Abstract
Background. Helicobacter pylori (H. pylori) are a causative agent of digestive disease. Although a proton pump inhibitor combined with amoxicillin-clarithromycin is the accepted drug treatment for H. pylori eradication in Japan, there is no consensus treatment for hemodialysis patients. Study. Seventy-seven hemodialysis patients underwent upper digestive tract endoscopy. Biopsy specimens were taken, and histological findings, culture, and rapid urease tests were performed to confirm the presence of H. pylori. H. pylori-positive patients were then administered at random either a seven-day lansoprazole (60 mg a day)-amoxicillin (750 mg a day)-clarithromycin (400 mg a day) (LAC) regimen or a seven-day lansoprazole (60 mg a day)-clarithromycin (400 mg a day) (LC) regimen. The success of H. pylori eradication was determined from histological findings, culture, and rapid urease tests. Results. In 13 of 77 patients (13.6%), ulcers and/or ulcer scars were seen by endoscopy. Thirty-one patients (40.3%) were positive for H. pylori, and 20 patients among them were randomized to one of two regimens: one is seven-day LAC regimen (eleven patients) and the other is seven-day LC regimen (nine patients). Eradication was successful in nine of the eleven patients (72.7%) receiving the LAC regimen, but in only three of the nine patients (33.3%) who underwent the LC regimen. No serious adverse effects were observed with either regimen, and 95% of the patients reported complete compliance. Conclusion. A seven-day low dose LAC regimen is safe and effective and recommended for treatment of H. pylori infection in hemodialysis patients.
Keywords:
INTRODUCTION
There is an increasing number of patients undergoing dialysis in Japan, and it is reported that in excess of 220,000 patients will receive dialysis therapy by the end of 2002. The life expectancy of hemodialysis patients has been extending with the progression of dialysis technology. Presently in hemodialysis patients, the incidence of ulcer is similar to that of patients who have not received hemodialysis.Citation[1–3] However, as the frequency of upper gastrointestinal tract bleeding is significantly higher in hemodialysis patients,Citation[4] the prevention of gastro-duodenal ulcers is important.
Because Helicobacter pylori (H. pylori) eradication therapy effectively prevents recurrent bleeding from peptic ulcers,Citation[5],Citation[6] the same therapy may also be effective for the treatment of upper digestive diseases in hemodialysis patients, as well as the general population. Among the many studies investigating H. pylori eradication treatments, few have examined H. pylori infection specifically in patients with hemodialysis,Citation[7] making detection and eradication of H. pylori in hemodialysis patients an important clinical issue.
The treatment regimen for H. pylori eradication that is approved in Japan includes a combination of clarithromycin (CAM), amoxicillin (AMOX), and a proton pump inhibitor for seven days.Citation[8] This treatment regimen is known to be effective for eradication in 80–90% of cases, with few adverse effects.Citation[9–11] However, there have been few reports about the efficacy and safety of eradication therapy for H. pylori in hemodialysis patients.Citation[12],Citation[13] CAM is metabolized both in the liver and kidney,Citation[14] while AMOX is metabolized mainly in the kidney.Citation[15] Therefore, the optimization of AMOX dosage is necessary in hemodialysis patients. To establish a safe and efficacious regimen for the eradication of H. pylori in hemodialysis patients, the outcome of a seven-day dual regimen consisting of lansoprazole and CAM was compared to a seven-day triple therapy regimen consisting of lansoprazole and half of a dosage AMOX and CAM.
PATIENTS AND METHODS
From August 1999 to May 2004, 77 chronic hemodialysis patients (51 males and 26 females) who underwent endoscopy for medical check participated in this study. All patients received regular hemodialysis therapy in Oji hospital. They were between 28 and 89 years of age (62.9 ± 12.1 years, mean ± SD). The duration of dialysis averaged 53.3 ± 52.6 months. The underlying disease in the majority of these patients was diabetic nephropathy.
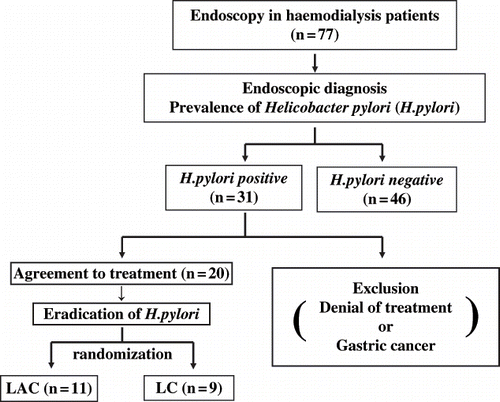
The study is outlined in . Written informed consent was obtained from all patients before endoscopy. All endoscopies were performed under local anesthesia (Xylocaine) using an Olympus XQ-200, XQ210, XQ230, XQ240, or XP260 endoscope. Six biopsy specimens were taken from the greater curvature of the antrum and the gastric body. Paired biopsy specimens from the antrum and gastric body were used for culture, histological analysis, and rapid urease testing. To culture bacteria, biopsy specimens were placed in transport medium immediately after the biopsy procedure and cultured in isolation for five to seven days. For histological identification of bacteria, specimens were stained with Giemsa. The PYLORITEK TEST KITR (Serim Research Corp., Elkhart, Indiana, USA) was used for rapid urease testing. The presence of H. pylori was confirmed by positive results in at least two of these tests or by a positive result in culture.
Figure 1. Demographic schema of study design. Seventy-seven hemodialysis patients underwent upper gastrointestinal endoscopy. The presence of Helicobacter pylori (H. pylori) was assessed by histology, rapid urease test and cultivation. Positive H. pylori status was assigned based upon at least two positive results. Patients who were diagnosed as being H. pylori-positive and wished to receive therapy were enrolled into this study. Patients were randomly assigned into two groups.
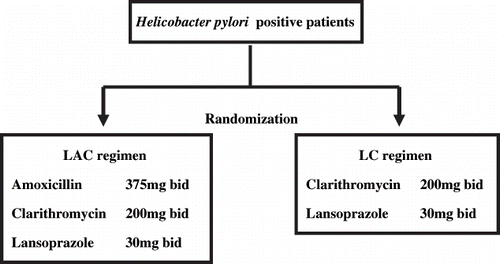
Patients with H. pylori infection were randomly assigned to seven-day regimens of either LAC or LC, consisting of 30 mg b.d lansoprazole, 375 mg b.d amoxicillin and 200 mg b.d. clarithromycin, or 30mg b.d lansoprazole and 200 mg b.d clarithromycin, respectively (see ). Blood exams (WBC, RBC, Hb, Hct, PLT, GOT, GPT, γ-GTP) were performed on all patients before and after therapy. At the completion of therapy, patients were interviewed to report adverse effects and compliance.
Three months after completion of the H. pylori eradication therapies, all patients underwent endoscopy to measure the success of eradication. Biopsies were taken from the six points examined in the previous endoscopy. Positive results for H. pylori in any of the tests described above were scored as evidence of a failure to eradicate.
For statistical analysis, univariate associations were first investigated using the Fisher's exact test. A p value less than 0.05 was regarded as statistically significant.
RESULTS
Prevalence of H. pylori in Hemodialysis Patients
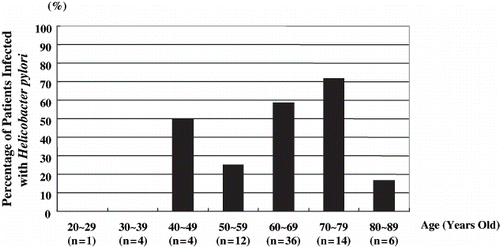
Thirty-one of 77 (40.3 %) of hemodialysis patients had H. pylori infection. The mean age of H. pylori positive patients was 64.1 ± 10.3 years old. H. pylori infection was first seen in patients forty years old or above (see ). The percent of H. pylori-infected patients increased in those over age, and 71.4% of patients seventy or older were positive.
Figure 3. Percentage of Helicobacter pylori-positive patients relative to age. Cases of Helicobacter pylori infection were not observed until age forty years or above.
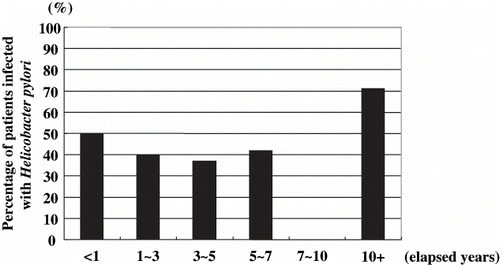
Gastric ulcers and/or ulcer scars were detected in 14.3% of patients, overall, and duodenal ulcer and/or ulcer scars were detected in 2.2% of patients. However, the percentage of patients infected with H. pylori was 53.8% among those with gastric ulcers and/or ulcer scars, and 66.7% among those with duodenal ulcers and/or ulcer scars. The percentage of H. pylori-positive patients was relatively constant regardless of the duration of hemodialysis (see ).
Figure 4. Percentage of patients infected with Helicobacter pylori relative to the number of elapsed years since beginning dialysis. There was no correlation between the duration of patients’ hemodialysis and the prevalence of H. pylori infection. In patients with prolonged duration of hemodialysis, the proportion of H. pylori-positive patients did not change.
Eradication of H. pylori When Following the LAC or LC Regimen
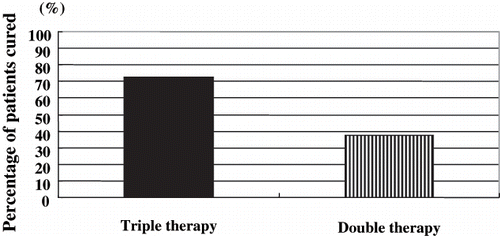
Twenty of 31 (64.5%) H. pylori-positive patients received eradication therapy (18 males, 13 females; mean age 65.8 ± 10.4 years, range 42–89 years). These patients were randomly assigned to one of two therapeutic regimens. Eradication was successful in nine of 11 patients (72.7%) receiving the LAC regimen and in three of the nine patients (33.3%) receiving the LC regimen (p = 0.064; see ).
Figure 5. Success of treatment in eradicating Helicobacter pylori. Of the eleven patients selected to receive the LAC regimen, eradication was successful in nine (72.7%). H. pylori were eradicated in three of the nine patients (33.3%) who received the LC regimen.
Among the 20 patients interviewed, 19 (95%) reported complete compliance, while one patient in the LC regimen took only three of the seven days of medication (42.9%). No adverse effects were reported in 95% (19 out of 20) of all patients following either regimen; one patient in the LC group reported taste disturbance (11%, 1 out of 9). Based on blood test findings, liver function disorder was recognized in one patient receiving the LC regimen. Prior to eradication therapy, his aminotransferase (AST) level was 34 IU/L and alanine aminotransferase (ALT) level was 20 IU/L, whereas after the treatment, AST was 42 IU/L and ALT was 31 IU/L. Enzyme levels indicative of liver function returned to the normal range in two weeks.
DISCUSSION
Nausea, abdominal discomfort and pain are frequent symptoms in uremic patients, and many patients undergoing hemodialysis experience complications of the upper gastrointestinal tract.Citation[16] Because patient reports of peptic ulcer incidence are generally controversial,Citation[17] it is not easy to predict whether the incidence of peptic ulcers would be higher in hemodialysis patients than in patients without hemodialysis. It has been reported that in western countries, the incidence of ulcer in patients who undergo hemodialysis is similar to the general population.Citation[1],Citation[2] However, there are no reports of the incidence of peptic ulcer in hemodialysis patients in Japan. In this study, gastric/duodenal ulcer and ulcer scarring were observed in 14.3% of patients.
Also regarding the prevalence of H. pylori infection in patients with hemodialysis, there are various reports available. The current authors surveyed and summarized the previous studies that studied the prevalence of H. pylori infection in patients receiving hemodialysis (see ).Citation[12], Citation[18–28] In the present study, 40.3% of hemodialysis patients were infected with H. pylori. Considering that the prevalence of H. pylori infection has been reported to be higher than 70% among Japanese adults over 50 years of age,Citation[29] these results indicate the relative percentage of H. pylori-positive hemodialysis patients could be lower than the general patient population. It has been reported that urea concentrations of six mmol/L or higher can inhibit the growth of H. pylori.Citation[30] Thus, the high levels of urea nitrogen and gastric juice urea reported in patients receiving dialysisCitation[31] may possibly inhibit the growth of H. pylori. However, the period of time on hemodialysis did not show any positive correlation with the incidence of H. pylori infection in the current study. Previously, it has been reported that there was no difference in the prevalence of H. pylori infection in dialysis patients in regard to age, dialysis period, or the timing of symptom appearance.Citation[32]
Table 1 Reported frequency of H.pylori infection in haemodialysis patients in 1991∼2005
It has been shown that H. pylori eradication regimens with higher antibiotic dosages provide a higher incidence of cure.Citation[33] However, because amoxicillin (AMOX) is mainly excreted from the kidney, especially high plasma concentrations of AMOX in patients on hemodialysis can be harmful,Citation[34],Citation[35] and it is necessary to adjust AMOX dosage for hemodialysis patients. In contrast, clarithromycin (CAM), which is primarily metabolized in the liver, is frequently used in eradication regimens because of its high toxicity to H. pylori. In Japanese patients without hemodialysis, it has been reported that the frequencies of cure when lansoprazole-AMOX-CAM regimens were applied using 800 versus 400 mg of CAM dailyCitation[36] were similar although adverse effects were significantly more frequent in the LAC regimen with 800 mg CAM.
Based on these findings, a daily dosage of CAM 400 mg daily was adopted, and a regimen was established that contained no AMOX. Accordingly, the two treatment regimens were compared. In one, the dosage of AMOX was reduced from 1500 to 750 mg daily (half of the usual dosage), and the other did not include any AMOX. It has been shown that a seven-day LAC regimen with 200 mg CAM b.d. is sufficient for cure among the general population of Japan.Citation[36] The present study showed that the eradication rate using the LAC regimen (with reduced dosage of AMOX) was far higher than the LC regimen in hemodialysis patients. The success of eradication of H. pylori using the LAC regimen was comparable to previous reports, though on the other hand, the regimen without AMOX provided only a 42.9% cure rate, which was far lower than the previous ones (see ).Citation[12],Citation[25],Citation[28] Unfortunately, this study failed to yield the statistically significant difference, perhaps due to the small number of the studied patients. However, the cure rate of LC regimen seems apparently insufficient.
Table 2 Reported percentages of cured H.pylori-infected haemodialysis patients treated from 1991–2005
Regarding compliance of the study drugs, complete compliance was reported in all interviewed patients in the LAC group, and only one patient in the LC group discontinued medication because of taste disturbance. In addition, as to adverse events, one patient in the LC group showed apparent mild liver dysfunction that did not affect diagnosis, and no adverse effects were noted among interviewed LAC group patients.
As shown in , there are few reports describing how to perform H. pylori eradication treatment to hemodialysis patients, which makes one hesitate to tailor the combination and dosage of the treatment drugs. In this study, it was clearly shown that LAC regimen, including lansoprazole proton-pump inhibitor, 400 mg CAM, and 750 mg AMOX, provides a high chance of H. pylori eradication with no adverse effects, and that such a regimen is recommended for H. pylori eradication treatment in hemodialysis patients.
REFERENCES
- Milito G, Taccone-Gallucci M, Brancaleone C, et al. The gastrointestinal tract in uremic patients on long-term hemodialysis. Kidney Int Suppl 1985; 17: 157–160
- Ala-Kaila K. Upper gastrointestinal findings in chronic renal failure. Scand J Gastroenterol 1987; 22: 372–376
- Chalasani N, Cotsonis G, Wilcox CM. Upper gastrointestinal bleeding in patients with chronic renal failure: role of vascular ectasia. Am J Gastroenterol 1996; 91: 2329–2332
- Chachati A, Godon JP. Effect of hemodialysis on upper gastrointestinal tract pathology in patients with chronic renal failure. Nephrol Dial Transplant 1987; 1: 233–237
- Gisbert JP, Khorrami S, Carballo F, Calvet X, Gene E, Dominguez-Munoz E. Meta-analysis: Helicobacter pylori eradication therapy vs. antisecretory non-eradication therapy for the prevention of recurrent bleeding from peptic ulcer. Aliment Pharmacol Ther 2004; 19: 617–629
- Holtmann G, Howden CW. Review article: management of peptic ulcer bleeding—the roles of proton pump inhibitors and Helicobacter pylori eradication. Aliment Pharmacol Ther 2004; 19: 66–70
- Tamura H, Tokushima H, Murakawa M, et al. Eradication of Helicobacter pylori in patients with end-stage renal disease under dialysis treatment. Am J Kidney Dis 1997; 29: 86–90
- Satoh K. Indications for Helicobacter pylori eradication therapy and first-line therapy regimen in Japan: recommendation by the Japanese Society for Helicobacter Research. J Gastroenterol 2002; 37: 34–38
- Miwa H, Nagahara A, Sato K, et al. Efficacy of one week omeprazole or lansoprazole-amoxycillin-clarithromycin therapy for Helicobacter pylori infection in the Japanese population. J Gastroenterol Hepatol 1999; 14: 317–321
- Miwa H, Ohkura R, Murai T, et al. Effectiveness of omeprazole-amoxicillin-clarithromycin (OAC) therapy for Helicobacter pylori infection in a Japanese population. Helicobacter 1998; 3: 132–138
- Miwa H, Ohkura R, Murai T, et al. Impact of rabeprazole, a new proton pump inhibitor, in triple therapy for Helicobacter pylori infection-comparison with omeprazole and lansoprazole. Aliment Pharmacol Ther 1999; 13: 741–746
- Tsukada K, Miyazaki T, Katoh H, et al. Seven-day triple therapy with omeprazole, amoxycillin and clarithromycin for Helicobacter pylori infection in hemodialysis patients. Scand J Gastroenterol 2002; 37: 1265–1268
- Tamura H, Tokushima H, Murakawa M, et al. Eradication of Helicobacter pylori in patients with end-stage renal disease under dialysis treatment. Am J Kidney Dis 1997; 29: 86–90
- Davey PG. The pharmacokinetics of clarithromycin and its 14-OH metabolite. J Hosp Infect 1991; 19: 29–37
- Humbert G, Spyker DA, Fillastre JP, Leroy A. Pharmacokinetics of amoxicillin: Dosage nomogram for patients with impaired renal function. Antimicrob Agents Chemother 1979; 15: 28–33
- Milito G, Taccone-Gallucci M, Brancaleone C, et al. Assessment of the upper gastrointestinal tract in hemodialysis patients awaiting renal transplantation. Am J Gastroenterol 1983; 78: 328–331
- Schlemper RJ, van der Werf SD, Vandenbroucke JP, Biemond I, Lamers CB. Peptic ulcer, non-ulcer dyspepsia and irritable bowel syndrome in The Netherlands and Japan. Scand J Gastroenterol. Suppl 1993; 200: 33–41
- Loffeld RJ, Peltenburg HG, Stobberingh E, vd Oever H. Prevalence of Helicobacter pylori antibodies in patients on chronic intermittent hemodialysis. Nephron 1991; 59: 250–253
- Jaspersen D, Fassbinder W, Heinkele P, et al. Significantly lower prevalence of Helicobacter pylori in uremic patients than in patients with normal renal function. J Gastroenterol 1995; 30: 585–588
- Luzza F, Imeneo M, Maletta M, et al. Helicobacter pylori-specific IgG in chronic hemodialysis patients: relationship of hypergastrinemia to positive serology. Nephrol Dial Transplant 1996; 11: 120–124
- Abu Farsakh NA, Roweily E, Rababaa M, Butchoun R. Brief report: evaluation of the upper gastrointestinal tract in uraemic patients undergoing hemodialysis. Nephrol Dial Transplant 1996; 11: 847–850
- Ozgur O, Boyacioglu S, Ozdogan M, Gur G, Telatar H, Haberal M. Helicobacter pylori infection in hemodialysis patients and renal transplant recipients. Nephrol Dial Transplant 1997; 12: 289–291
- Munoz de Bustillo E, Sanchez Tomero JA, Sanz JC, et al. Eradication and follow-up of Helicobacter pylori infection in hemodialysis patients. Nephron 1998; 79: 55–60
- Yildiz A, Besisik F, Akkaya V, et al. Helicobacter pylori antibodies in hemodialysis patients and renal transplant recipients. Clin Transplant 1999; 13: 13–16
- Huang JJ, Huang CJ, Ruaan MK, Chen KW, Yen TS, Sheu BS. Diagnostic efficacy of (13)C-urea breath test for Helicobacter pylori infection in hemodialysis patients. Am J Kidney Dis 2000; 36: 124–129
- Wang YL, Sheu BS, Huang JJ, Yang HB. Noninvasive stool antigen assay can effectively screen Helicobacter pylori Infection and assess success of eradication therapy in hemodialysis patients. Am J Kidney Dis 2001; 38: 98–103
- Nakajima F, Sakaguchi M, Amemoto K, et al. Helicobacter pylori in patients receiving long-term dialysis. Am J Nephrol 2002; 22: 468–472
- Nakajima F, Sakaguchi M, Oka H, et al. Prevalence of Helicobacter pylori antibodies in long-term dialysis patients. Nephrology (Carlton) 2004; 9: 73–76
- Asaka M, Kimura T, Kudo M, et al. Relationship of Helicobacter pylori to serum pepsinogens in an asymptomatic Japanese population. Gastroenterology 1992; 102: 760–766
- Gladziwa U, Haase G, Handt S, et al. Prevalence of Helicobacter pylori in patients with chronic renal failure. Nephrol Dial Transport 1993; 8: 301–306
- Lieber CS, Lefèvre A. Ammonia as a source of gastric hypoacidity in patients with uremia. J Clin Invest 1959; 38: 1271–1277
- Giachino G, Sallio-Bruno F, Chiappero F, et al. Helicobacter pylori in patients unfergoing periodic hemodialysis. Minerva Urol Nefrol 1994; 46: 213–215
- Huang J, Hunt RH. The importance of clarithromycin dose in the management of Helicobacter pylori infection: a meta-analysis of triple therapies with a proton pump inhibitor, clarithromycin and amoxicillin or metronidazole. Aliment Pharmacol Ther 1999; 13: 719–729
- Humbert G, Spyker DA, Fillastre JP, Leroy A. Pharmacokinetics of amoxicillin: Dosage nomogram for patients with impaired renal function. Antimicrob Agents Chemother 1979; 15: 28–33
- Jones RH, Cundy T, Bullock R, et al. Concentrations of amoxicillin in serum and dialysate of uremic patients undergoing peritoneal dialysis. J Infect 1979; 1: 235–242
- Miwa H, Murai T, Sato K, et al. Comparison of the efficacy of 400 mg and 800 mg of clarithromycin used with lansoprazole and amoxicillin in eradication regimens for Helicobacter pylori infection in a Japanese population. J Gastroenterol 2000; 35: 536–539