Abstract
Acute rejection is the most important threat to transplanted kidneys in the early phase after transplantation. With the advances in renal transplant surgery and immunosuppressive therapies, one-year graft survival rates reached 90%, but long-term graft survival did not improve to a similar degree. To prevent acute rejection more effectively and decrease the risk of chronic nephropathy development, the pathogenesis and effects of acute rejection on renal grafts should be further explored.
This study aimed to examine the glomerular and tubular changes ultrastructurally. Tissues were obtained from 11 renal allografts with acute rejection, fixed in 1% Osmium tetra oxide embedded in Epon. The changes in glomerular basement membrane, podocyte, mesangium, and proximal tubules were examined by electron microscope.
Tubular changes such as tubular basement membrane multi-lamellation, MN and PMN cells in peritubular capillaries, tubular vacuolization, mitochondrial changes (increase in number, alterations in cristae organization, or cristae effacement), and infiltration of tubular epithelium by MN cells (mainly lymphocytes) were found statistically significant (p < 0.01) when compared to those of control group. Some forms of endothelial injury (swelling of endothelial cells or fenestrae loss) were also statistically significant (p < 0.01).
Acute rejection is an important predictor of long-term graft survival, and there may be no clinical clue to make diagnosis easier. Therefore ultrastructural changes may help solve this problem together with molecular studies.
INTRODUCTION
Despite improvements in surgical techniques, new problems have arisen in renal transplantation. It has been found that surviving the graft is as important as good surgery. The recipient's body can react to the new tissue with different mechanisms immediately or later, and these reactions may be so severe that graft loss occurs in minutes. Acute rejection (AR) is one of the most important threats to transplanted kidneys in the early phase after transplantation. In addition, acute rejection episodes are considered a risk factor for short-term and long-term allograft survival.Citation[1]
Acute rejection begins with injury to the endothelium. The endothelial damage induces a complex cascade of events involving complement activation and results in ischemic necrosis of the allograft and vascular thrombosis and ischemic necrosis of the allograft within a few hours or days after transplantation.
In the Banff schema for renal allograft pathology, acute rejection is recognized by the presence of tubulitis and intimal arteritis. Tubulitis is defined as the infiltration of the tubular epithelium by leucocytes, usually lymphocytes. Infiltration of the arterial intima is referred to as intimal arteritis. The intensity of the infiltrate and the severity of tubulitis and intimal arteritis are used to classify rejection into mild, moderate, and severe acute rejection categories.Citation[2]
Because electron microscopy is not routinely used in renal allograft biopsy examinations, a complementary approach to the ultrastructure of all units in allografts has seldom been investigated. The present study aimed to detect all changes in tubular and glomerular subunits and give an overall ultrastructural perspective, hence contributing to other ultrastructural studies regarding differential diagnosis of allograft related pathologies.Citation[3],Citation[4]
MATERIAL AND METHODS
All 11 patients with acute rejection underwent renal transplantation at Baskent University Medical Faculty between 1999 and 2001. In all, 31 biopsies of 11 patients were included.
The diagnosis of AR was made on the basis of clinical criteria (i.e., acute allograft dysfunction with normal or subtherapeutic levels of cyclosporine and normal findings by renal ultrasound) with histopathology of the biopsies (i.e., presence of tubulitis and intimal arteritis or transplant glomerulitis).
For a control group, seven renal biopsy specimens were collected showing normal histology from patients with renal cell carcinoma.
Renal biopsy specimens were obtained using a bioptygun with an 18-gauge needle. They were fixed in 2.5% glutaraldehyde solution and 1% Osmium tetra oxide embedded in Epon, and examined by LEO 906 E transmission electron microscope to detect the changes in both glomeruli and tubules.
Acute rejection and control groups were compared by Mann-Whitney U test according to the presence of tubular (i.e., focal tubular basement membrane multi lamellation, tubular vacuolization, mitochondrial changes, and infiltration of tubular epithelium by mononuclear cells) and glomerular (i.e., inflammatory cells in glomerular capillaries, endothelial injury) changes. A p value less than 0.05 was considered statistically significant. Statistical analysis was performed using the SPSS 13.0 for Windows.
RESULTS
Of 11 patients, eight were male and three were female, with ages ranging from 17 to 47 (mean ± standard error of mean was 26.18 ± 2.66; median was 22). Two of control biopsies showed polymorphonuclear (PMN) cells in glomerular capillaries; one showed endothelial injury as a swollen endothelial cell, and one showed tubular vacuolization. Control biopsies showed neither PMN or mononuclear (MN) cells in tubules, nor tubular basement membrane (TBM) thickening.
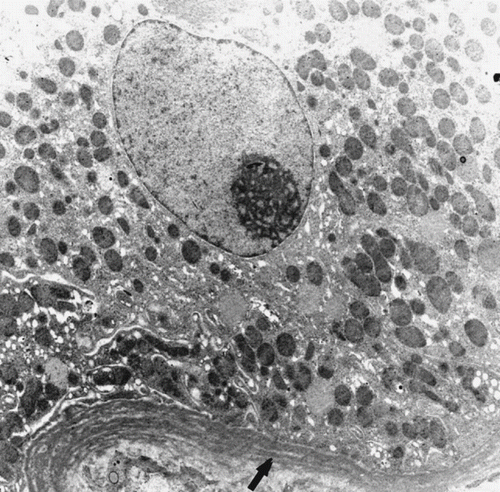
In patients with AR, tubular findings were focal tubular basement membrane multi-lamellation (see ), MN and PMN cells in peritubular capillaries (PCs; see ), postcapillary venule-like transformation of PCs (see ), endothelial cell layer abnormalities (see and ), tubular vacuolization, mitochondrial changes (i.e., an increase in number, alterations in cristae organization, or cristae effacement), and infiltration of tubular epithelium by MN cells. All of these tubular changes were found statistically significant (p < 0.01) when compared to those of control group. The nuclei of tubules generally shrank, stained densely, and had irregular nucleus borders. There was no evident brush border loss.
Figure 2. MN cell in PCs (arrow) and an apoptotic endothelial cell (arrowhead) (Uranyl acetate, lead citrate 3597X).
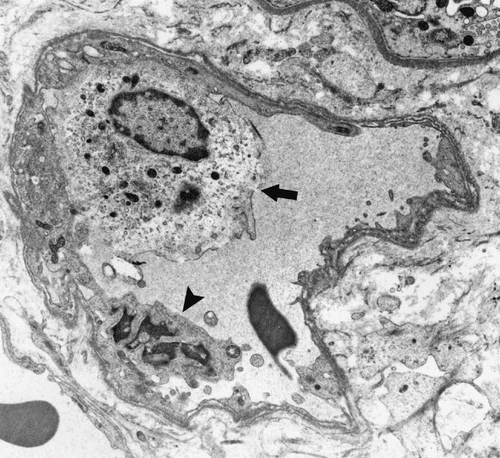
Figure 3. An MN cell in PCs (arrow), postcapillary venule-like transformation (limited by arrowheads), and hypertrophic endothelial cells (*) (Uranyl acetate, lead citrate 1670X).
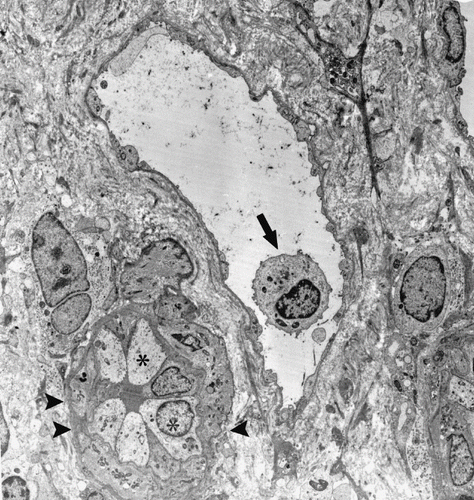
Figure 4. An MN cell (arrow) and detached endothelial cell (arrowhead) in a PC. (Uranyl acetate, lead citrate 2156X).
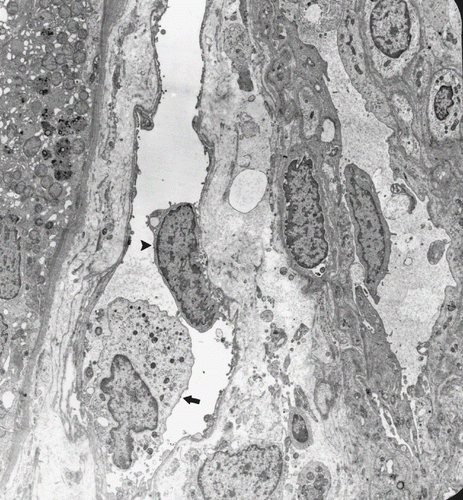
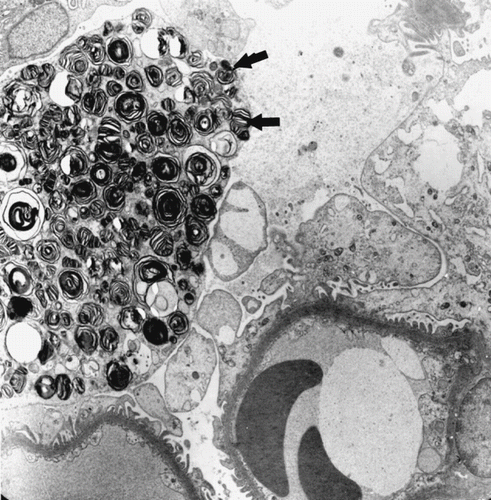
In glomeruli, inflammatory cells were also present statistically more frequently in the AR group than the control group. Podocyte foot processes were generally preserved and glomerular basement membrane appeared mostly normal. In one patient with AR, one podocyte cytoplasm was completely filled with circumferential lamellar bodies (see ). No organelles or nucleus could be seen. Glomerular capillaries (GCs) were congested with numerous erythrocytes. In addition, some forms of endothelial injury (swelling of endothelial cells or fenestrae loss) were also statistically significant (p < 0.01).
DISCUSSION
Acute rejection episodes are a major predictor of renal allograft survival, and the improvement of the transplantation results in the last 20 years mostly depends on a progressive decrease in the incidence of acute rejection. It was previously reported that acute rejection remained a powerful risk factor for late transplant failure.Citation[5]
After kidney transplantation, the initial encounter between recipient T cells and donor antigen can occur on the apical surface of endothelial cells lining the graft vasculature. Some inflammatory factors were secreted by endothelial cells and tubular epithelium as a consequence of transplantation process or presensitization.Citation[6] Robertson et al. reported the presence of some chemokines, primary motivators for the vectorial recruitment of specific immune cell populations from the blood through the endothelium and renal tubules, in graft tubular epithelial cells.Citation[7] This is why the diagnosis mostly depends on tubular changes. In the present study, there was a predominance in tubular findings.
Ivanyl et al.Citation[8] reported postcapillary venule-like transformation in the PCs endothelium, suggesting cytotoxic injury to capillaries and subendothelial lymphocyte infiltration during acute renal allograft rejection. They pointed out that peritubular capillary endothelium, which shows postcapillary venule-like transformation, bound five times more lymphocytes compared to normal control kidneys. This event precedes immunologic rejection responses. If it repeats, the increased surface area of the activated and enlarged endothelial cells induces the secretion of cytokines and adhesion molecules. Both the increased endothelial surface area and incoming inflammatory cells induce secretion of growth factors and causes fibrosis. Baboolal et al.Citation[9] reported that early developing structural injuries were partly mediated by prefibrotic growth factors such as thrombospondin, fibronectin, and TGF-beta. Also, repeated damage to endothelial cells results in the multi-lamellation of peritubular capillary basement membrane. Fibrosis and multi-lamellation obliterate the peritubular capillary network, resulting in tubular injury. The multi-lamellation of TBM, postcapillary venule-like transformation of PCs, and significant tubular vacuolization as a sign of tubular injury were also observed in the present study. Liptak et al.,Citation[10] though, did not report a high endothelial venule-like transformation of endothelial cells in patients with acute humoral rejection.
Studies mostly have focused on PCs. Aita et al.Citation[11] reported that peritubular capillaritis in early allograft is associated with the development of chronic rejection and chronic allograft nephropathy. Peritubular capillaritis was defined as the existence of inflammatory cells (of any kind) in the capillary lumens, with the swelling of endothelial cells. In the present study, there were inflammatory cells, mainly MN cells, and endothelial cell abnormalities (endothelial cell detachment, endothelial cell layer fragmentation) in PCs. The dominance of MN cells might have originated from the day of the biopsy, as Liptak et al.Citation[10] indicated. The current study's biopsies also were taken at least one week after the clinical onset of graft disfunction, though there was no scoring system in place, unlike Aita et al.Citation[11] These ultrastructural findings in PCs were also supported by Liptak et al.,Citation[10] who described various forms of PC damage. They described three distinct patterns of PC injury—lysis, apoptosis, and fragmentation—and suggested that those different patterns could be brought on by different pathologic mechanisms, the first two being mediated by alloantibodies and the third by real ischemia. In the present study, only the presence of morphologic changes is described, but it is not classified. Instead of focusing on one unit, the entire ultrastructure was examined.
The combination of intracapillary MN cells and endothelial injury is a lesion referred to as transplant glomerulitis in the Banff schema.Citation[12] This change is commonly observed in conjunction with acute rejection. In the present study, most capillary loops involved MN and PMN cells. In addition, different forms of endothelial injury such as cytoplasmic blebbing or swelling were seen.
Schimizu et al.Citation[13] reported a case with acute rejection that showed transplant glomerulitis and tubulitis in a protocol biopsy one year after kidney transplantation. They noted endothelial cell swelling, subendothelial enlargement, mesangial interposition to subendothelial space, and infiltration of PMN cell in glomerular capillary. In peritubular capillary basement membranes, mild multilayering was observed. The present study found MN and PMN cells in glomerular capillary, some signs of endothelial injury, tubulitis, and multilayering of TBM. Such a case revealed the importance of pathological findings in diagnosis because there was no evidence of allograft dysfunction.
As stated earlier, acute rejection is an important predictor of long-term graft survival, but there may be no clinical symptoms to make for an easier diagnosis. Therefore, an overall look at ultrastructural changes may help solve this problem together with molecular studies.
REFERENCES
- Meier-Kriesche HU, Ojo AO, Hanson JA, Cibrik DM, Punch JD, Leichtman AB, et al. Increased impact of acute rejection on chronic allograft failure in recent era. Transplantation 2000; 70(7)1098–1100
- Trpkov K, Campbell P, Pazderka F, Cockfield S, Solez K, Halloran PF. Pathologic features of acute renal allograft rejection associated with donor-specific antibody: Analysis using the Banff grading schema. Transplantation 1996; 61(11)1586–1592
- Kiyici H, Demirhan B, Ozdemir BH, Aktas G, Karabay G, Haberal M. Significance of peritubular capillary basement membrane multilamellation in diagnosis of chronic allograft nephropathy. Transplant Proc 2003; 35(7)2643–2644
- Ozdemir BH, Karabay G, Ozdemir FN, Demirhan B, Haberal M. Early detection of amyloidosis in renal allografts: electron microscopic, histochemical, immunohistochemical findings and relationship with graft survival. Transplant Proc 2003; 35(7)2639–2640
- Mateu LMP CA, Plaza LC, Esteve AF. Acute rejection and late renal transplant failure: Risk factors and prognosis. Nephrol Dial Transplant 2004; 19(Suppl.3)iii38–iii42
- Robertson H, Morley AR, Talbot D, Callanan K, Kirby JA. Renal allograft rejection: beta-chemokine involvement in the development of tubulitis. Transplantation 2000; 69(4)684–687
- Robertson H, Wheeler J, Morley AR, Booth TA, Talbot D, Kirby JA. Beta-chemokine expression and distribution in paraffin-embedded transplant renal biopsy sections: analysis by scanning laser confocal microscopy. Histochem Cell Biol 1998; 110(2)207–213
- Ivanyi B, Hansen HE, Olsen TS. Postcapillary venule-like transformation of peritubular capillaries in acute renal allograft rejection. An ultrastructural study. Arch Pathol Lab Med 1992; 116(10)1062–1067
- Baboolal K, Jones GA, Janezic A, Griffiths DR, Jurewicz WA. Molecular and structural consequences of early renal allograft injury. Kidney Int 2002; 61(2)686–696
- Liptak P, Kemeny E, Morvay Z, Szederkenyi E, Szenohradszky P, Marofka F, et al. Peritubular capillary damage in acute humoral rejection: an ultrastructural study on human renal allografts. Am J Transplant 2005; 5(12)2870–2876
- Aita K, Yamaguchi Y, Horita S, Ohno M, Tanabe K, Fuchinoue S, et al. Peritubular capillaritis in early renal allograft is associated with the development of chronic rejection and chronic allograft nephropathy. Clin Transplant 2005; 19(Suppl. 14)20–26
- Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, et al. The Banff ’97 working classification of renal allograft pathology. Kidney Int 1999; 55(2)713–723
- Shimizu T, Tanabe K, Tokumoto T, Kanemitsu I, Tsunoyama K, Ishida H, et al. A case of acute humoral rejection with various depositions of C4d, IgG, IgM, and C3c in peritubular capillaries and/or glomerular capillaries. Clin Transplant 2005; 19(Suppl.14)65–70