Abstract
The role of the kidney in the control of blood pressure has been convincingly demonstrated by several studies. Recent evidence has suggested that subtle acquired tubulointerstitial injury may cause a defect in sodium excretion function, thus leading to salt-sensitive hypertension. There are no reports, however, examining the effect of experimental chronic pyelonephritis on renal sodium handling and arterial pressure. Thus, to examine the influence of salt intake and unilateral nephrectomy, unanesthetized, unrestrained rats were randomly assigned to one of two separate groups: sham-operated rats (CO) or chronic unilateral pyelonephritic rats (CP). After twenty one days, the pyelonephritic group was subdivided in two: one subgroup continued with water intake (CPw), while the other was changed to 0.9% NaCl intake (CPs), like the control group (COs). After seven days, all rats were submitted to unilateral nephrectomy of the left normal kidney. Data presented herein show that chronic pyelonephritis produced an increase in mean arterial pressure (CO: 121.4 ± 1.0 mmHg to CP: 127.0 ± 0.9 mmHg, p = 0.000) that was enhanced by saline ingestion (COs: 121.6 ± 1.4 mmHg; CPw: 127.0 ± 1.8 mmHg; CPs: 132.1 ± 1.2 mmHg, p = 0.000) and further aggravated by unilateral nephrectomy (CO: 125.2 ± 2.6 mmHg; CPw: 127.5 ± 0.9 mmHg; CPs: 139.2 ± 1.1 mmHg, p = 0.000). Unchanged blood pressure measurements (120.2 ± 2.3 mmHg) were observed beyond 21 days in control rats maintained on water regimen when compared with saline-drinking groups. These changes in mean arterial pressure were observed despite an increased fractional sodium excretion in the CPs group compared to the other groups before uninephrectomy (COs: 0.125 ± 0.025%; CPw: 0.045 ± 0.013%; CPs: 0.292 ± 0.046%; p = 0.000), as compared to CPw after uninephrectomy (COs: 0.249 ± 0.077%; CPw: 0.062 ± 0.011%; CPs: 0.363 ± 0.195%, p = 0.019). In addition, it was shown that daily liquid intake was higher in CPs than in CPw but similar to COs, both before uninephrectomy (COs: 42.8 ± 2.6 ml/d; CPw: 34.3 ± 3.5 ml/d; CPs: 51.8 ± 3.7 ml/d, p = 0.006) and after uninephrectomy (COs: 40.9 ± 5.5 ml/d; CPw: 33.8 ± 1.4 ml/d; CPs: 53.0 ± 3.5 ml/d, p = 0.004). The current data suggest that chronic pyelonephritis promotes an inability of renal tubules to handle sodium excretion when exposed to sodium overload and aggravated by uninephrectomy, thus constituting a model for salt-sensitive hypertension.
INTRODUCTION
Although arterial hypertension is one of the most common diseases worldwide, its pathogenesis is not completely understood. The etiologic mechanism is complex and probably does not have a single cause. It has been postulated that the kidneys play a pivotal role in the pathogenesis of essential hypertension, perhaps as a consequence of a primary defect in kidney function and/or renal hemodynamics that promote the retention of sodium.[Citation[1–8]] Although the precise mechanism by which blood pressure rises in the hypertensive models remains to be elucidated, the renal control of the fluid and electrolyte balance is thought to play a dominant role in the long-term control of arterial blood pressure.[Citation[2]]
The transplantation of kidneys from genetically hypertensive rats to normotensive rats leads to the development of hypertension, and when the kidneys of normal rats are transplanted to hypertensive rats, the level of blood pressure can be normalized.[Citation[1],Citation[5],Citation[6],Citation[9]] Moreover, the kidneys of hypertensive patients may have a physiologic inability to perform sodium excretion in response to increases in blood pressure, an impairment represented as a rightward shift in the “pressure-natriuresis” curve.[Citation[3],Citation[10]] In addition, there is controversy over the role of sodium intake in hypertension. In some groups (e.g., black individuals, elderly individuals with hypertension) and strains of rats (e.g., Dahl and spontaneously hypertensive rats [SHR]), blood pressure increased in response to an increase in sodium intake, a condition known as salt-sensitivity.[Citation[11–14]]
Recent evidence suggests that subtle acquired renal injury, such as tubulointerstitial and microvascular damage caused by chronic infusion of angiotensin[Citation[15–17]] or phenylephrine,[Citation[18]] and the chronic use of cyclosporine[Citation[19]] could be responsible for the renal inability to excrete additional amounts of sodium, thus leading to the development of salt-sensitive hypertension.[Citation[20],Citation[21]] Chronic pyelonephritis is a disease characterized by inflammatory tubulointerstitial damage and therefore could be a model of salt-sensitive hypertension. Because the changes in urinary sodium excretion associated with chronic pyelonephritis have not been examined in detail, the following study was undertaken to investigate (1) the effects of acquired renal tubulointerstitial injury on arterial pressure, and (2) tubular sodium-handling effects, evaluated by lithium clearance, in unanesthetized, unrestrained experimental and their sham-operated appropriate control rats.
MATERIALS AND METHODS
The general guidelines established by the Brazilian College of Animal Experimentation (COBEA) were followed throughout the investigation. Male Wistar-Hannover rats (250–350g) were maintained under controlled temperature and lighting conditions with free access to tap water and standard rat laboratory chow (Purina rat chow: Na+ content: 135 ± 3 µEq/g; K+ content: 293 ± 5 µEq/g).
Experimental Design
Chronic pyelonephritis (CP) was induced by direct inoculation of a nephritogenic strain of Escherichia coli in the right renal parenchyma. Another group was sham-operated (CO). After twenty-one days, renal clearances and arterial pressure measurements were performed. The pyelonephritic group was then subdivided in two: one subgroup continued to drink water (CPw), while the other switched to a saline solution (0.15 M NaCl) instead of water (CPs). The same saline solution was offered to the control group (COs). After seven days, renal clearances and arterial pressure measurements were performed. All rats were then submitted to uninephrectomy of the contralateral kidney and maintained on the same liquid intake regime for seven days, at which point renal clearances and arterial pressure measurements were performed again. Kidneys were removed, weighed, and fixed in 10% formalin for histological analysis. Liquid and solid intakes were recorded daily.
Pyelonephritis-Induced Model
Briefly, the animals were anesthetized with an intraperitoneal injection of sodium pentobarbital (50 mg kg−1 body weight), adequate asepsis of the region was performed, and a 1 cm incision was made immediately below the last right rib. The right kidney was carefully exposed, and pyelonephritis was induced by direct inoculation in renal parenchyma of a hypodermic needle imbibed in a solution containing 106 CFU/ml of a nephritogenic strain of Escherichia coli (O75:K15). Three punctures were carefully performed in each renal lobe of both sides of the kidney. After certifying the absence of excessive bleeding, the incision was sutured.
Renal Function Tests
The renal function tests were performed 21, 28, and 35 days of follow-up in unanesthetized, unrestrained rats. Fourteen hours before the renal test, 60 mmol LiCl/100 g body weight was given by means of gavage. The rats were subsequently individually housed in metabolic cages with free access to tap water but no food. After an overnight fast, each animal received a load of tap water by gavage (5% of the body weight), followed by a second load of the same volume one hour later. Thirty minutes after the second load, spontaneously voided urine was collected over 120 min into a graduated centrifuge tube. At the end of the experiment, blood samples were drawn through the tail vein or cardiac puncture in anesthetized rats, and urine and plasma samples were collected for analysis.
Blood Pressure Measurement
Systolic blood pressure (SBP) was estimated each week, one day before the renal test, in conscious, restrained rats by the tail-cuff method using an electrosphygmomanometer (Narco Bio-System, Austin, Texas, USA). This indirect approach permits repeated measurements with a close correlation (correlation coefficient = 0.975) compared to direct intra-arterial recording.[Citation[22]] The SBP values were assessed from the mean of three consecutive readings.
Unilateral Nephrectomy
Animals were anesthetized with an intraperitoneal injection of sodium pentobarbital (50 mg kg−1 body weight); adequate asepsis of the region was performed and a 1 cm incision was made immediately below the last left rib. The left kidney was carefully removed, drained, and weighed on a precise balance. The adrenal was kept in place intact. After certifying the absence of excessive bleeding, the incision was sutured.
Biochemical Analysis
Plasma and urine sodium, potassium, and lithium concentrations were measured by flame photometry (Micronal, B262, São Paulo, Brazil), while creatinine concentration was determined spectrophotometrically (Instruments Laboratory, Genesys V, Lexington, Massachusetts, USA).
Statistical Analysis and Calculations
The results are reported as means ± SEM per gram kidney weight. Renal clearance (C) was calculated by a standard formula (C = UV/P) using the plasma creatinine and lithium levels for each period. Creatinine clearance was used to estimate the glomerular filtration rate, and lithium clearance (CLi+) was used to assess proximal tubule output. Fractional sodium (FENa+) and potassium (FEK+) excretion was calculated as CNa+/CCr and CK+/CCr, respectively, where CNa+ and CK+ are the ion clearances and CCr is the creatinine clearance. Fractional proximal (FEPNa+) and post-proximal (FEPPNa+) sodium excretion was calculated as CLi+/CCr × 100 and CNa+/CLi+ × 100, respectively.[Citation[2],Citation[23],Citation[24]] All data are reported as means ± SEM. Data obtained over time were analyzed using appropriate ANOVA. Post hoc comparisons between selected means were performed with Bonferroni's contrast test when initial ANOVA indicated statistical differences between experimental groups. A p value < 0.05 was considered to indicate significance.
RESULTS
Renal function test and systolic blood pressure data for all experimental groups are summarized in . As shown in , tail arterial pressure (mmHg) in CPs was significantly higher than in control and CPw from 21 to 35 days of treatment (p < 0.001). During the study, CPs systolic blood pressure increased from 127 ± 0.9 mmHg to 132 ± 1.2 and 139 ± 1.1 mmHg in CPs and CPs+UNx, respectively, as compared with a slower rise from 121 ± 1.0 mmHg to 121 ± 1.4 in COs and 139 ± 1.1 mmHg in COs+UNx. The continuously increased blood pressure in CPs was significantly and progressively enhanced by saline intake and unilateral nephrectomy (p < 0.001) (see ) at 28 days of follow-up. Unchanged blood pressure measurements (120.2 ± 2.3 mmHg) were observed beyond 21 days in control rats maintained on water regimen when compared with saline-drinking groups (data not included in ). This increased blood pressure was associated with a significant increase in urinary sodium excretion and a fall in post-proximal sodium reabsorption.
Figure 1 Systolic blood pressure (SBP), creatinine clearance (CCr), fractional sodium (FENa), potassium excretion (FEK), and proximal (FEPNa) and post-proximal (FEPPNa) fractional sodium excretion for all experimental groups. The data are reported as the means ± SEM. *p value ≤ 0.05 was considered to indicate statistical significance between experimental groups over time data (ANOVA and Bonferroni's contrast test).
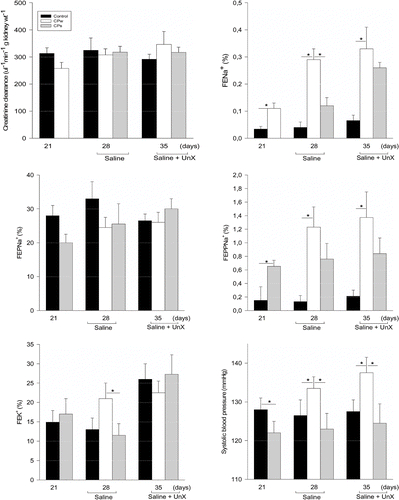
In general, food and (therefore) sodium intake were similar, when normalized by body weight, in all experimental groups during the investigation. At 28 days after offering saline to control and pyelonephritic rats, the daily liquid intake was significantly higher in CPs (51.8 ± 3.7 ml/d) than in the CPw group (34.3 ± 3.5 ml/d) (p < 0.05) but unaffected in COs (42.8 ± 2.6). The same behavior for liquid intake was observed after unilateral nephrectomy as well (CPs: 53.0 ± 3.5 vs. CPw: 33.8 ± 1.4 ml/d), but not significantly when related to COs (40.9 ± 5.5 ml/d).
The data for renal function over the five-week period are summarized in –e. Urinary flow rates (data not included) and the glomerular filtration rate estimated by CCr did not significantly differ among the groups during the renal tubule sodium handling studies (see ).
As shown in , fractional urinary sodium excretion (FENa) was significantly higher in pyelonephritic 0.15M NaCl-treated rats (CPs, 0.29 ± 0.04%) when compared to the pyelonephritic non-treated group (CPw, 0.04 ± 0.01%, p < 0.0001) and control 0.15M NaCl-treated rats (COs: 0.12 ± 0.02%, p < 0.0001). After unilateral nephrectomy, FENa was increased in pyelonephritic 0.15M NaCl-treated rats (CPs+UNx: 0.36 ± 0.19%) when compared with the pyelonephritic non-saline treated group (CPw+UNx, 0.06 ± 0.01%, p < 0.006) but unchanged in relation to the control 0.15M NaCl-treated age-matched group (COs+UNx: 0.895 ± 0.314%).
The increased FENa in CPs and CPs+UNx was accompanied by a significant increase in post-proximal sodium excretion (FEPPNa: CPs, 1.19 ± 0.16 % and CPs+UNx, 1.31 ± 0.36%) compared with the unchanged urinary sodium excretion response observed in the age-matched control uninephrectomyzed group (COs+UNx, 0.89 ± 0.31%, p<0.05). This increase occurred despite unchanged CCr and FEPNa (see ). Thus, this consistently enhanced post-proximal sodium excretion produced by saline intake was not followed by any additional increase in this nephron segment of sodium rejection in the unilateral nephrectomyzed pyelonephritic-induced rats. The renal natriuretic responses for pyelonephritic-induced control groups (water intake) were not altered by uninephrectomy and were similar in all studied groups up to the thirty-fifth experimental day. Likewise, CCr, proximal sodium handling, and kaliuresis were unaffected at any time in the present investigation (see , panels A, C, and E).
The present study shows that CP excreted less sodium than CO during the first three-week observation period (p < 0.05). Of particular interest is the observation that the reduced excretion by CP during the initial three weeks (on drinking water, before saline) occurred while sodium in chow intake did not appreciably differ between groups. This is further highlighted by the significant differences in fractional sodium excretion during the same time period (see ). As a result of the relative retention of sodium, CP may have exhibited a higher cumulative sodium balance throughout this experimental period. Data from experiments performed evaluating the arterial blood pressure and renal function in control rats kept on water intake regimen were similar throughout five weeks of follow-up (data not shown).
There were no significant differences between the daily solid rat chow, body weights, plasma sodium, potassium, and lithium levels (see ) in the pyelonephritic rats compared with the other groups. The pyelonephritic renal scarring and unchanged renal parenquima in the control group was confirmed by histological analysis.
Table 1 Body weight as related to age, sodium intake, serum sodium, potassium, and lithium data for all experimental groups fed a standard diet
DISCUSSION
The present study was designed to evaluate the influence of 0.15M NaCl intake and unilateral nephrectomy effects on arterial blood pressure and renal urinary sodium handling, determined by lithium clearance, in the pyelonephritis-induced model compared with appropriate age-matched control subjects. In the current study, it was demonstrated that pyelonephritis induced developing hypertension, while uninephrectomized and salt-treated rats retain, up to the thirty-fifth day of follow-up, more sodium than age-matched control rats. The present investigation also shows pronounced differences in the renal natriuretic response. Of particular interest is the observation that the reduced excretion by CP during the initial three weeks (on drinking water, before saline) occurred while sodium in chow intake did not appreciably differ between groups. The offer of additional salt (0.15M NaCl intake) followed by unilateral nephrectomy in pyelonephritic rats produced natriuresis and significant increases in post-proximal fractional urinary sodium rejection compared to other groups, indicating a direct distal tubule effect. However, the authors hypothesized that a post-proximal natriuretic response may not be enough to excrete adequate sodium intake load, resulting in an absolute retention of sodium through the experimental periods in PCs + UNx rats. Moreover, the significant differences in urinary sodium excretion and retained sodium in these hypertensive animals compared to age-matched controls may be attributed to renal abnormal mechanisms induced by pyelonephritic renal scarring, despite the unchanged glomerular filtration rate estimated by creatinine clearance.
Since Goldblatt's[Citation[12]] and Wilson and Byrom's[Citation[25]] demonstration that renal injury could lead to the development of hypertension, the kidney has been an area of interest for the study of the pathogenesis of hypertension. The fact that the transplantation of kidneys from hypertensive to normal rats could increase blood pressure created the concept that hypertension “follows” the kidney. Guyton et al.[Citation[10]] proposed that kidneys of hypertensive subjects might have a reduced sodium excretion function in response to elevations in blood pressure. The exact mechanism by which the kidneys lose their capacity for long-term control of blood pressure is not known. Several mechanisms are hypothesized, including reduced numbers of nephrons[Citation[4]] and a disruption in the balance of intra-renal vasoactive substances, leading to renal ischemia.[Citation[20],Citation[21],Citation[24]]
The twenty-one-day pyelonephritis-induced effect per se increased arterial blood pressure when compared to adequate control rats. Additionally, when drinking water was substituted by a 0.15M NaCl solution in a subgroup of pyelonephritic rats, blood pressure showed a substantial increase that was further aggravated by uninephrectomy of the intact kidney. These results and previous studies in SHR kidneys indicate that a higher arterial pressure than that of the kidneys of normotensive rats is required to excrete the same amount of salt under basal conditions. However, it is possible that, as shown previously, when renal perfusion pressure is reduced to the range observed in the normotensive strain, urinary sodium excretion will also be reduced in PCs+UNx.[Citation[2],Citation[5]] The present findings, before the development of hypertension beyond the twenty-first experimental day, were consistent with this view.
Salt sensitivity is defined as an increase in blood pressure in response to an increased sodium intake. The relationship between blood pressure increases and duration and load of sodium intake for defining salt-sensitive hypertension are still controversial both in human and in animal models. Criteria vary from a 5% to 10% increment in blood pressure in response to variable quantity of sodium diary intake.[Citation[12],Citation[26]] The majority of studies in animals have used a 4% NaCl diet. In the present study, a 0.9% NaCl solution, substituting water intake, was offered, and increases in blood pressure of 8.6% after seven days of saline intake and 11.2% after the seven days of saline intake plus unilateral nephrectomy in pyelonephritis-induced rats, compared to the control group, were observed. Many mechanisms have been implicated in explaining how pyelonephritic renal scarring injury may cause renal tubule inability for sodium handling. Three major renal mechanisms were proposed, leading to the development of hypertension: an increased pre-glomerular vascular resistance, a decrease in whole kidney ultrafiltration, and an increase in tubular sodium reabsorption.[Citation[4],Citation[27]] In the present study, the disruption of the balance between vasoconstrictors and vasodilator substances in PCs+UNx renal parenchyma could impair an adequate reabsorption.
Thus, sodium retention could contribute to the development of hypertension by interacting with a variety of mechanisms, such as vasoconstriction,[Citation[28]] effective extracellular volume expansion,[Citation[29]] or hyperactivity of the sympathetic nervous system.[Citation[8],Citation[30]] However, human studies are scarce, and mechanisms by which sodium retention occurs in the pyelonephritis-induced model remain obscure. Evidence supporting mechanisms involving the functional impairment of glomerular vasculature associated with reduced glomerular filtration rate and renal blood flow as well as in potassium metabolism is provided by other hypertensive studies.[Citation[31],Citation[32]] However, the present investigation saw no significant changes in the CCr, plasma potassium levels, and urinary potassium excretion, suggesting that enhanced blood pressure has a more direct tubule antinatriuretic effect in the proximal segment than at the sodium-potassium exchange site, as demonstrated in the present sodium handling study.
Additionally, the possibility exists that several humoral factors may be involved in mediating the decreased natriuresis observed in the current study. Results from several investigations suggest that the involvement of humoral factors in the pathogenesis of experimental hypertension may be mediated, at least in part, by its influence on renal function. Children with focal renal scarring due to pyelonephritis are at high risk of serious long term consequences.[Citation[33]] Studies by Jacobson et al. have demonstrated that patients with pyelonephritic renal scarring are at risk of developing renal failure and hypertension.[Citation[16],Citation[33],Citation[34]] However, patients with renal scarring had the same capacity to excrete sodium and water during transition to volume expansion as the healthy controls. Conversely, Levison et al.[Citation[35]] demonstrated that an inability to attain maximum urine flow after overnight dehydration is the earliest functional abnormality in human and experimental pyelonephritis caused by diverse microorganisms.[Citation[35]] Indeed, the attenuated prostaglandin synthesis associated with an abnormally activated renin-aldosterone system is probably more important than hypervolemia in the development of hypertension in this group of patients.[Citation[16],Citation[33],Citation[34],Citation[36],Citation[37]]
The novel finding of the present investigation suggests that progressive natriuresis in PCs rats associated with an increased sodium rejection in the post-proximal tubule is incompletely compensated by additional ion excretion by all nephron segments after unilateral nephrectomy. Although the precise mechanism responsible for the subsequent attenuated natriuretic response observed in PCs+UNx is still unclear, one might speculate that one of the pyelonephritis-induced defects may result in the inability of renal tubules to handle the hydro-electrolyte balance, consequently developing arterial hypertension and supporting the hypothesis that PCs+UNx rats may constitute a model for salt-sensitive hypertension.
Grants from CNPq (no. 500868/91-3), PRONEX (0134/97), and FAPESP (12606/03-9 and 00/12216-8) supported this work.
References
- Bianchi G, Fox U, Di Francesco GF, et al. Blood pressure changes produced by kidney cross-transplantation between spontaneously hypertensive rats and normotensive rats. Clin. Sci. Mol. Med. 1974; 47: 435–448, [INFOTRIEVE]
- Boer PA, Morelli JM, Figueiredo JF, Gontijo JAR. Early altered renal sodium handling determined by lithium clearance in spontaneously hypertensive rats (SHR): role of renal nerves. Life Sci. 2005; 76: 1805–1815, [INFOTRIEVE], [CROSSREF]
- Borst JGG, Borst-de-Geus A. Hypertension explained by Starling's theory of circulatory homeostasis. Lancet 1963; I: 677–682, [CROSSREF]
- Brenner BM, Garcia DL, Anderson L. Glomeruli and blood pressure: less of one, more the other?. Am. J. Hypertens. 1988; 1: 335–347, [INFOTRIEVE]
- Cowley AW, Jr, Roman JR. The role of kidney in hypertension. JAMA 1996; 275: 1581–1589, [INFOTRIEVE], [CROSSREF]
- Frey BAJ, Grisk O, Bandelow N, Wussow S, Bie P, Rettig R. Sodium homeostasis in transplanted rats with a spontaneously hypertensive rat kidney. Am J Physiol. 2000; 279: R1099–R1104
- Goldblatt H. The renal origin of hypertension. Physiol. Rev. 1947; 27: 120–165
- Strazzullo P, Galletti F, Barba G. Altered renal handling of sodium I human hypertension: short review of the evidence. Hypertension 2003; 41: 1000–1005, [INFOTRIEVE], [CROSSREF]
- Dahl LK, Heine M. Primary role of renal homografts in setting chronic blood pressure in rats. Circ. Res. 1975; 36: 692–696, [INFOTRIEVE]
- Guyton AC, Coleman TG, Cowley AW, Jr, et al. Arterial pressure regulation: overriding dominance of the kidneys in long-term regulation and in hypertension. Am. J. Med. 1972; 52: 584–594, [INFOTRIEVE], [CROSSREF]
- Campese VM, Romoff MS, Levitan D, et al. Abnormal relationship between sodium intake and sympathetic nervous system activity in salt-sensitive patients with essential hypertension. Kidney Int. 1982; 21: 371–378, [INFOTRIEVE]
- Chrysant GS, Bakir S, Oparil S. Dietary salt reduction in hypertension—what is the evidence and why is it still controversial?. Prog. Cardiovasc. Dis. 1999; 42: 23–38, [INFOTRIEVE], [CROSSREF]
- Midgley JP, Matthew AG, Greenwood CM, et al. Effect of reduced dietary sodium on blood pressure: a meta-analysis of randomized controlled trials. JAMA 1996; 275: 1590–1597, [INFOTRIEVE], [CROSSREF]
- Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension 1996; 27: 481–490, [INFOTRIEVE]
- Fine LG, Orphanides C, Norman JT. Progressive renal disease: the chronic hypoxia hypothesis. Kidney Int. 1998; 65(Suppl.)S74–S78
- Jacobson SH, Eklof O, Eriksson CG, Lins LE, Tidgren B, Winberg J. Development of hypertension and uraemia after pyelonephritis in childhood: 27-year follow-up. BMJ 1989; 299: 703–706, [INFOTRIEVE]
- Lombardi D, Gordon KL, Polinsky P, et al. Salt-sensitive hypertension develops after short-term exposure to angiotensin II. Hypertension 1999; 33: 1013–1019, [INFOTRIEVE]
- Johnson RJ, Schreiner GF. Hypothesis: the role of acquired tubulo-interstitial disease in the pathogenesis of salt-dependent hypertension. Kidney Int. 1997; 52: 1169–1179, [INFOTRIEVE]
- Curtis JJ, Luke RG, Jones P, et al. Hypertension in cyclosporine-treated renal transplant recipient is sodium dependent. Am. J. Med. 1988; 85: 134–138, [INFOTRIEVE]
- Johnson RJ, Gordon KL, Suga S, et al. Renal injury and salt-sensitive hypertension after exposure to cathecolamines. Hypertension 1999; 34: 151–159, [INFOTRIEVE]
- Johnson RJ, Herrera-Acosta J, Schreiner GF, et al. Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. N. Engl. J. Med. 2002; 346: 913–923, [INFOTRIEVE], [CROSSREF]
- Lovenberg W. Techniques for measurements of blood pressure. Hypertension 1987; 9: 15–16
- Cesar de Oliveira P, Boer-Lima PA, Figueiredo JF, Gontijo JAR. Effect of nitric oxide synthase inhibition and saline administration on blood pressure and renal sodium handling during experimental sepsis in rats. Ren Fail. 2003; 25: 897–908, [INFOTRIEVE], [CROSSREF]
- Xavier F, Magalhães AMF, Gontijo JAR. Effect of inhibition of nitric oxide synthase on blood pressure and renal sodium handling in renal denervated rats. Brazilian J. Med. Biol. Res. 2000; 33: 347–354
- Wilson C, Byrom FB. The vicious circle in chronic Bright's disease: experimental evidence from the hypertensive rat. Q. J. Med. 1941; 10: 65–93
- Chrysant SG, Walsh GM, Kem DC, et al. Hemodynamic and metabolic evidence of salt sensitivity in spontaneously hypertensive rats. Kidney Int. 1979; 15: 33–37, [INFOTRIEVE]
- Kimura G, Brenner BM. A method for distinguishing salt-sensitive from non-salt-sensitive forms of human and experimental hypertension. Curr. Opin. Nephrol. Hypertens. 1993; 2: 341–349, [INFOTRIEVE]
- Sofola OA, Knill A, Hainsworth R, Drinkhill M. Change in endothelial function in mesenteric arteries of Sprague-Dawley rats fed a high salt diet. J. Physiol. 2002; 543(1)255–260, [INFOTRIEVE], [CROSSREF]
- Schafer JA. Abnormal regulation of ENaC: syndromes of saltretention and salt wasting by the collecting duct. American Journal of Physiology 2002; 283: F221–F235, [INFOTRIEVE]
- Strazzullo P, Barbato A, Vuotto P, Galletti F. Relationships between salt sensitivity of blood pressure and sympathetic nervous system activity: a short review of evidence. Clin. Exp. Hypertension 2001; 23: 25–33, [CROSSREF]
- Dilley JR, Stier CT, Arendshorst WJ. Abnormalities in glomerular function in rats developing spontaneous hypertension. Am. J. Physiol. 1984; 246: F12–F20, [INFOTRIEVE]
- Zhou X, Frohlich ED. Functional and structural involvement of afferent and efferent glomerular arterioles in hypertension. Am. J. Kidney Dis. 2001; 37: 1092–1097, [INFOTRIEVE]
- Jacobson SH. Blood pressure regulation, peripheral renin activity and aldosterone in patients with pyelonephritic renal scarring. Acta. Physiol Scand. 1987; 131: 242–248, [INFOTRIEVE]
- Jacobson SH, Kjellstrand CM, Lins LE. Role of hypervolemia and renin in the blood pressure control of patients with pyelonephritis renal scarring. Acta. Med. Scand. 1988; 224(1)47–53, [INFOTRIEVE]
- Levison SP, Pitsakis PG, Levison ME. Free water reabsorption during saline diuresis in experimental enterococcal pyelonephritis in rats. J. Lab. Clin. Med. 1982; 99(4)474–480, [INFOTRIEVE]
- Kucherenko A, Markov C. Urinary excretion of prostanoids in children with chronic pyelonephritis. Adv. Exp. Med. Biol. 1997; 400B: 963–969, [INFOTRIEVE]
- Natochin YV, Bogolepova AE, Kuznetsova AA, Shakhmatova EI. Study of the role of prostaglandin E2 in urine flow regulation in chronic renal failure. Scand. J. Urol. Nephrol. 2000; 34: 327–330, [INFOTRIEVE], [CROSSREF]