Abstract
The correct diagnosis of renal allograft rejection may be difficult using only clinical and/or histopathological criteria. Immunological assays should be considered in order to evaluate the phenotype of inflammatory infiltrate in renal allograft biopsies. Immunohistochemical studies were performed to detect mononuclear cells, CD4 and CD8 T lymphocytes, B lymphocytes, macrophages, null cells, and positive cells for interleukin-2 receptors. A total of 41 allograft biopsies classified into three groups were studied: acute cellular rejection (28 biopsies/22 patients), borderline (7 biopsies/5 patients) and control (6 biopsies/6 patients).
In the rejection group (RG), increased cellularity was found mainly at the tubulo-interstitial level. Expression of CD8 positive cells was higher in RG when compared to borderline (BG) and control (CG) groups, respectively (0.9 vs. 0.0 vs. 0.35 cells/mm2; p < 0.001). Expression of macrophages was not statistically significant among the three groups (RG = 0.6 vs. BG = 0.2 vs. CG = 0.0 cells/mm2; p < 0.02). In the BG, CD4 + cells predominated (BG = 0.2 vs. RG = 0.05 vs. CG = 0.0 cells/mm2; p < 0.05). Clinically these patients were treated as cases of acute rejection. The numbers and different types of infiltrating cells did not correlate with patient's clinical outcome.
INTRODUCTION
Kidney transplantation is the treatment of choice for chronic renal failure, although numerous processes, such as acute rejection, nephrotoxic drugs, infections, and obstruction of the urinary or blood flow, cause allograft dysfunction and limit graft survival.Citation[1] The accurate diagnosis of an episode of renal allograft rejection is critical to the successful clinical management of organ transplant recipients, while renal biopsy is the most reliable method for evaluation of rejection.Citation[2–4] Acute rejection is an immune response against the graft involving cellular and humoral reactions, where T lymphocyte is the central component of the process. A lymphomononuclear inflammatory infiltrate with invasion of tubular and vascular walls are the main criteria of histological rejection. These morphological criteria were introduced by Banff's classification, which provides a practical and reproducible means of assessing renal allograft rejection and clinical orientation.Citation[5] Therefore, the differential diagnosis of acute rejection and borderline cases can be difficult, and new diagnostic methods are needed. Several immunohistological studies have focused on the characterization of the cellular infiltrate, cytokine production, and leukocyte adhesion molecular expression. With the development of monoclonal antibodies directed against leukocyte subsets, the knowledge of the types of cells involved in allograft rejection has increased significantly. Once these cells are activated, they are capable of producing and releasing cytokines, which are critical as regulators of the immune response.Citation[2],Citation[6],Citation[7] The immunophenotyping of biopsy specimens has confirmed the predominance of macrophages and T cells in interstitial infiltrates. The receptors for interleukin-2 are essential for lymphocyte interaction, cell growth, and new synthesis of other cytokines.Citation[8] Knowledge of the phenotype of the mononuclear cell population infiltrating the transplanted kidney has proved to be of great value in establishing the precise diagnosis of post-transplant renal dysfunction as well as determining its severity and reversibility. The allograft survival is dependent on the detection and treatment of acute rejection episodes as early as possible.
The aim of the present study was to analyze the phenotype of inflammatory infiltrate in borderline cases and of acute rejection with a correlation to the clinical outcome.
SUBJECTS AND METHODS
Study Groups
This study included the evaluation of 41 kidney transplant biopsy specimens from 33 patients submitted to renal transplant at the Clinical Hospital of Botucatu Medical School from 1987 to 2000.
All biopsies were obtained for clinical indication of renal allograft dysfunction. The biopsies were divided into three groups: rejection (n = 28 biopsies/22 patients), borderline (n = 7 biopsies/5 patients), and control (n = 6 biopsies/6 patients). Rejection episodes were diagnosed on clinical grounds and confirmed by transplant biopsy in all individuals. In the rejection groups, 63.6% of the patients rejected the graft in the first month post-transplant (50% in the first 15 days and 50% after 15 days). Eight patients rejected after one month (mean 109 days; range 33 to 240 days). The control group included cases of acute tubular necrosis.
The mean recipient age was 33.9 ± 11.9 years (range 14–54) in the RG, 36.0 ± 10.5 years (range 26–49) in the BG, and 34.0 ± 12.9 years (range 16–50) in the CG. The renal allograft recipient population received 21 living donor allografts and 12 cadaver renal allografts. Twenty-three patients received standard triple therapy maintenance immunosuppression (azathioprine, prednisone, Csa) and 10 received azathioprine and prednisone. The acute rejection episodes were treated with intravenous pulse methylprednisolone (9 patients) and resistant cases with pulse, OKT3 and/or Csa (12 patients). In the BG, two patients were treated with pulse and two with pulse plus OKT3. The patients were monitored for 0.5 to 155 months (mean 48.6 ± 45). Graft failure was defined as return to maintenance dialysis, transplant nephrectomy, or patient death. The characteristics of the studied population are outlined in .
Table 1 Characteristics of the study population
Morphological and Immunophenotypic Study
Percutaneous renal allograft biopsies were performed with ultrasound guidance. Histological features were analyzed in Duboscq-Brazil-fixed, paraffin-embedded tissue. Sections 3μm thick were stained with hematoxylin-eosin, periodic-acid-Schiff, methenamine silver, and Masson's trichrome. All of the biopsies had 10 or more glomeruli, and at least two arteries as recommended by Banff 97 classification.
The criteria for the histologic diagnosis was also performed using Banff's Schema.Citation[5]
Paraffin-embedded tissue sections 3μm thick were immunostained using avidin-biotin peroxidase method.Citation[9] Briefly, slides were incubated with primary antibody and secondary biotinylated anti-mouse IgG antibody (Vector Laboratories, California, USA) for 1h at room temperature. Endogeneous peroxidase was blocked with H2O2 for 15 min, and the antigen recovery was achieved with 0.01M citrate buffer or 1M EDTA. The slides were washed in PBS and incubated with the avidin-biotin complex reagent (Vector Laboratories) for 45 min. After washing, stains were developed with diaminobenzidine-tetrahydrochloride (Sigma, Steinheim, Germany) for 5 min. The slides were counterstained with Harris hematoxylin and mounted. Monoclonal antibodies used for labeling included LCA for CD45 (common leukocyte antigen), C8/144B for CD8 (CD8 + T lymphocyte), L26 for CD20 (pan B cells) from Dako (Glostrup, Dinamarca), 1F6 clone Ab-2 for CD4 (CD4 + T lymphocyte), Ab-2, clone 14 CO2 for CD14 (monocytes / macrophages), NCAM-1 Ab-a,56CO4 for CD56 (null cells), and IL-2Rα Ab-1 for CD25-IL2 receptor (Interleukin-2 receptor), all from NeoMarkers, California, USA.
Image Analysis
Computerized image analysis of immunohistochemically positive-stained cells in the infiltrate was used. Cells showing either peripheral, membrane-bound, or cytoplasmic staining were considered positive. The entire renal cortex in the biopsy was analyzed by an operator blind to the clinical status of the patient. Analysis of 10 to 25 fields (mean 21 ± 0.4) was done at high magnification (×40). The analysis was performed in a Leica QwinLite 2.5 image analyzer attached to a Leica DMLB microscope. The number of cells were expressed as positive cells/mm2. The cells were counted in the glomerular, periglomerular, tubular, interstitial, vascular, and perivascular compartments.
Statistical Analysis
Statistical comparisons between values were analyzed non-parametrically by Kruskal-Wallis test. Data were expressed as mean ± SEM and median values; a p value less than 0.05 was considered significant.
RESULTS
Phenotypic Composition of Cellular Infiltrate
The immunoreactivity of CD45, CD4, CD8, CD20, CD14, CD56, and IL-2R was along the outer cell membrane of inflammatory cells; IL-2R was also positive in the cytoplasm of tubular cells.
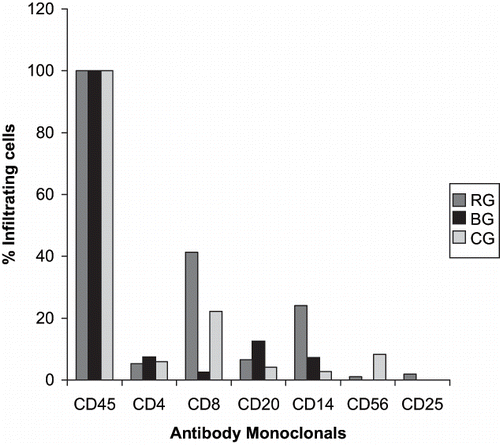
All cases in the three studied groups showed cells positive for panleukocyte marker (CD45). The percentages of cases with CD8-positive cells (41.3%) and macrophages (24%) were higher in the RG than in the BG and CG. The percentage of positive cases in the BG was 12.5% for B cells, 7.5% for CD4 T cells, 7.2% for macrophages, and 2.6% for CD8 T cells. B lymphocytes, null cells and IL-2R-positive cells were infrequent or absent in all groups. The positive cells were present mainly in the tubular and interstitial compartments.
The proportions of positive cases in all groups for CD45, CD4, CD8, B cells, macrophages, null cells, and IL-2R-positive cells are shown in .
Quantification of Positive Cells in the Renal Allograft Biopsies
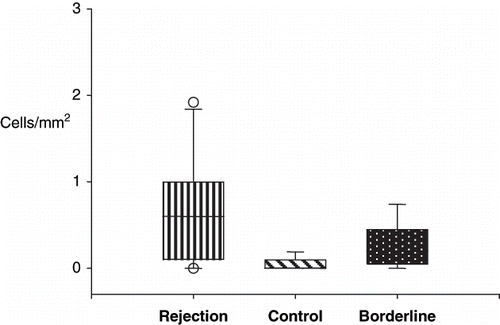
summarizes the results of the quantification of positive cells/mm2 in the different groups. The expression of several surface markers defining cellular subsets were different in the rejecting compared with the control groups. As expected, the number of CD45-positive cells infiltrating the cortex was significantly higher in the rejecting and borderline groups versus control group (md 2.6 vs. 4.4 vs. 1.35 cells/mm2). Likewise, macrophages, CD4, and B cells were increased in the RG and BG when compared to BG. During rejection, the proportions of CD8 T lymphocytes were increased (md 0.9 vs. 0.0 vs. 0.35; p < 0.001; see ). No significant differences were found in the number of macrophages between RG and BG (see ). Null cells and IL-2R-positive cell expression were similar in the three groups.
Table 2 Comparison of cellular infiltrates (cells/mm2) among renal transplant groups
Figure 2. Quantitative analysis of CD8+ cells in the rejection, borderline, and control groups. Rejection (*) and borderline groups (#) were statistically significant.
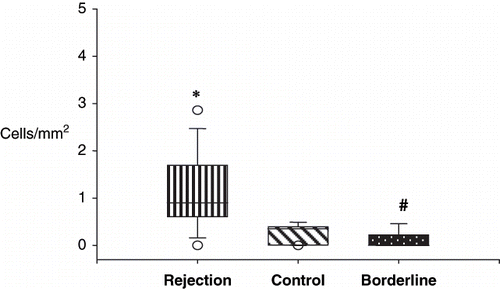
Figure 3. Quantitative analysis of CD14 + cells in the rejection, control, and borderline groups. Rejection and borderline groups were not statistically significant.
Using Banff criteria in the RG, 18 biopsies (64.3%) were classified as grade I (8 IA, 10 IB), 6 (21.4%) as grade II (5 IIA, 1 IIB), and 4 (14.3%) as grade III. The pattern of inflammatory infiltrate was similar among the three grades.
Clinicopathological Correlations
In the rejection group, no significant differences were found in the composition of the cellular infiltrates when the subgroups with rejection in the first 15 days were compared to those from 16 days to 1 month and those after 1 month post-transplant. In the follow-up of the RG, six patients lost the graft in the first six months, and five patients within four years; out of eleven, five died with normal renal function and six were still on follow-up. The inflammatory infiltrate did not show any difference among these three subgroups.
No significant correlation was found between the quantitative analysis of different kinds of cells and the patients who received a living donor or cadaver allografts in the rejection and borderline groups.
After the treatment, 19 of 27 patients from the rejection and borderline groups presented basal serum creatinine less than 2.0 mg/dL, and 8 patients maintained elevated serum creatinine ((2.0 mg/dL). The phenotype of inflammatory cells was similar in both groups.
The clinicopathological correlations were expressed in and .
Table 3 Comparison of cellular infiltrates (cells/mm2) among graft donors, time from transplant to biopsies in the RG and BG, and Banff analysis in the RG
Table 4 Comparison of cellular infiltrates (cells/mm2) among patients with basal creatinine <2.0 and >2.0 mg/dL, and patients outcome, in the rejection and borderline groups
DISCUSSION
The aim of the present study was to elucidate whether clinical rejection was associated with a different phenotype and activation status than those of the graft infiltrating cells. LCA-positive cells were found in all biopsies, even in the control cases with acute tubular necrosis. A possible explanation may be that unknown antigens are released from the damaged tissue, and that these, in turn, are capable of stimulating the immune system.Citation[8]
As expected the inflammatory infiltrates were more severe in acute rejection and borderline cases when compared to the control group. The results showed that the number of CD8+ lymphocytes and macrophages were greater in the rejection group versus the borderline and control groups (p < 0.001). The CD8+ cells were demonstrated mainly in the tubulointerstitial compartment, characterizing the tubulitis. CD4, CD20, and CD56-positive cells were infrequent in the rejection group.
In previous studies of rejected grafts, T lymphocytes accounted for up to 90% of the infiltrate, with increases in both CD4+ and CD8+ populations.Citation[10–12] Ibrahim et al.Citation[11] found that biopsies from patients with acute rejection had higher counts of CD3-, CD4-, CD8-, CD45RA-, and CD45RO-positive cells compared with graft biopsies showing no rejection. The greatest difference was the increased frequency of CD8, CD45RO, and CD68 cells.Citation[13] T suppressor-cytotoxic CD8+ cells in a diffuse pattern and with invasion of tubular wall were considered the preponderant cells in acute rejection.Citation[6],Citation[11] MacrophagesCitation[7],Citation[14] comprised between 30–60% of the total infiltrating leukocytes in renal allograft rejection. Variable numbers of B lymphocytes (2–10%) and null cells (2–30%) have been demonstrated in cases of rejection.Citation[6],Citation[7] Hancock et al.Citation[7] showed a transient increase in NK/K cell infiltration at days 2 and 3 post-transplant.
In this study, the IL-2R expression was not significant in the rejection and borderline groups, and the prevalence in acute rejection was lower than that reported in previous studies.Citation[8],Citation[10] Some authors think that the IL-2R expression in allograft biopsies was the most reliable marker in discriminating acute cellular rejection from other abnormalities.Citation[8] However, other studies have demonstrated IL-2R expression before the clinical dysfunction of the graft. Seron et al.Citation[10] found IL-2R very early after immunological stimulation, with a peak 48H after the stimulation had taken place. And the IL-2 gene product appeared before acute rejection was detectable by clinical or biochemical changes.Citation[15] These can explain the infrequent positivity in some reports.
No correlation was found between the numbers of different subsets of mononuclear cells and the severity of the rejection. There was no difference in the inflammatory infiltrate between cases with grade I versus grades II and III in Banff's classification. Some reports have demonstrated association of CD8+ T cells and macrophages with more severe rejection.Citation[16]
Most of the patients (68.2%) presented a rejection episode in the first month post-transplant. No significant differences were found in the number of different subpopulations of cells in the cases with rejection up to 15 days, in those from 16 days to 1 month, or those after 1 month post-transplant. Some studies have reported greater number of CD4, CD8, and null cells in the initial stages of inflammatory process, with later increase of macrophages.Citation[7],Citation[14] An examination of sequential biopsies should provide additional insight into the rejection process.Citation[7] In human studies as well as in the rat model of acute renal allograft rejection, the number and proportion of macrophages were found to increase as rejection progressed, such that macrophages were the dominant cell type present during severe rejection at day 14.Citation[14]
The correlation between mononuclear cell infiltrates and outcome has been less clear. Initial reports utilizing phenotypic and functional assays to identify allograft infiltrates suggested an association between severe rejection and the type of cellular response.
A diffuse cortical inflammatory infiltrate with CD8+ cells and macrophages has been related to severe rejection and poor prognosis of the graft . A preponderance of macrophages has been associated with more severe forms of rejection and may be helpful in diagnosing and predicting a poor outcome of kidney allograft.Citation[16]
No statistically significant differences were found in the inflammatory infiltrate between the patients that had lost the graft until 6 months, from 6 months to 4 years, and the patients that are still on follow-up. The small number of cases in the different groups can explain these results.
In some cases, the intensity and pattern of the inflammatory infiltrate were not conclusive for rejection and were interpreted as borderline cases. The quantitative analysis of different subsets of cells in the borderline group showed a greater number of CD20 cells and a smaller number of CD8+ T cells when compared to rejection group. Although this group did not show histological criteria for rejection, the patients were treated and their cases clinically interpreted as rejection. In the literature, there is a very small number of studies with a small number of borderline cases.Citation[17],Citation[18] that have been studied phenotypically. In general, these cases are really rejection and have a good response to immunosuppression. Tubulitis involved the various tubular segments to a varying degree, so sampling error becomes a potential problem. Gibson et al.Citation[19] agreed that tubulitis, critical to the diagnosis of acute allograft rejection, was detected as reliably by PAS staining as by immunohistochemistry. They concluded that for routine diagnostic examination of renal transplant biopsies, the standard histological methods are adequate for the distinction of acute rejection versus no acute rejection in the majority of cases. However, routine staining of CD8+ cytotoxic T cells may prove useful as a diagnostic aid in cases where the amount of tubulitis identified is insufficient for the diagnosis of borderline cases.Citation[19],Citation[20]
In conclusion, the present study demonstrated that a significant interstitial infiltration with LCA-positive cells occurred in the kidney graft and was more severe when acute rejection was noted. The results are consistent with previous findings demonstrating marked increases in the number of CD8 + T cells and macrophages in the rejection group. The borderline cases evolve clinically as rejection. No significant correlations were found between the inflammatory infiltrate and the graft outcome. The immunological diagnosis of rejection requires a combination of several markers.
ACKNOWLEDGMENTS
The authors thank Paulo Cury, MD, PhD, and Reinaldo José da Silva, Biol. PhD, for assistance with statistical and image analysis, respectively.
This work was supported by FAPESP (02/06285-2), FUNDUNESP (785/2002DFP) and CNPq (130156/2003-5) grants.
REFERENCES
- Pestana JOM, Ramos OL, Ajzen H. Guia clinico para transplante renal. J. Bras. Nefrol 1992; 14(2)66–86
- Veronese EV, Centeno AD, Almeida AG, Fritsch A, Mello AG, Webber A, Zucatto AE, Perini SC, Manfro RC, Gonçalves LF. Percutaneous renal allograft biopsy: Where are we going?. Rev. Assoc. Med. Bras 1992; 45(2)169–174
- McCarthy GP, Roberts ISD. Diagnosis of acute renal allograft rejection: evaluation of the Banff '97 guidelines for slide preparation. Transplantation 2002; 73(9)1518–1521
- Dean DE, Kamath S, Peddi VR, Schroeder TJ, First MR, Cavallo T. A blinded retrospective analysis of renal allograft pathology using the Banff schema. Implications for clinical management. Transplantation 1999; 68(5)642–645
- Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldeberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Marcussen N, Mihatsch MJ, Nadasdy T, Nickerson P, Olsen TS, Papadimitriou JC, Randhawa PS, Rayner DC, Roberts I, Rose S, Rush D, Salinas-Madrigal L, Salomon DR, Sund S, Taskinen E, Trpkov K, Yamaguchi Y. The Banff '97 working classification of renal allograft pathology. Kidney Int 1999; 55: 713–723
- McWhinnie DL, Thompson JF, Taylor HM, Chapman JR, Bolton EM, Carter NP, Wood RFM, Morris PJ. Morphometric analysis of cellular infiltration assessed by monoclonal antibody labeling in sequential human renal allograft biopsies. Transplantation 1986; 42(4)352–358
- Hancock WW, Gel D, De Moerloose P, Rickels FR, Ewan VA, Atkins RC. Immunohistological analysis of serial biopsies taken during human renal allograft rejection. Transplantation 1985; 39(4)430–438
- Noronha IL, Eberlein-Gonska M, Hartley B, Stephens S, Cameron JS, Waldherr R. In situ expression of tumor necrosis factor-alpha, interfero-gamma, and interleukin-2 receptors in renal allograft biopsies. Transplantation 1992; 54(6)1017–1024
- Hsu SM, Rainn L, Fanger M. Use of avidin-biotin-peroxidase complex (ABC) and unlabeled antibody (RAP) procedure. J. Histochem. Cytochem 1981; 29(4)577–580
- Serón D, Alexopoulos E, Raftery MJ, Hartley RB, Cameron JS. Diagnosis of rejection in renal allograft biopsies using the presence of activated and proliferating cells. Transplantation 1989; 47(5)811–816
- Ibrahim S, Dawson DV, Sanfilippo F. Predominant infiltration of rejecting human renal allografts with T cells expressing CD8 and CD45RO. Transplantation 1995; 59(5)724–728
- Tötterman TH, Hanas E, Bergström R, Larsson E, Tufveson G. Immunologic diagnosis of kidney rejection using FACS analysis of graft-infiltrating functional and activated T and NK cell subsets. Transplantation 1989; 47(5)817–823
- Grimm PC, McKenna R, Nickerson P, Russell ME, Gough J, Gospodarek E, Liu B, Jeffery J, Rush DN. Clinical rejection is distinguished from subclinical rejection by increased infiltration by a population of activated macrophages. J. Am. Soc. Nephrol 1999; 10: 1582–1589
- Le Meur Y, Jose MD, Mu W, Atkins RC, Chadban SJ. Macrophage colony-stimulating factor expression and macrophage accumulation in renal allograft rejection. Transplantation 2002; 73(8)1318–1324
- Dallman MJ, Roake J, Hughes D, Toogood G, Morris PJ. Sequential analysis of IL-2 gene transcription in renal transplants. Transplantation 1992; 53(3)683–685
- Sanfilippo F, Kolbeck PC, Vaughn WK, Bollinger RR. Renal allograft cell infiltrates associated with irreversible rejection. Transplantation 1985; 40(6)679–685
- Strehlau J, Paulakis M, Lipman M, Shapiro M, Vasconcellos L, Harmon W, Strom TB. Quantitative detection of immune activation transcripts as a diagnostic tool in kidney transplantation. Proc. Natl. Acad. Sci USA 1997; 94: 695–700
- Lipman ML, Stevens AC, Bleackley RC, Helderman JH, McCune TR, Harmon WE, Shapiro ME, Rosen S, Strom TB. The strong correlation of cytotoxic T lymphocyte-specific serine protease gene transcripts with renal allograft rejection. Transplantation 1992; 53(1)73–79
- Gibson IW, Marcussen N, Brown RW, Solez K, Truong LD. The use of immunocytochemistry (LCA and Leu-7) in diagnosis of renal allograft rejection. Transplantation Proceedings 1996; 28(1)457–464
- Beschorner WE, Burdick JF, Williams GM, Solez K. The presence of Leu-7-reactive lymphocytes in renal allografts undergoing acute rejection. Transplantation Proceedings 1985; 17: 618–682