Abstract
Background. Anti-neutrophil cytoplasmic antibodies (ANCA) are classified into perinuclear (P)-ANCA and cytoplasmic-ANCA by an indirect immunofluorescence (IIF) test with ethanol-fixed neutrophils. Circulating P-ANCA with specificity for myeloperoxidase (MPO) are frequently found in patients with pauci-immune necrotizing glomerulonephritis. P-ANCA without a specificity for MPO are also found in a minority of patients with this form of glomerulonephritis, but their clinicopathological features remain poorly delineated. Methods. The clinical data, the renal pathology, and the outcome were compared between 48 patients with MPO-specific P-ANCA-associated glomerulonephritis (MPO-specific group) and five patients with MPO-nonspecific P-ANCA-associated glomerulonephritis (MPO-nonspecific group). In the MPO-nonspecific group, antibodies against bactericidal/permeability-increasing protein were detected in one patient, but the other known antibodies that can produce a P-ANCA pattern on the IIF test were not detected in the remaining patients. All patients in the two groups were treated with steroids with or without cyclophosphamide. Results. There were no remarkable differences in the degree of hematuria and serum levels of C-reactive protein and creatinine between the two groups. In contrast, proteinuria levels and the rate of glomerular crescent formation were higher in the MPO-nonspecific group than in the MPO-specific group. While the patient survival rate was similar between the two groups, the renal survival rate was lower in the MPO-nonspecific group. Conclusions. This pilot analysis suggests that there are clinicopathological differences between patients with MPO-specific and -nonspecific P-ANCA-associated pauci-immune necrotizing glomerulonephritis. Renal lesions appear to be more active in patients with MPO-nonspecific P-ANCA than in patients with MPO-specific P-ANCA.
INTRODUCTION
Circulating anti-neutrophil cytoplasmic antibodies (ANCA) were first reported in 1982 in eight patients with pauci-immune necrotizing glomerulonephritis,Citation[1] an important cause of rapidly progressing renal failure. Thereafter, two major categories of ANCA have been recognized by an indirect immunofluorescence (IIF) test with ethanol-fixed neutrophils: one with cytoplasmic staining (C-ANCA) and the other with perinuclear staining (P-ANCA).Citation[2] ANCA have been widely used as serological markers for small vessel vasculitides, such as Wegener's granulomatosis, microscopic polyangiitis (MPA), Churg-Strauss syndrome, and renal-limited vasculitis.Citation[3] Patients with Wegener's granulomatosis most often have C-ANCA, whereas patients with MPA, Churg-Strauss syndrome, and renal-limited vasculitis most often have P-ANCA.Citation[2]
Many of the target antigens of ANCA are located in the primary granules of neutrophils. C-ANCA almost always have a specificity for proteinase 3 (PR3).Citation[4] The major P-ANCA target is myeloperoxidase (MPO),Citation[5] but antibodies against other neutrophil antigens (for example, elastase,Citation[6] cathepsin G,Citation[7] lactoferrin,Citation[8] lysozyme,Citation[9] bactericidal/permeability-increasing protein (BPI),Citation[10] and azurocidinCitation[10]) can also produce a P-ANCA pattern on the IIF test. Among them, cathepsin G-, lactoferrin-, lysozyme-, BPI-, and azurocidin-ANCA were found to be closely associated with inflammatory bowel and rheumatic diseases, but not specifically associated with renal disease.
Circulating P-ANCA/MPO-ANCA are frequently found in patients with pauci-immune necrotizing glomerulonephritis.Citation[2],Citation[11] P-ANCA without specificity for MPO are also found in a minority of patients with this form of glomerulonephritis,Citation[12],Citation[13] but their clinicopathological features remain poorly delineated. This study compared the clinical data, renal pathology, and outcome between 48 patients with MPO-specific P-ANCA-associated glomerulonephritis and five patients with MPO-nonspecific P-ANCA-associated glomerulonephritis.
PATIENTS AND METHODS
Patients
Renal biopsies were performed in 3,550 patients at Akita University Hospital and its affiliated related hospitals from 1990–2004. In all, 58 patients were found with pauci-immune necrotizing P-ANCA-associated glomerulonephritis. The following patients were excluded: one with propylthiouracil-induced vasculitis,Citation[14] one with cholesterol embolism-related vasculitis,Citation[15] and three patients in whom more than 10 glomeruli were not observed in their biopsy specimens. The remaining 53 patients were evaluated in this study.
P-ANCA were detected by conventional methods using ethanol-fixed neutrophils.Citation[2] Serum PR3- and MPO-ANCA levels were measured by enzyme-linked immunosorbent assay (ELISA) kits (Nipro, Osaka, Japan) in the laboratory of Special Reference Laboratories, Tokyo, Japan. In patients in whom PR3- and MPO-ANCA were not detected, antibodies against six known ANCA antigens (azurocidin, BPI, cathepsin G, elastase, lactoferrin, and lysozyme) were measured using an ELISA kit (Wieslab, Lund, Sweden).
The clinical vasculitis activity was assessed with clinical and biological parameters at diagnosis using the Birmingham Vasculitis Activity Score (BVAS).Citation[16] Hematuria was graded as: 1–4/high-power field (HPF), score 0; 5–10/HPF, score 1; 11–50/HPF, score 2; 51–99/HPF, score 3; and >100/HPF, score 4. The total urinary protein for 24 hours, the peripheral leukocyte counts, and serum levels of C-reactive protein (CRP) and creatinine were also measured. Values are expressed as means ± SD.
All 53 patients included in this study were treated with corticosteroids with or without cyclophosphamide. Among them, detailed follow-up data were available in 48 patients. The patient and renal survival rates after treatments were evaluated in these patients. Death was considered to be therapy-related if it was caused by an opportunistic infection or bleeding, secondary to drug-induced leukocytopenia or thrombocytopenia. Patient and renal survival curves were constructed according to the Kaplan-Meier method.
Renal Biopsy Findings
All renal biopsies were evaluated by the same observer, who was unaware of the P-ANCA specificity. The percent of crescent formation was calculated in the remaining glomeruli, excluding global sclerotic glomeruli. Interstitial cell infiltration and interstitial fibrosis were graded as: <25%, score 1; 25–49%, score 2; and >50%, score 3. The presence or absence of fibrinoid necrosis and/or inflammatory changes in small arteries and arterioles were also assessed.
RESULTS
In this study, 53 patients with pauci-immune necrotizing P-ANCA-associated glomerulonephritis were selected. Among them, MPO-ANCA were detected in 48 patients (MPO-specific group), but not detected in five patients (MPO-nonspecific group). In both groups, PR3-ANCA were negative. In the MPO-nonspecific group, a search for correspondence antigens revealed that one patient had BPI-ANCA. This patient did not have inflammatory bowel disease nor an associated disorder. Azurocidin-, cathepsin G-, elastase-, lactoferrin-, or lysozyme-ANCA were not detected in the MPO-nonspecific group.
shows comparative clinicopathological findings between the MPO-specific and -nonspecific groups. summarizes the clinicopathological findings of five cases in the MPO-nonspecific group.
Table 1 Comparison of clinicopathological findings between MPO-specific and -nonspecific groups
Table 2 Clinicopathological findings in MPO-nonspecific group
There were no remarkable differences in age, peripheral leukocyte counts, serum CRP levels, degree of hematuria, serum creatinine levels, and BVAS between the two groups. BVAS scores excluding renal scores were higher in the MPO-specific group than in the MPO-nonspecific group (5.1 ± 6.3 versus 2.4 ± 3.3). On the other hand, proteinuria levels were higher in the MPO-nonspecific group than in the MPO-specific group (2.8 ± 1.9 g/day versus 1.6 ± 1.3 g/day).
Regarding renal pathological parameters, the crescent formation rate was higher in the MPO-nonspecific group than in the MPO-specific group (71.8 ± 18.4% versus 44.8 ± 27.1%). There were no remarkable differences in interstitial cell infiltration/fibrosis scores between the two groups. Patients in the MPO-specific group had small arteritis (19.4%) or arteriolitis (35.7%), while no patients in the MPO-nonspecific group had arteritis or arteriolitis.
All 53 patients were treated with corticosteroids including methylprednisolone pulse therapy with or without cyclophosphamide. Detailed follow-up data were available except for five patients in the MPO-specific group. The causes of the 18 deaths in these patients are shown in . In the MPO-specific group, two patients died of progressive vasculitis and seven patients died of infectious side effects of treatment. The remaining eight patients died of various other causes. In the MPO-nonspecific group, one patient died of gastrointestinal hemorrhage.
Table 3 Causes of the eighteen deaths in MPO-specific and -nonspecific groups
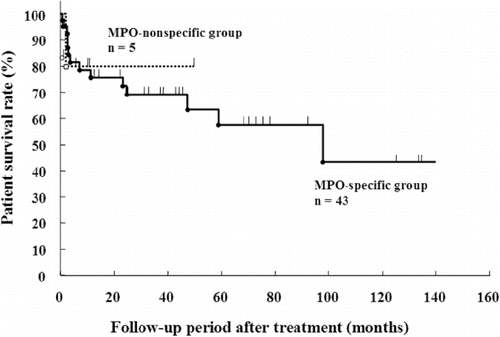
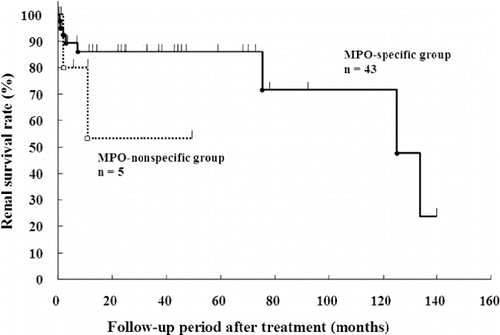
The survival curves assessing the death or end-stage kidney disease are shown in and , respectively. The patient survival rate was similar between the MPO-specific and -nonspecific groups (see ). In contrast, the renal survival rate in the MPO-nonspecific group was lower than that in the MPO-specific group (see ).
DISCUSSION
ANCA are a heterogeneous group of autoantibodies with a broad spectrum of clinically associated diseases. To date, many clinical studies have confirmed that C-ANCA/PR3-ANCA and P-ANCA/MPO-ANCA are highly specific for Wegener's granulomatosis and MPA including a renal-limited form, respectively.Citation[2],Citation[11] Regarding the differences between PR3-ANCA- and MPO-ANCA-associated renal disease, Franssen et al.Citation[17] performed a retrospective study of clinicopathological features in 92 patients. They found that renal lesions were more active and renal function deteriorated more rapidly in the PR3-ANCA group than in the MPO-ANCA group. Despite these differences, there was no difference in outcomes between the two groups. On the other hand, Hauer et al.Citation[12] and Vizjak et al.Citation[13] reported discordant results.
The antigen specificity for MPO is negative in a minority of patients with P-ANCA-associated pauci-immune necrotizing glomerulonephritis.Citation[12],Citation[13] There does not appear to be any research comparing clinicopathological differences between patients with P-ANCA-associated glomerulonephritis with and without specificity for MPO. Therefore, this retrospective study of the clinical features, renal morphology, and outcome in 48 patients with MPO-specific P-ANCA-associated glomerulonephritis and five patients with MPO-nonspecific P-ANCA-associated glomerulonephritis was performed.
In this study, there were no remarkable differences in BVAS, the degree of hematuria, and serum levels of CRP and creatinine between both antibody groups. To assess the degree of extra-renal lesions, the BVAS scores excluding renal scores between the two groups were compared. These scores were higher in the MPO-specific group than in the MPO-nonspecific group, which suggests more frequent extra-renal involvement in the MPO-specific group than in the MPO-nonspecific group.
In contrast, proteinuria levels and the rate of glomerular crescent formation were higher in the MPO-nonspecific group than in the MPO-specific group. Renal pathological lesions of small arteritis or arteriolitis were not observed in the MPO-nonspecific group, suggesting the main involvement of glomerular capillaries in this group. Patients in both groups were treated with steroids with or without cyclophosphamide. While the patient survival rate was similar between the two groups, the renal survival rate was lower in the MPO-nonspecific group than in the MPO-specific group. These results suggest that renal lesions in P-ANCA-associated glomerulonephritis are more active in patients with MPO-nonspecific P-ANCA than in patients with MPO-specific P-ANCA.
In the patients with MPO-nonspecific P-ANCA, six kinds of described ANCA (azurocidin-, BPI-, cathepsin G-, elastase-, lactoferrin-, and lysozyme-ANCA) were tested that can produce a P-ANCA pattern on the IIF test. One patient was found to have BPI-ANCA. However, serologic detection of BPI-ANCA is not diagnostically specific in patients with renal disease.Citation[10]
In conclusion, there appear to be clinicopathological differences between patients with MPO-specific and -nonspecific P-ANCA-associated pauci-immune necrotizing glomerulonephritis. This pilot analysis suggests that while extra-renal involvement is less frequent, glomerular lesions are more active and renal function deteriorates more rapidly in patients with MPO-nonspecific P-ANCA-associated glomerulonephritis. Further studies are required to determine the target antigens of ANCA in these patients.
REFERENCES
- Davies DJ, Moran JE, Niall JF. Segmental necrotizing glomerulonephritis with antineutrophil antibody: possible arbovirus aetiology?. Br Med J. 1982; 285: 606
- Savige J, Davies D, Falk RJ, Jennette JC, Wiik A. Antineutrophil cytoplasmic antibodies and associated diseases: a review of the clinical and laboratory features. Kidney Int. 2000; 57: 846–862
- Saleh A, Stone JH. Classification and diagnostic criteria in systemic vasculitis. Best Pract Res Clin Rheumatol. 2005; 19: 209–221
- Niles JL, McCluskey RT, Ahmad MF, Arnaout MA. Wegener's granulomatosis autoantigen is a novel neutrophil serine proteinase. Blood. 1989; 74: 1888–1893
- Falk RJ, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med. 1988; 318: 1651–1657
- Gallicchio MC, Savige JA. Detection of anti-myeloperoxidase and anti-elastase antibodies in vasculitides and infections. Clin Exp Immunol. 1991; 84: 232–237
- Halbwachs-Mecarelli L, Nusbaum P, Noel LH, et al. Antineutrophil cytoplasmic antibodies (ANCA) directed against cathepsin G in ulcerative colitis, Crohn's disease and primary sclerosing cholangitis. Clin Exp Immunol. 1992; 90: 79–84
- Peen E, Almer S, Bodemar G, et al. Anti-lactoferrin antibodies and other types of ANCA in ulcerative colitis, primary sclerosing cholangitis, and Crohn's disease. Gut. 1993; 34: 56–62
- Schmitt WH, Csernok E, Flesch BK, Hauschild S, Gross WL. Autoantibodies directed against lysozyme: a new target antigen for anti-neutrophil cytoplasmic antibodies (ANCA). Adv Exp Med Biol. 1993; 336: 267–272
- Yang JJ, Tuttle R, Falk RJ, Jennette JC. Frequency of anti-bactericidal/permeability-increasing protein (BPI) and anti-azurocidin in patients with renal disease. Clin Exp Immunol. 1996; 105: 125–131
- Csernok E. Anti-neutrophil cytoplasmic antibodies and pathogenesis of small vessel vasculitides. Autoimmun Rev. 2003; 2: 158–164
- Hauer HA, Bajema IM, van Houwelingen HC, et al. Renal histology in ANCA-associated vasculitis: differences between diagnostic and serologic subgroups. Kidney Int. 2002; 61: 80–89
- Vizjak A, Rott T, Koselj-Kajtna M, Rozman B, Kaplan-Pavlovcic S, Ferluga D. Histologic and immunohistologic study and clinical presentation of ANCA-associated glomerulonephritis with correlation to ANCA antigen specificity. Am J Kidney Dis. 2003; 41: 539–549
- Dolman KM, Gans ROB, Vervaat TJ, et al. Vasculitis and antineutrophil cytoplasmic autoantibodies associated with propylthiouracil therapy. Lancet. 1993; 342: 651–652
- Aviles B, Ubeda I, Blanco J, Barrientos A. Pauci-immune extracapillary glomerulonephritis and atheromatous embolization. Am J Kidney Dis. 2002; 40: 847–851
- Luqmani RA, Bacon PA, Moots RJ, et al. Birmingham vasculitis activity score (BVAS) in systemic necrotizing vasculitis. Q J Med. 1994; 87: 671–678
- Franssen CFM, Gans ROB, Arends B, et al. Differences between anti-myeloperoxidase- and anti-proteinase 3-associated renal disease. Kidney Int. 1995; 47: 193–199