA at the constant temperature of 50°C. The laser power was 9.9 mW. Electropherograms were analyzed with the standard Gene Scan software supplied for the Sequencer Analyzer (Applied Biosystems Inc.).
Statistical Analysis
Data were analyzed through statistic software for the Windows operating system. In particular, the χ2 test with Yates's correction was used, as well as multivariate analysis of multiple regression through Cox test. p<0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Data obtained while researching SENV-H and SENV-D showed a moderate presence in both of the examined categories, blood donors and dialysis patients, though SENV-D demonstrated less of a presence. Indeed, on 187 samples, 51 (27.7%) were positive at n-PCR for SENV-H and 21 (11.2%) for SENV-D. SENV-H was found positive in 24 samples of blood donors and 27 samples of dialysis patients. The prevalence rate was 24.24% (CI 95% = 15.80–32.67) for blood donors and 30.68% (CI 95% =21.08–40.28) for dialysis patients. This difference in prevalence was not statistically significant, as showed by χ2 test (p = .32). SENV-D was positive in 21 samples, 9 blood donors and 12 dialysis patients; the prevalence rates were respectively 9.1% (CI 95% = 3.43–14.75) and 13.6% (CI 95% = 6.47–20.81). Again, in this case, the difference was not statistically significant (χ2 test p = .35).
As to gender, blood donors showed a difference in prevalence of SENV-H between men (31.58%) and women (14.28%) that was statistically significant (p = 0.047). No difference was found relative to gender for SENV-D; indeed, prevalence for SENV-D positive patients was 8.8% (CI 95% = 1.43–16.12) in men and 9.5% (CI 95% = 0.65–18.40) in women, without a statistically significant difference (p = 0.89). As to the age in blood donors, there were no differences statistically relevant for patients positive for SENV-H (p = 0.12) or SENV-D (p = 0.44). In this regard, the sample was divided into two groups using the age of 40 as a cutoff because it was close to the median age. A more detailed analysis of the results obtained in blood donors positive for SENV-H showed how the difference in sex and age was due to the lower prevalence observed in women of an age <40 (p = 0.53 for men and p = 0.42 for women). Indeed, this was roughly six times lower than the prevalence observed in men of the same age and in women >41 years old, and eight times lower in men of an age <40. The same variables applied for SENV-D did not show any difference statistically significant for men (p = 0.85) and women (p = 0.39).
SENV-H and SENV-D were also studied in dialysis patients because this population is at high risk of infection for parental transmission, as confirmed by the frequency of HBV- and especially HCV-positive patients observed in these subjects (see ). In the three dialysis units (A, B, and C), the prevalence rate for HBV-positive patients, which were all men, was 11.1%, 4.3%, and 2.1%, respectively, while for HCV-positive patients, it was 4.3% (unit C) and 50% (unit A), with no case of co-infection for both viruses. Moreover, there was no difference in gender prevalence for HCV-positive patients (men, 29.2%; women, 22.5%).
Table 1 Dialysis patients: HBV- and HCV-positive patients
The present authors also observed that HBV and HCV infection represents risk factors for SENV-H infection, as shown in . Unfortunately, due to its poor presence in the HBV-positive patient, an association with SENV-H could not be evaluated; nevertheless, it is important to note that three of four HBV-positive subjects were also positive for SENV-H. It is clear from the same table how so strong an association between hepatitis virus with parental route and the presence of SENV-H influences the epidemiological measure of relative risk (RR) and attributable risk (AR), confirming the common route of transmission of SENV-H, HBV, and HCV.
Table 2 Contingence tables and risk measures for SENV-H
SENV-D-positive samples showed the same prevalence in patients positive for the hepatitis virus as SENV-H-positive samples (60%), and it was double (100%) in patients positive only for HCV, with the p value highly significant (see ). Because no patients were found to be co-infected with HBV and SENV-D, and because the values of RR and AR are markedly higher than in SENV-H samples (positive for both hepatitis viruses or only for HCV), one can conclude that HCV infection is more responsible for the transmission of SENV-D than SENV-H.
Table 3 Contingence tables and risk measures for SENV-D
Moreover, in SENV-H-positive samples, the small difference in prevalence between sex confirms the importance of parental transmission (see ); indeed, in dialysis patients, because of the common route of exposure, the prevalence of viral infection is almost the same in both sexes. On the contrary, in SENV-D-positive samples, the prevalence in females was significantly higher than in males (see ). The stratification of dialysis patients for age did not show a statistically significant difference for χ2 test in both SENV-H and SENV-D patients. Moreover, SENV-H-positive patients have the highest prevalence in people in middle age (40–60 years), the same as observed for HBV- and HCV-positive subjects (see ), while in SENV-D samples, the highest prevalence was in patients over the age of 61.
Table 4 Dialysis patients: gender distribution in SENV-H and SENV-D positive patients
Table 5 Dialysis patients: age distribution in HBV− and HCV-positive patients
In the three dialysis units, the current authors found the same age distribution regarding the prevalence of SENV-H, SENV-D, HBV, and HCV (see ); indeed, the difference in prevalence is due to the different age compositions among the patients in the three dialysis units. As shown in these tables, dialysis unit B, where the median age was 73, showed the lowest virus prevalence, while the other two units (with younger patients) showed the highest virus prevalence rate. The difference of prevalence at χ2 test in the three dialysis units were not statistically significant (p = 0.0736 for SENV-H and 0.09 for SENV-D), but the comparison between Unit B and C, which had a different age composition, showed a statistically significant difference (p = 0.024 for SENV-H and 0.035 for SENV-D).
Table 6 Dialysis patients: SENV-H and SENV-D results in dialysis units (A, B, and C)
Using the multiple regression test, multivariate analysis in dialysis patients confirmed that HBV and HCV positivity, being a strong indicator of parental transmission for the virus, is usually responsible for the SENV-H infection; for this independent variable, the β value is 0.364 (p = 0.00088), while the other variables (age, gender, unit of dialysis, SENV-D infection) were unrelated to SENV-H infection. The same statistical analysis applied for SENV-D, showing that only gender (beta =0.263, p = 0.013) and HCV infection (beta = 0.226, p = 0.004) were correlated with SENV-D positivity.
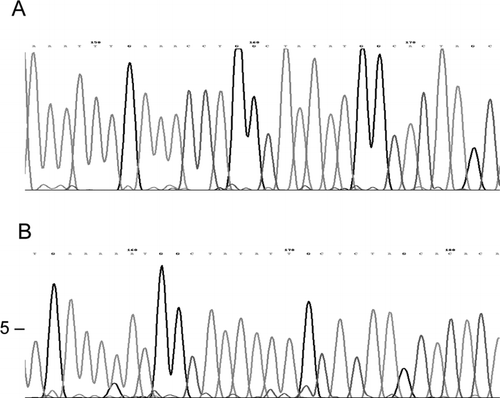
Finally, the nucleotide sequences confirmed the homology of the amplified regions (see ).
DISCUSSION
On the basis of these results, some conclusions can be drawn, especially considering the poor epidemiological and etiological knowledge of SENV. It is also useful to point out that the little data available in the literature about the spread of the SEN virus are extremely heterogeneous (as previously shown), particularly to a healthy population, and the rare differences are only due to a different circulation of virus agents in various geographical areas. Indeed, with regard to blood donors, considered by most authors as representative of a seemingly healthy population,Citation[6],Citation[9],Citation[11],Citation[15] the majority ranges from ∼2% for the American and Italian populations to 28.6% for the Japanese population. Considering that a similar exposure risk may be observed due to the same lifestyle of these people, the dissimilarity could be simply due to the different analytical sensitivity of the diagnostic test performed—the classic PCR in single step used by Umemura and SottiniCitation[11],Citation[15] and the nested PCR by Schröter et al.Citation[8] This is confirmed by our results obtained through the most sensitive nested PCR techniques. According to this technique, the majority in blood donors in Messina, with a 24.24% value, was much higher than that estimated by Umemura et al. and by Sottini but overlaps that reported by Mikumi et al.Citation[6] (28.6%), who used this technique. This led, on one hand, to underestimate the majority data reported in our early investigation, but on the other hand, it undoubtedly reduces the likely etiological function of the SEN virus, as such a high, positive percentage in a seemingly healthy population is a sign of poor pathogenicity of such virus agents. However, it is likely that despite the great emphasis placed on the discovery of such a new human virus in the scientific community, including the SEN virus, the role it plays in the pathogenesis of NANE hepatitis may rapidly decrease—similar to what happened previously with HGVCitation[18],Citation[23] and only recently with the TT virus.Citation[24] On this point, the present authors agree with Simmonds et al.,Citation[24] who, with reference to the TT virus, phylogenically related it to the SEN virus and described them both as absolutely not pathogens. Instead, they should considered as “simple guests,” although it may be difficult to attribute the term “guest” or “endosymbiont” to viral agents which, due to their biological characteristics, alter normal cell functions. This theory has then later developed by Mushahwar.Citation[7] According to the author, the lack of pathogenicity is due to the long agent-guest interaction and is responsible for a mutual adaptation. This results, in most subjects, in a quite asymptomatic, even chronic, infection, and only in a very small number of infected subjects is there an appearance of NANE hepatitis, due to a change in the balance between agent and guest. On the basis of the results obtained from blood donors and dialysis patients at high and low parental exposure risk, respectively, and with reference to the second aim of this work (i.e., the evaluation of the transmission/circulation of the virus and the high spread of infection in a healthy population), it leads one to believe in the existence of other routes of transmission. On the other hand, the initial high incidence of the virus genome in HIV-, HCV-, and HBV-positive patients, plus the evidence of vertical transmission together with an initially low incidence of positivity in blood donors, stressed the importance of the parental route of transmission, though such a way no longer seems the only way to explain the high frequency of patients being virus-positive even in healthy populations at low or no risk of blood-associated infections. On this subject, the current authors point out that parental transmission is one of the most general routes of direct transmission, and perhaps the most insidious one in that it is not easily identified despite being the most common and “community acquired.” (To this end, it is also referred to as “parental invisible.”) All of the other different ways of infection, though less effective then the parental, can explain the high prevalence observed in both categories of subjects, even taking into account SEN virus spreading in the population.
On the basis of these results, this may be confirmed by the significantly higher prevalence among male donors, who are at a higher risk of exposure to such infections due to lifestyle, and even among both sexes aged between 41–60. As to the latter group, it is worth mentioning the increase of HBV- and HCV-positive patients, especially in the “pre-AIDS” era, in our areas, which could be due to the massive adoption of medical and dental treatments lacking any preventive measures.
This assertion finds confirmation in the prevalence observed for HBV- and HCV-positive dialysis patients aged between 41–60, and this clearly points out that the risk of iatrogenic infection has been particularly high and unacceptable in the recent past.
REFERENCES
- Primi D, Fiordalisi G, Mantero JL, Mattioli S, Sottini A, Bonelli F, Vaglini L. Identification of SEN-V genotypes. World Intellectual Property Organization. Available at: http://ep.espacenet.com, (International patent application published under the patent cooperation treaty.)
- Umemura T, Tanaka Y, Kiyosawa K, Alter HJ, Shih JWK. Observation of positive selection within hypervariable regions of a newly identified DNA virus (SEN virus). FEBS Letters 2002; 510: 171–174, [INFOTRIEVE], [CSA]
- Tanaka Y, Primi D, Wang RY, Umemura T, Yeo AE, Mizokami M, Alter HJ, Shih JW. Genomic and molecular evolutionary analysis of a newly identified infectious agent (SEN virus) and its relationship to the TT Virus family. J Infect Dis 2001; 183: 359–367, [INFOTRIEVE], [CSA], [CROSSREF]
- Kao J-H, Chen W, Chen P-J, Ming-Yang LJ, Ding-Shinn C. Prevalence and implication of a newly identified infectious agent (SEN virus) in Taiwan. J Infect Dis 2002; 185: 389–392, [INFOTRIEVE], [CSA], [CROSSREF]
- Kobayashi NJ, Tanaka EJ, Umemura T, Matsumoto AJ, Lijima TJ, Higuchi M, Hora K, Kiyosawa K. Clinical significance of SEN virus in patients on maintenance hemodialysis. Nephrol Dial Transplant 2003; 18(2)348–352, [INFOTRIEVE], [CSA], [CROSSREF]
- Mikuni M, Moriyama M, Tanaka N, Abe K, Arakawa Y. SEN virus infection does not affect the progression of non -A to -E liver disease. J Med Virol 2002; 67(4)624–629, [INFOTRIEVE], [CSA], [CROSSREF]
- Mushahwar IK. Recently discovered blood-borne viruses: are they hepatitis viruses or merely endosymbiont?. J Med Vir 2000; 62: 399–404, [CSA], [CROSSREF]
- Schröter M, Laufs R, Zöllner B, Knödler B, Schäfer P, Stemeck M, Fischer L, Feucht HH. Prevalence of SENV-H viremia among healthy subjects and individuals at risk for parenteral transmitted diseases in Germany. Journal of Virai Hepatitis 2002; 9(6)455, [CSA], [CROSSREF]
- Shibata M, Wang RYH, Yoshiba M, Shih WK, Alter HJ, Mitamura K. The presence of a newly identified infectious agent (SEN virus) in patient with liver disease and in blood donors in Japan. J Infect Dis 2001; 184: 400–404, [INFOTRIEVE], [CSA], [CROSSREF]
- Umemura T, Yeo AE, Sottini A, Moratto D, Tanaka Y, Wang RYH, Shih JW, Donahue P, Primi D, Alter HJ. SEN virus infection and its relationship to transfusion-associated hepatitis. Hepatology 2001; 33: 1303–1311, [INFOTRIEVE], [CSA], [CROSSREF]
- Umemura T, Donahue P, Sottini A, et al. The incidence of SEN virus infection in transfusion-associated hepatitis. Antivir Ter 2000; 5(Suppl 1)G.11, [CSA]
- Umemura T, Yeo AE, Shih JV, et al. The prevalence of SEN virus infection in Japanese patients with viral hepatitis and liver disease. Hepatology 2000; 32(Suppl)381A, [CSA]
- Wong SG, Primi D, Kojima H, Sottini A, Giulivi A, Zhang M, Uhanova J, Minuk GY. Insights into SEN virus prevalence, transmission and treatment in community-based person and patients with liver disease referred to a liver disease unit. Clinical Infectious Diseases 2002; 35: 789–795, [INFOTRIEVE], [CSA], [CROSSREF]
- Schröter M, Laufs R, Zöllner B, Knödler B, Schäfer P, Feucht HH. A novel DNA virus (SEN) among patients on maintenance hemodialysis: prevalence and clinical importance. Journal of Clinical Virology 2003; 27(1)69–73, [CSA], [CROSSREF]
- Sottini A, Mattioli S, Fiordalisi G, Mantero G, Imberbi L, Moratto D, De Primi D. Molecular and biological characterization of SEN viruses: a family of viruses remotely related to the original TTV isolates. Proceedings of the 10th International Symposium on Viral Hepatitis and Liver Disease, H. Margolis. Meditech Media, Atlanta 2001
- Umemura T, Yeo AE, Wang RY, et al. The incidence of SEN virus infection in transfusion-associated hepatitis. Hepatology 2000; 32(Suppl.)52A, [CSA]
- Wong SG, Primi D, Giulivi A, Minuk GY. Insights into SEN-V transmission. Hepatology 2000; 32(2)382A, [CSA], [CROSSREF]
- Lefrere J-J, Roudot-Thoraval F, Morand-Joubert L, Brossard Y, Parnet-Mathieu F, Mariotti M, Agis F, Ronet G, Lerable J, Lefevre G, Girot R, Loiseau P. Prevalence of GB virus type C/hepatitis G virus RNA and of anti-E2 antibody in individuals at high or low risk for blood-borne or sexually-transmitted viruses: evidence of sexual and parenteral transmission. Transfusion 1999; 39: 83–94, [INFOTRIEVE], [CSA], [CROSSREF]
- Pirovano S, Bellinzoni M, Matterelli A, Ballerini C, Cariani E, Duse M, Alberini A, Imberbi L. Trasmission of SEN virus from mothers to their babies. J Med Virol 2002; 66: 421–427, [INFOTRIEVE], [CSA], [CROSSREF]
- Kao JH, Chen W, Chen PJ, Lai MY, Chen DS. Prevalence and implication of newly identified infectious agent (SEN virus) in Taiwan. J Infect Dis 2002; 185(3)389–392, [INFOTRIEVE], [CSA], [CROSSREF]
- Tangkijvanich P, Theamboonlers A, Sriponthong M, Thong-Ngam D, Kullavanijaya P, Poovorawan Y. SEN virus infection in patients with chronic liver disease and hepatocellular carcinoma in Thailand. J Gastroenterol 2003; 38(2)142–148, [INFOTRIEVE], [CSA], [CROSSREF]
- Sagir A, Kirschberg O, Heintges T, Erhardt A, Haussinger D. SEN virus infection. Rev Med Virol 2004; 14(3)141–148, [INFOTRIEVE], [CSA], [CROSSREF]
- Prati D, Zanella A, Bosoni P, Rubella P, Farma E, De Mattei C, Capelli C, Mozzi F, Gallismi D, Mangano C, Melivendi C, Sirchia G. The incidence and natural course of transfusion-associated GB virus C/hepatitis G virus infection in a cohort of thalassemic patients. Blood 1998; 91: 774–777, [INFOTRIEVE], [CSA]
- Simmonds P, Prescott LE, Logue C, Davidson F, Thomas AE, Ludlam CA. TT virus—part of the normal human flora?. J Infect Dis 1999; 180: 1748–1750, [INFOTRIEVE], [CSA], [CROSSREF]