Abstract
The present work was designed to study Na+ K+ ATPase α1-subunit phosphorylation in rats with chronic renal failure (CRF) in comparison with normal rats. Na+ K+ ATPase α1-subunit phosphorylation degree was measured by binding the McK-1 antibody to dephosphorylated Ser-23 in microdissected medullary thick ascending limb of Henle (mTAL) segments. In addition, the total Na+ K+ ATPase α1-subunit expression and activity were also measured in the outer renal medulla homogenates and membranes.
CRF rats showed a higher Na+ K+ ATPase activity, as compared with control rats (18.95 ± 2.4 vs. 11.21 ± 1.5 μmol Pi/mg prot/h, p < 0.05), accompanied by a higher total Na+ K+ ATPase expression (0.54 ± 0.04 vs. 0.27 ± 0.02 normalized arbitrary units (NU), p < 0.05). When McK-1 antibody was used, a higher immunosignal in mTAL of CRF rats was observed, as compared with controls (6.3 ± 0.35 vs.4.1 ± 0.33 NU, p < 0.05). The ratio Na+ K+ ATPase α1-subunit phosphorylation / total Na+ K+ ATPase α1-subunit expression per μg protein showed a non-significant difference between CRF and control rats in microdissected mTAL segments (2.11 ± 0.12 vs.2.26 ± 0.18 NU, p = NS). The PKC inhibitor RO-318220 10−6M increased immunosignal (lower phosphorylation degree) in mTAL of CRF rats to 128.43 ± 7.08% (p < 0.05) but did not alter McK1 binding in control rats. Both phorbol 12-myristate 13-acetate (PMA) 10−6M and dopamine 10−6M decreased immunosignal in CRF rats, corresponding to a higher Na+ K+ ATPase α1-subunit phosphorylation degree at Ser-23 (55.26 ± 11.17% and 53.27 ± 7.12% compared with basal, p < 0.05). In mTAL of CRF rats, the calcineurin inhibitor FK-506 10−6M did not modify phosphorylation degree at Ser-23 of Na+ K+ ATPase α1-subunit (100.21 ± 3.00% compared with basal CRF). In control rats, FK 506 10−6M decreased the immunosignal, which corresponds to a higher Na+ K+ ATPase α1-subunit phosphorylation degree at Ser-23. The data suggest that the regulation of basal Na+ K+ ATPase α1-subunit phosphorylation degree at Ser-23 in mTAL segments of CRF rats was primarily dependent on PKC activation rather than calcineurin dependent mechanisms.
INTRODUCTION
Sodium homeostasis in chronic renal failure (CRF) is maintained until late stages of the disease by mechanisms that have only been partially characterized. Thus, when total renal mass is reduced, single nephron glomerular filtration rate in the remaining nephrons augmentsCitation[1] and is paralleled by an increase in fractional sodium excretion (FENa).Citation[2],Citation[3] Several authors have found a reduction in proximal tubule fractional reabsorption of sodium in CRF,Citation[4–6] which results in an increased delivery to both loop segments and distal tubules.Citation[7],Citation[8] The increased sodium delivery to the loop of Henle is associated with a compensatory increment in sodium reabsorption in this segment, though FENa remains elevated.Citation[8] Changes in the ionic transport events described above have been associated with an increase in both Na+ K+ ATPase expression and activity in the outer medulla after unilateral nephrectomyCitation[9] and in CRF animals.Citation[10],Citation[11]
Three isoforms of the catalytic α-subunit and two β‐subunit isoforms constitute the multigene family encoding the functional Na+ K+ ATPase α/β heterodimer. Na+ K+ ATPase isoforms exhibit tissue-specific patterns of expression. In the mTAL of rats, only α1/β 1 proteins are expressed under normal physiological conditions.Citation[12] In CRF rats, an increase in renal Na+ K+ ATPase activity was accompanied by an increase in the mRNA of α1-subunit,Citation[13] so different tissues display different patterns of isoform expression for Na+ K+ ATPase. Independently of the particular isoforms expressed in each tissue, it has been described as an important and specific role for post-translational regulation as well. When cation activation kinetics was explored in different tissues expressing different Na+ K+ ATPase isoforms, it was found that tissue-specific kinetic was at least as important as isoform-specific predicted activation for Na+ K+ ATPase function.Citation[14] In line with this, recent studies in the normal kidney have well established that phosphorylation/dephosphorylation processes at the α1-subunit of Na+ K+ ATPase contribute to different post-translational Na+ K+ ATPase regulatory rates.Citation[15–19] Thus, rat Na+ K+ ATPase α1-subunit is phosphorylated in vitro by protein kinase C (PKC), predominantly at Ser-23.Citation[17] It has recently been demonstrated in rats with normal renal function that Na+ K+ ATPase phosphorylation degree at Ser-23 was lower in microdissected medullary thick ascending limb of Henle (mTAL) segments, as compared with proximal tubules (PCT), accompanied by an increase in Na+ K+ ATPase activity.Citation[20] This Na+ K+ ATPase phosphorylation heterogeneity in the normal kidney was associated with different regulatory intracellular mechanisms. Thus, while Na+ K+ ATPase activity was strongly dependent of PKC activation in PCT, it was mostly determined by calcineurin-dependent phosphorylation in mTAL.Citation[20]
Thus, factors that could potentially affect Na+ K+ ATPase function in CRF, such as tissue-specific activation kinetic, segmental variations in the rate of phosphorylation/dephosphorylation in the nephron, and compensatory changes in ionic transport shown by mTAL in CRF, prompted this investigation into whether the modifications observed in electrolyte transport in CRF could be a consequence of changes in regulatory intracellular pathways that command the post-translational regulation of Na+ K+ ATPase in CRF. Therefore, the present study was designed to focus on the regulation by both PKC and calcineurin of Na+ K+ ATPase α1-subunit in mTAL microdissected segments in rats with CRF. The results indicate that Na+ K+ ATPase α1-subunit phosphorylation in mTAL of CRF rats was determined by an increase in PKC-dependent phosphorylation, rather than on a calcineurin dependent mechanism, as has been found in normal rats.Citation[20]
METHODS
Male Wistar rats (Animal Care Laboratory, Alfredo Lanari Institute of Medical Research, Buenos Aires, Argentina), weighing 250–350 g with either normal renal function or CRF, on a free diet (Ganave, Buenos Aires, Argentina) containing 24% protein, 0.5% sodium chloride and 1.25% potassium, with tap water ad libitum and under controlled room temperature and light conditions, were used. The protocol was approved by the local Committee for Animal Research. Renal insufficiency was produced by suppressing a major portion of renal tissue, as previously described.Citation[21] Briefly, under pentobarbital anesthesia (25 mg/kg body wt, i.p.), the left kidney was exposed by a flank incision and then dipped in hot water for 14 seconds to burn the outer cortex. Previous studies have demonstrated that this procedure results in a predominantly loss of cortical nephrons with preserved medullary nephrons showing an intact diluting capacity. After seven days of recovery and under light anesthesia, the right kidney was surgically removed. The rats were studied one week later. Control rats were subjected to sham operation with the surgical procedures described above.
In Vivo Studies
Both animal groups, those with normal renal function and after induction of CRF, were anesthetized and prepared for clearance studies. Briefly, after tracheotomy, suitable catheters were placed at the carotid artery, jugular vein, and urinary bladder. Enough inulin (Inutest, Linz, Austria) to provide 0.2 mg/mL plasma concentrations was administered in saline at 0.025 mL/min through the jugular vein to measure glomerular filtration rate (GFR). After 45 minutes of equilibrium, three consecutive periods of 30 minutes for urine collections were done. Blood samples from the carotid artery were taken at the midpoint of each period. Inulin was determined in both plasma and urine samples by anthrone method.Citation[22] Urea, electrolytes, and osmolality were also measured in urine and plasma.
Preparation of Samples
Control and CRF rats were anesthetized with intraperitoneal sodium pentobarbital, 50 mg/kg body wt i.p. After a midline incision, the left kidney was perfused and the tubules were microdissected as described previously.Citation[23] The kidney was perfused with a modified Hank's solution (mM): NaCl 137, KCl 5, MgSO4 0.8, Na2HPO4 0.33, KH2PO4 0.44, CaCl2 1, MgCl2 1 and Tris-HCl 10, to which 0.05% collagenase (Sigma Chemical Co., St. Louis, Missouri, USA) and 0.1% bovine serum albumin (BSA) had been added. The pH was adjusted to 7.4. The kidney was removed and cut along its corticopupillary axis into small pyramids. The pyramids were incubated at 35oC for 20 min in 10 ml perfusion solution, which was bubbled with 100% oxygen. Butyrate 10−3 M was added to the perfusion fluid to optimize mitochondrial respiration of the tubules. The tissue was rinsed and transferred to the same perfusion solution, except that collagenase and bovine serum albumin were omitted and the CaCl2 concentration was reduced to 0.25 mM (microdissection solution). Butyrate (10−3 M) was also added to the microdissection solution. mTAL segments were manually microdissected from outer medulla. Dissection was carried out on ice and under stereomicroscopic observation as described.Citation[24]
Outer medulla homogenates from control and CRF rats were prepared as described.Citation[25] Outer medulla slices were isolated and homogenized with 300 μl of buffer containing (in mM): 20 Tris, 2 EGTA, 2 EDTA, 1 phenylmethylsulnyl fluoride (PMSF), 10 β-mercaptoethanol and 100 KIE/mL aprotinin (pH 7.4). The sample was stored at −70°C until assay. The protein content for each sample was measured by Lowry's method.Citation[26]
Incubation of Samples for Electrophoresis
Dissected tubules were drawn for measurement of length by using a camera lucida device. mTAL segments were incubated in a final volume of 15 μl with dissection buffer alone or with phorbol 12-myristate 13-acetate (PMA) 10−6 M, RO-312880 10−6 M, FK-506 10−6 M or dopamine (DA) 10−6 M for 10 min at room temperature.Citation[27] The incubation was stopped by adding lysis buffer containing 10% 2-mercaptoethanol, 8% sodium dodecyl sulfate (SDS), 0.25 M Tris-HCl, pH 6.8, 50% glycerol, 50 mM NaF, 1 mM phenylmethylsulphonyl fluoride (PMSF), and 0.001% phenol blue. Aliquots of this solution, containing 10 to 12 microdissected segments with a total length ranging between 9 and 12 mm, were immediately placed onto an 8% SDS-PAGE. The protein content was 2–3 μg for each sample, which is in agreement with previous data.Citation[24] For each gel, an identical gel was run in parallel and subjected to Coomassie staining to ensure identical protein loading. The amount of protein was chosen after the linearity of detection had been verified. After electrophoresis, proteins were electrophoretically transferred to PVDF membranes (Hybond, PVDF, Amersham Pharmacia Biotech, Buckinghamshire, England). Then, the membranes were rinsed in Tris buffered saline (TBS) and quenched with 5% fat-free dry milk in TBS containing 0.1% Tween 20.
Phosphorylation of PKC sites in Na+ K+ ATPase α1‐subunit was detected with a specific monoclonal antibody, McK-1. This antibody binds a sequence, DKKSKK, located in the N- terminus of the rat α1-subunit of Na+ K+ ATPase. This sequence contains the serine residue Ser-23.Citation[28] McK-1 binds Ser-23 when this residue is dephosphorylated and not when it is phosphorylated by PKC.Citation[17] Thus, a higher immunosignal indicates that Na+ K+ ATPase is more dephosphorylated at α1-subunit, Ser 23. In rat Na+ K+ ATPase α1-isoform, McK-1 also binds Ser-11, another PKC phosphorylation site, but with a lower stoichiometry than Ser-23.Citation[17] This antibody, constructed at Dr. K. Sweadner's laboratory, has been validated as a good probe to evaluate phosphorylation/dephosphorylation degree in rat Na+ K+ ATPase α1-subunit as seen with the 32P loading method by several other works.Citation[17],Citation[29],Citation[30] McK-1 was diluted 1:1000 in TBS containing 0.1% Tween 20.
The outer medulla homogenates were also placed onto an 8% SDS-PAGE, loading an equal amount of protein per lane (∼15 μg). The expression of total Na+ K+ ATPase was assessed by using a common monoclonal antibody (mouse anti-Na+ K+ ATPase α1-subunit). This antibody recognizes Na+ K+ ATPase α1-subunit irrespective of its phospho-state and was chosen because α1-subunit represents 99% of the α-isoforms in the kidney.Citation[31] Common antibody was diluted 1:5000 in TBS containing 0.1% Tween 20.
After incubation with secondary antibody, proteins were visualized with an enhanced chemiluminescence detection kit (ECL, Amersham Pharmacia Biotech, Buckinghamshire, England). Thereafter, the PVDF membranes were stained with Amido Black and scanned to confirm uniform protein loading of the lanes. Densitometric analyses of films and membranes were performed on a PC computer using the BioRad Laboratories Molecular Analyst Software (Bio-Rad, Model GS-670 Imaging Densitometer). Channel width and height selected for densitometric analyses were equal in each gel, and the background was also considered. Samples under comparison were run on the same gel and comparisons among band intensities were performed within the same membrane. The magnitude of the immunosignal is given as normalized figures, referring to common antibody signal or ug protein, when appropriate.
Na+ K+ ATPase Activity
Membranes of outer renal medulla from control and CRF rats were prepared by homogenizing slices in 25 mM phosphate buffer (pH 7.4) as previously described.Citation[11] Homogenates were centrifuged at 2,000 rpm for 10 min at 4°C. Then, supernatants were centrifuged at 12,000 rpm for 30 min at 4°C and pellets resuspended in the original buffer to the same volume. The membranes obtained were incubated during 15 min at 37°C in the absence or presence of 4 mM ouabain. Na+ K+ ATPase activity was measured in a buffer containing (in mM): 140 NaCl, 5 KCl, 5 MgCl2, 1 EGTA, 30 Tris-HCl, 3 Na2ATP, and trace amounts of [γ-32P] ATP. When ouabain was present, NaCl and KCl were omitted from the incubation medium. The phosphate liberated by hydrolysis of [γ-32P] ATP was separated by centrifugation after adsorption of the unhydrolyzed nucleotide on 15% activated charcoal in trichloroacetic acid. Radioactivity of the supernatant was measured in a liquid scintillation spectrometer. Total and ouabain insensitive ATPase activities were measured and the difference between them was expressed in micromoles of [32P] hydrolyzed per mg protein and per hour.
Statistical Analysis
Results are given as means ± SEM in normalized arbitrary units (NU) or percentage of NU as compared with basal condition. Na+ K+ ATPase activity is expressed as micromoles of [32P] hydrolyzed per mg protein and per hour and results are given as means ± SEM. p < 0.05 was considered significant. Data were analyzed by Student's t-test.
Chemicals
Common monoclonal antibody against total α1-subunit Na+ K+ ATPase was purchased from Upstate Biotechnology (Waltham, Massachusetts, USA); McK-1, antibody against dephosphorylated Ser-23 of rat α1-subunit Na+ K+ ATPase was a kind gift from Dr. K Sweadner. Dopamine (DA), phorbol 12-myristate 13-acetate (PMA), and ouabain were purchased from SIGMA. RO-318220 from Roche Products LTD (London, UK); FK-506 from Calbiochem, Calbiochem-Novabiochem, Corporation (La Jolla, California, USA). [γ-32P]ATP was from PerkinElmer, Life Sciences, Inc. (Boston, Massachusetts, USA).
RESULTS
shows baseline parameters in experimental and control animals, where CRF rats display values consistent with a ∼60% decrease in renal function.
Table 1 Body weight, plasma and urine parameters in control and CRF rats
The activity and expression of Na+ K+ ATPase in outer renal medulla homogenates were compared between control and CRF rats. As shown in , Na+ K+ ATPase activity was ∼70% higher in CRF, as compared with control rats (18.95 ± 2.4 vs.11.21 ± 1.5 μmol Pi / mg prot . hr, respectively, n = 9, p < 0.05). When a common monoclonal antibody, which recognizes total Na+ K+ ATPase α1-subunit expression, was used in outer medulla homogenates, CRF rats showed a ∼100% increased in the immunosignal, as compared with control homogenates (0.54 ± 0.036, n = 5 vs.0.27 ± 0.018 NU, n = 6, p < 0.05; see ). The results agree with the notion that CRF is associated both with a higher Na+ K+ ATPase activity and expression in the outer medulla, as earlier reported.Citation[9],Citation[10]
Figure 1. Differences in Na+ K+ ATPase activity and total Na+ K+ ATPase expression in outer renal medulla of control and CRF rats: (a) Na+ K+ ATPase activity was measured in outer renal medulla membranes by hydrolysis of [γ-32P]ATP; values are means ± SEM and expressed as μmol Pi / mg. prot . hr, and (b) Na+ K+ ATPase expression was measured in outer renal medulla homogenates using a common monoclonal antibody that recognizes total Na+ K+ ATPase α1-subunit independently of its phosphorylation degree; values are means ± SEM and were expressed as normalized arbitrary units. Normalized arbitrary units were obtained as total Na+ K+ ATPase α1-subunit expression with common antibody per μg protein. Na+ K+ ATPase activity and total Na+ K+ ATPase expression were increased in CRF rats as compared with control rats. *p < 0.05, as compared with control rats.
![Figure 1. Differences in Na+ K+ ATPase activity and total Na+ K+ ATPase expression in outer renal medulla of control and CRF rats: (a) Na+ K+ ATPase activity was measured in outer renal medulla membranes by hydrolysis of [γ-32P]ATP; values are means ± SEM and expressed as μmol Pi / mg. prot . hr, and (b) Na+ K+ ATPase expression was measured in outer renal medulla homogenates using a common monoclonal antibody that recognizes total Na+ K+ ATPase α1-subunit independently of its phosphorylation degree; values are means ± SEM and were expressed as normalized arbitrary units. Normalized arbitrary units were obtained as total Na+ K+ ATPase α1-subunit expression with common antibody per μg protein. Na+ K+ ATPase activity and total Na+ K+ ATPase expression were increased in CRF rats as compared with control rats. *p < 0.05, as compared with control rats.](/cms/asset/ce4dcd62-622e-46c0-8cad-6ad8a8117c79/irnf_a_203756_uf0001_b.gif)
Outer medullary homogenates contain both inner and outer stripe zones. Among renal tubule segments in the outer medulla, the higher Na+ K+ ATPase activity is found in the thick ascending limb,Citation[32] which accounts for ∼90% of the total Na+ K+ ATPase activity of the outer medulla. Thus, changes in Na+ K+ ATPase expression in the outer medulla are most likely due to changes in enzyme expression in the thick ascending limb, as previously shown.Citation[33]
In order to determine whether the increase in Na+ K+ ATPase activity in CRF rats is paralleled by changes in the phosphorylation degree, Na+ K+ ATPase α1-subunit phosphorylation degree at Ser-23 in microdissected mTAL in control and CRF rats was measured. shows a higher immunosignal in mTAL of CRF rats when McK-1 antibody was used (6.3 ± 0.35 vs.4.1 ± 0.33 NU, n = 5, p < 0.05). This increment indicates an increase in the dephosphorylation degree of >50% in CRF rats. However, the ratio between Na+ K+ ATPase α1-subunit phosphorylation degree at Ser-23 / total Na+ K+ ATPase α1-subunit expression showed no significant differences between CRF and control rats in microdissected mTAL segments (2.11 ± 0.12 vs. 2.26 ± 0.18 NU, p = NS; see ), suggesting a major contribution of total Na+ K+ ATPase α1-subunit to the observed changes.
Figure 2. Differences in α1-subunit Na+ K+ ATPase expression at Ser-23 in mTAL microdissected segments of control and CRF rats. The immunoblot was performed with a monoclonal antibody (McK-1) against dephosphorylated PKC site, Ser-23, of the Na+ K+ ATPase α1-subunit. Values are means ± SEM and were expressed as normalized arbitrary units. Normalized arbitrary units were obtained as (a) Na+ K+ ATPase α1-subunit expression with McK-1 antibody per μg protein, and (b) Na+ K+ ATPase α1-subunit expression with McK-1 antibody / total Na+ K+ ATPase α1-subunit expression with common antibody per μg protein in microdissected tubules. (see ). Densitometric analysis of all samples revealed a higher immunosignal (decreased phosphorylation) of Na+ K+ ATPase α1-subunit expression in CRF rats, as compared with control rats. *p < 0.05, as compared with control rats.
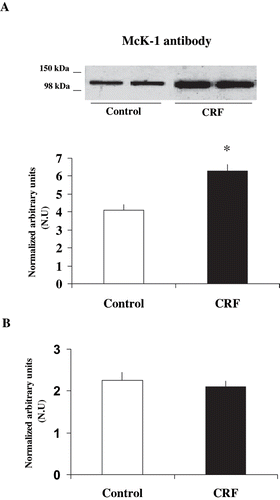
Table 2 Expression of α1-subunit Na+ K+ ATPase phosphorylation degree at Ser-23 in microdissected mTAL in control and CRF rats
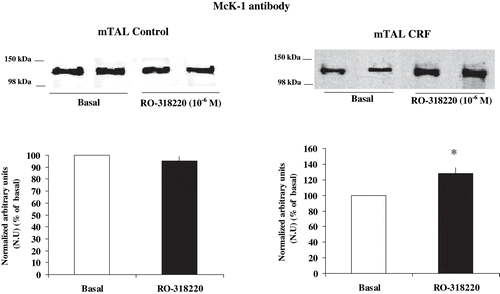
and show the effect of RO-318220 10−6 M, a specific PKC inhibitor, on the degree of phosphorylation at Ser-23 α1-subunit Na+ K+ ATPase in mTAL from control and CRF rats. The addition of the PKC inhibitor caused a significant decrease in the degree of phosphorylation in CRF rats, as given by a higher immunosignal (128.43 ± 7.08 %, n = 6, p < 0.05), as compared with basal CRF. In control rats, the PKC inhibitor did not alter the immunosignal, as compared with basal values (95.32 ± 3.56 %, n = 5, p = NS). These results suggest that Na+ K+ ATPase α1-subunit degree of phosphorylation at Ser-23 in mTAL of CRF rats is dependent, at least in part, on a constitutive activation of PKC.
Figure 3. Effect of PKC inhibition on α1-subunit Na+ K+ ATPase phosphorylation degree at Ser-23 in mTAL microdissected from control and CRF rats. Na+ K+ ATPase α1-subunit phosphorylation degree at Ser-23 in immunoblots of mTAL segments from control and CRF rats were treated with RO-318220 10−6 M, a specific PKC inhibitor. The immunoblot was performed with a monoclonal antibody (McK-1) against dephosphorylated PKC site, Ser-23, of the Na+ K+ ATPase α1-subunit. Values are means ± SEM and were expressed as percentage of normalized arbitrary units over basal. Normalized arbitrary units were obtained as Na+ K+ ATPase α1-subunit expression with McK-1 antibody / total Na+ K+ ATPase α1-subunit expression with common antibody per μg protein in microdissected tubules (see ). Densitometric analysis of all samples revealed an increase in immunosignal of Na+ K+ ATPase α1-subunit (decreased phosphorylation) under RO-318220 in mTAL segments of CRF rats. No changes were observed with RO-318220 in mTAL segments of control rats (p = NS). *p < 0.05, as compared with basal in CRF rats.
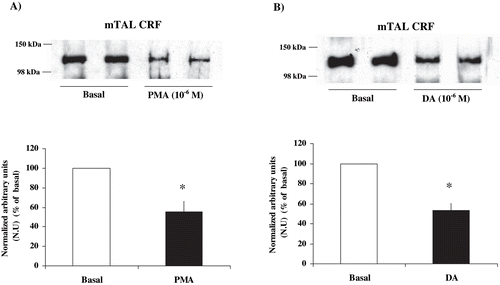
The ability of PKC to phosphorylate Na+ K+ ATPase α1-subunit at Ser-23 in mTAL was examined by incubating microdissected mTAL segments with PMA 10−6 M, a direct PKC activator, and DA. As can be seen in , control rats with PMA and DA showed a significant decrease in the immunosignal, revealing a higher degree of Na+ K+ ATPase α1-subunit phosphorylation at Ser-23 (PMA: 40.19 ± 6.4 % vs. basal, n = 4, p < 0.05; DA: 41.85 ± 9.6 % vs. basal, n = 5, p < 0.05). and show a significant decrease in the immunosignal in CRF rats by PMA, corresponding to a higher degree of Na+ K+ ATPase α1-subunit phosphorylation at Ser-23 (55.26 ± 11.2% compared with basal CRF, n = 5, p < 0.05). Besides, when microdissected mTAL segments of CRF rats were incubated with dopamine (DA) 10−6 M, a decrease was observed in the immunosignal (increased phosphorylation) of Na+ K+ ATPase α1-subunit in mTAL (53.27 ± 7.1%, compared with basal CRF, n = 4, p < 0.05; see ). These results suggest that Na+ K+ ATPase in microdissected mTAL segments from CRF rats can be phosphorylated by the same direct and indirect mechanisms that affect PKC in control rats.
Figure 4. Effect of PKC stimulation on α1-subunit Na+ K+ ATPase phosphorylation degree at Ser-23 in mTAL microdissected segments from CRF rats. Na+ K+ ATPase α1-subunit phosphorylation degree at Ser-23 in immunoblots mTAL segments in CRF rats and under (a) phorbol 12-myristate 13-acetate (PMA) 10−6 M, a specific PKC agonist, or (b) dopamine (DA) 10−6 M. The immunoblot was performed with a monoclonal antibody (McK-1) against dephosphorylated PKC site, Ser-23, of the Na+ K+ ATPase α1-subunit. Values are means ± SEM and were expressed as percentage of normalized arbitrary units over basal. Normalized arbitrary units were obtained as Na+ K+ ATPase α1-subunit expression with McK-1 antibody / total Na+ K+ ATPase α1-subunit expression with common antibody per μg protein in microdissected tubule (see ). Densitometric analysis of all samples revealed a decrease in immunosignal (higher phosphorylation) of Na+ K+ ATPase α1-subunit under PMA and DA in mTAL segments of CRF rats. *p < 0.05, as compared with basal in CRF rats.
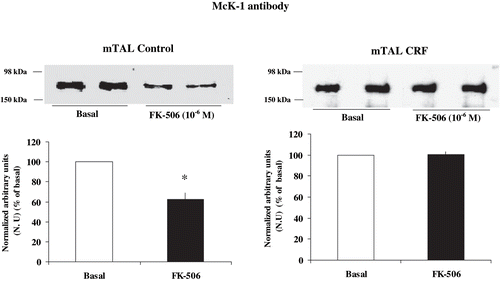
and show the effect of FK-506, a specific calcineurin inhibitor,Citation[34] on the degree of phosphorylation at Ser-23 α1-subunit Na+ K+ ATPase in mTAL from control and CRF rats. Treatment with FK-506 10−6 M caused no changes in the immunosignal when microdissected mTAL segments from CRF rats were tested (100.21 ± 3.00%, compared with basal CRF, n = 4, p = NS). Conversely, FK-506 decreased the immunosignal in control rats (62.46 ± 6.3 % compared with basal condition, n = 5, p < 0.05). These results suggest that phosphorylation degree of α1-subunit Na+ K+ ATPase at Ser-23 in CRF rats is not dependent upon constitutive calcineurin activation.
Figure 5. Effect of calcineurin inhibition on α1-subunit Na+ K+ ATPase phosphorylation degree at Ser-23 in mTAL microdissected segments of control and CRF rats. Na+ K+ ATPase α1-subunit phosphorylation degree at Ser-23 in immunoblots of mTAL segments from control and CRF rats were treated with FK-506 10−6 M, a specific calcineurin inhibitor. The immunoblot was performed with a monoclonal antibody (McK-1) against dephosphorylated PKC site, Ser-23, of the Na+ K+ ATPase α1-subunit. Values are means ± SEM and were expressed as percentage of normalized arbitrary units over basal. Normalized arbitrary units were obtained as Na+ K+ ATPase α1-subunit expression with McK-1 antibody / total Na+ K+ ATPase α1-subunit expression with common antibody per μg protein in microdissected tubules (see ). Densitometric analysis of all samples revealed no changes in immunosignal of α1-subunit Na+ K+ ATPase at Ser-23 under FK-506 in mTAL segments of CRF rats (p = NS). The calcineurin inhibitor FK-506 decreased immunosignal (higher phosphorylation) in mTAL of control rats. *p < 0.05, as compared with basal in control rats.
DISCUSSION
The data presented in this study indicate, for the first time, that a reduction in renal mass is accompanied by PKC-dependent phosphorylation of the α1-subunit Na+ K+ ATPase at Ser-23 in mTAL, as compared with control rats, and corroborate earlier data of an increase in both Na+ K+ ATPase activity and expression in CRF. As shown in , PKC inhibition by RO-318220 10−6 M brought about an increase in the immunosignal and therefore a diminution in the phosphorylation rate. Conversely, and as shown in , the inhibition of protein phosphatase calcineurin by FK-506 10−6 M was not associated with changes in the phosphorylation signal. This type of response contrasts sharply with that observed in control rats, where basal phosphorylation of Na+ K+ ATPase was not dependent on a constitutive action of PKC, but rather on a constitutive activation of calcineurin.Citation[20]
The data must be put in the context of an increase in Na+ K+ ATPase expression and activity observed by reduction of renal mass, as observed here and as already reported,Citation[9],Citation[10] Although speculative, the fact that in CRF animals a common monoclonal antibody revealed an increment of ∼100% in Na+ K+ ATPase α1-subunit expression (see and ), whereas with McK1 the increment in Na+ K+ ATPase α1-subunit was ∼50% (see and ), would indicate a proportional higher expression of phosphorylated Na+ K+ ATPase.
When mTAL samples from CRF rats were treated with the specific PKC inhibitor RO-318220 10−6 M, McK1 immunosignal increased ∼25% over basal CRF, revealing that Na+ K+ ATPase α1-subunit at Ser-23 was more extensively phosphorylated by PKC in CRF as compared with normal rats (see and ). This indicates that PKC constitutively controls basal phosphorylation of Na+ K+ ATPase α1-subunit at mTAL in CRF rats and might also suggest that the Na+ K+ ATPase α1-subunit at Ser-23 in mTAL of CRF rats is shifted to a predominant phosphorylation state, unlike that observed in normal rats.
The McK-1 antibody recognizes a sequence DKKSKK localized at the α1-subunit Ser-23 of the Na+ K+ ATPase, which has been identified as the PKC site. As Ser-23 is one of the PKC phosphorylation sites, the antibody thus binds to this sequence when it is dephosphorylated and not when it is phosphorylated.Citation[17] Thus, the changes observed in this study should be regarded as representative only for the α1-subunit phosphorylation degree at Ser-23 and not as phosphorylation rates of other sites.[35,36] However, phosphorylation at the Ser-23 is responsible for most of the PKC dependent phosphorylation of the enzyme in the rat, since it has been demonstrated that PKC dependent Na+ K+ ATPase phosphorylation is almost suppressed when the Ser-23 site is mutated.Citation[35],Citation[36]
Though in the current experiments PKC activity was not measured, results in the CRF experimental model would suggest a constitutive activation of the enzyme in mTAL segments. In this setting, PKC seems to play a dual role in the regulation of Na+ K+ ATPase by phosphorylation. When inhibited (RO-318220), Na+ K+ ATPase α1-subunit dephosphorylation degree increases, pointing to a basal and tonic activation of the kinase; and when stimulated, PKC can further increase the phosphorylation degree of Na+ K+ ATPase α1-subunit in CRF. In fact, both PMA (a direct PKC activator) and DA (a PKC activator through the binding of G-protein coupled receptors) increased Na+ K+ ATPase α1-subunit phosphorylation at PKC site Ser-23 (see and ). In addition, DA results show the integrity of mTAL epithelium in spite of the injury imposed to kidney tissues by the underlying disease, as a change in the phosphorylation degree of Na+ K+ ATPase α1-subunit Ser-23 by DA requires that DA binds specific receptors with the subsequent triggering of the corresponding intracellular signaling cascade. In other pathological conditions, it has been described that DA does not modify Na+ K+ ATPase activity because some steps in the signaling cascade are disrupted.Citation[37],Citation[38] In addition, previous resultsCitation[39] have demonstrated that basal levels of intracellular cAMP in the microdissected mTAL are increased in this CRF model. Thus, vasopressin and calcitonin stimulated mTAL adenylate-cyclase in a dose-dependent manner in control rats but failed to stimulate it in CRF. Maximal adenylate-cyclase stimulation with IBMX plus forskolin increased cAMP in control rats but not in CRF. The findings that neither forskolin nor vasopressin were able to augment intracellular cAMP would suggest that stimulatory pathways of the adenylate-cyclase system were activated in the basal state in CRF rats. In this regard, Cheng et al. have reported that in transfected COS cells phosphorylation of Ser-23 site of Na+ K+ ATPase α1-subunit by PKC was enhanced when the enzyme was either previously phosphorylated at PKA site Ser-943 or when PKA site was mutated to Asp-943, mimicking a constitutive phosphorylation.Citation[40]
On the other hand, Silva et al. observed that increasing urea concentrations inhibit Na+ K+ ATPase activity in medulla membrane fractions from normal pig kidney, and that cAMP is not able to inhibit Na+ K+ ATPase in the presence of urea.Citation[41] The authors suggest that the increase in NaCl reabsorption in the mTAL is modulated by the combined effect of increased urea concentration and cAMP.Citation[41] Taken together, these data could suggest, at least in part, that a complex interaction among cAMP, urea, and PKC is operative, indicating that other regulatory phosphorylation mechanisms are potentially present in the CRF model. Alternatively, the rearrangements in Na+ K+ ATPase α1-subunit phosphorylation degree at PKC site Ser-23, which is under PKC control in CRF, may serve to rapid adjustments in extracellular space homeostasis posed by intercurrences in CRF.
In summary, PKC-dependent Na+ K+ ATPase phosphorylation in mTAL is regulated by different intracellular mechanisms in CRF, as compared with control rats. The data may help in the design of new experiments looking at the role of Na+ K+ ATPase post-translational changesCitation[14] in states where both injury associated events and increased demands for Na+ reabsorption are present.
ACKNOWLEDGMENTS
The authors are very grateful to Dr. K. Sweadner (Laboratory of Membrane Biology, Neuroscience Center, Massachusetts General Hospital, Charlestown), for the generous gift of the McK1 antibody, and to the Ministerio de Salud, Argentina, for the grant Carrillo-Oñativia.
Notes
*The first author is currently in the Department of Cellular and Molecular Physiology, Yale University School of Medicine.
**The last two authors contributed equally to this work.
REFERENCES
- Hayslett JP. Functional adaptation to reduction in renal mass. Physiol. Rev 1979; 59: 137–164
- Hayslett JP, Kashgarian M, Epstein FH. Functional correlates of compensatory renal hypertrophy. J. Clin. Invest 1968; 47: 774–799
- Schon DA, Silva P, Hayslett JP. Mechanism of potassium excretion in renal insufficiency. Am. J. Physiol 1974; 227: 1323–1330
- Wen S-F, Wong NL, Evanson RL, Lockhart EA, Dirks JJ. Micropuncture studies of sodium transport in the remnant kidney of the dog. Clin. Invest 1973; 52: 386–397
- Lubowitz H, Purkerson ML, Rolf DB, Weisser F, Bricker NS. Effect of nephron loss on proximal tubular bicarbonate reabsorption in the rat. Am. J. Physiol 1971; 220: 457–461
- Schltze RG, Weisser F, Bricker NS. The influence of uremia on fractional sodium reabsorption by the proximal tubule of rats. Kidney Int 1972; 2: 59–65
- Wong NL, Quamme GA, Dirks JH. Tubular handling of bicarbonate in dogs with experimental renal failure. Kidney Int 1984; 25: 912–918
- Buerkert J, Martin D, Prasad J, Chambless S, Klahr S. Response of deep nephrons and the terminal collecting duct to a reduction in renal mass. Am. J. Physiol 1979; 236: F454–F464
- Finkelstein FO, Hayslett JP. Role of medullary structures in the functional adaptation of renal insufficiency. Kidney Int 1974; 6: 419–425
- Jacobson M, Rodriguez H, Hogan W, Klahr S. Mechanism of activation of renal Na+-K+-ATPase in the rat: effects of reduction of renal mass. Am. J. Physiol 1980; 239: F281–F288
- Bertuccio CA, Ibarra FR, Toledo JE, Arrizurieta EE, Martin RS. Endogenous vasopressin regulates Na+-K+-ATPase and Na+-K+-Cl−-cotransporter rbsc-1 in rat outer medulla. Am. J. Physiol 2002; 282: F256–F270
- Tumlin JA, Hoban CA, Medford RM, Sands JM. Expression of Na-K-ATPase alpha- and beta-subunit mRNA and protein isoforms in the rat nephron. Am J Physiol 1994; 266: F240–F245
- Bofill P, Goecke IA, Bonilla S, Alvo M, Marusic ET. Tissue-specific modulation of Na, K-ATPase alpha-subunit gene expression in uremic rats. Kidney Int 1994; 45(3)672–678
- Therien AG, Nestor NB, Ball WJ, Blostein R. Tissue-specific versus isoform-specific differences in cation activation kinetics of the Na, K-ATPase. J. Biol. Chem 1996; 271(22)7104–7112
- Cheng X, Fisone G, Aizman O, Aizman R, Levenson R, Greengard P, Aperia A. PKA-mediated phosphorylation and inhibition of Na+, K+-ATPase in response to β-adrenergic hormone. Am. J. Physiol 1997; 273: C893–C901
- Chibalin AV, Ogimoto G, Pedemonte CH, Pressley TA, Katz AI, Feraille E, Berggren PO, Bertorello AM. Dopamine-induced endocytosis of Na+, K+-ATPase is initiated by phosphorylation of Ser-18 in the rat alpha subunit and is responsible for the decreased activity in epithelial cells. J. Biol. Chem 1999; 274: 1920–1927
- Feschenko MS, Sweadner KJ. Phosphorylation of Na+ K+ ATPase by protein kinase C at Ser-18 occurs in intact cells but does not result in direct inhibition of ATP hydrolysis. J. Biol. Chem 1997; 272: 17726–17733
- Vasilets LA. Regulatory phosphorylation of the Na+ K+ ATPase from mammalian kidneys and Xenopus oocytes by protein kinases. Characterization of the phosphorylation site for PKC. Ann. N. Y. Acad. Sci 1997; 834: 585–587
- Asghar M, Kansra V, Hussain T, Lokhandwala MF. Hyperphosphorylation of Na-pump contributes to defective renal dopamine response in old rats. J. Am. Soc. Nephrol 2001; 12: 226–232
- Bertuccio CA, Cheng SX, Arrizurieta EE, Martín RS, Ibarra FR. Mechanisms of Na+, K+-ATPase phosphorylation by PKC in the medullary thick ascending limb of Henle in the rat. Pflügers Arch 2003; 447: 87–96
- Arrizurieta de Muchnick EE, Wiersba CR, Paz RA. Contribución de los nefrones yuxtamedulares al balance de agua y sodio en la rata. Medicina (Bs As) 1977; 37(Suppl. 2)145–153
- Hilger HH, Klumper JD, Ullrich KJ. Water resorption and ion transport through collecting tubule cells of mammalian kidney: microanalytic studies. Pflugers Arch 1958; 267(3)218–237
- Morel F, Chabardes D, Imbert-Teboul M. Vasopressin action sites along the nephron. J Physiol (Paris) 1981; 77(4–5)615–620
- Bertuccio C, Ibarra FR, Toledo J, Paz L, Arrizurieta EE, Martin RS. cAMP regulation in thick ascending limb of Henle in rats with chronic renal failure. A microdissection study. Acta Phys. Scand 1998; 64: 107–114
- Kim GH, Ecelbarger C, Knepper MA, Packer RK. Regulation of thick ascending limb ion transporter abundance in response to altered acid/base intake. J Am Soc Nephrol 1999; 10(5)935–942
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem 1951; 193: 265–267
- Ibarra FR, Cheng SX, Agren M, Svensson LB, Aizman O, Aperia A. Intracellular sodium modulates the state of protein kinase C phosphorylation of rat proximal tubule Na+, K+-ATPase. Acta Physiol Scand 2002; 175(2)165–171
- Feschenko MS, Sweadner KJ. Structural basis for species-specific differences in the phosphorylation of Na-K-ATPase by protein kinase C. J. Biol. Chem 1995; 270: 14072–14077
- Cheng X, Aizman O, Nairn AC, Greengard AC, Aperia A. Ca2+ determines the effects of protein kinase A and C on activity of rat renal Na+, K+-ATPase. J. Physiol 1999; 518: 37–46
- Li D, Cheng SX, Fisone G, Caplan M, Ohtomo Y, Aperia A. Effects of okadaic acid, calyculin A and PDBu on state of phosphorylation of rat renal Na+, K+-ATPase. Am. J. Physiol 1998; 275: F863–869
- Lucking K, Nielsen JM, Pedersen PA, Jorgensen PL. Na-K-ATPase isoform (alpha 3, alpha 2, alpha 1) abundance in kidney estimated by competitive RT-PCR and ouabain binding. Am. J. Physiol 1996; 271: F253–F260
- Knepper MA, Rector FC, Jr. Urinary concentration and dilution. The Kidney, BM Brenner, FC Rector, Jr. Saunders, Philadelphia, PA 1995; 532–570
- Fernández-Llama P, Andrews P, Ecelbarger CA, Nielsen S, Knepper MA. Concentrating defect in experimental nephrotic syndrome: Altered expression of aquaporins and thick ascending limb Na+ transporters. Kidney Int 1998; 54(1)170
- Ibarra F, Aperia A, Svensson L-B, Eklöf A-C, Greengard P. Bidirectional regulation of Na+, K+-ATPase activity by dopamine and an α-adrenergic agonist. Proc. Natl. Acad. Sci 1993; 90: 21–24
- Belusa R, Wag ZM, Matsubara T, Sahlgren B, Dulubova I, Nairn AC, Ruoslahti E, Greengard P, Aperia A. Mutation of the protein kinase C phosphorylation site on rat alpha1 Na+-K+-ATPase alters regulation of intracellular Na+ and PH and influences cell shape and adhesiveness. J. Biol. Chem 1997; 272: 20179–20184
- Vasilets LA, Postina R, Kirichenko SN. Mutations of the alpha-1 subunit of the rat Na-K-ATPase to negatively charged amino acid residues mimic the functional effect of PKC-mediated phosphorylation. FEBS Letters 1999; 455: 8–12
- Asghar M, Hussain T, Lokhandwala MF. Higher basal serine phosphorylation of D1A receptors in proximal tubules of old Fischer 344 rats. Am J Physiol 2002; 283: F350–F355
- Horiuchi A, Albrecht FE, Eisner GM, Jose PA, Felder RA. Renal dopamine receptors and pre- and post-cAMP-mediated Na+ transport defect in spontaneously hypertensive rats. Am. J. Physiol 1992; 263: F1105–F1111
- Bertuccio CA, Ibarra FR, Pignataro O, Toledo J, Arrizurieta EE, Martin RS. Cellular adaptation of the rat medullary thick ascending limb to chronic renal failure. Medicina (Buenos Aires) 1995; 55: 329–333
- Cheng X-J, Höög J-O, Nairn AC, Greengard P, Aperia A. Regulation of rat Na+- K+- ATPase activity by PKC is modulated by state of phosphorylation of Ser-943 by PKA. Am J Physiol Cell Physiol 1997; 273: 1981–1986
- Silva IV, Caruso-Neves C, Azeredo IM, Carvalho TL, Lara LS, de Mello MC, Lopes AG. Urea inhibition of renal Na+ K+ ATPase activity is reversed by cAMP. Arch. Biochem. Biophys 2002; 406: 183–189