Abstract
Purpose. The aim of this pilot study was to compare the effect of heparin anticoagulation with and without iloprost administration during continuous renal replacement therapy (CRRT) in critically ill patients. Material and methods. In a prospective, randomized, controlled pilot study at an intensive care unit at a university hospital, 20 patients requiring CRRT were investigated. Patients were allocated into two groups: group 1, the heparin group; and group 2, the heparin plus 1 ng/kg/min iloprost. In both groups, activated partial thromboplastin time (aPTT) was adjusted to 40–50 sec. Observation time was a maximum of 7 days. Results. Median filter run time was significantly prolonged by iloprost administration to a median of 14 h (13–26 h) compared to 10 h (4–12 h) in the heparin group (p = 0.004). A decrease in platelet count was attenuated by iloprost administration (p = 0.012). There were no bleeding complications in either group. Hemofiltration efficiency did not differ significantly between the groups. Conclusion. Additional administration of iloprost prolonged the filter run time of continuous veno-venous hemofiltration (CVVH) in this setting and attenuated the fall in platelet count during CRRT.
INTRODUCTION
Extracorporeal blood circulation, and continuous renal replacement therapy (CRRT) in particular, necessitates anticoagulation to prevent thromboembolic events and consequent filter clotting or catheter occlusion. Heparin is the current gold standard anticoagulant for this purpose. Improvements in the prevention of filter clotting would reduce CRRT-associated blood loss and consequent transfusion as well as reduce staff workload and overall costs. As a result, the ability of a number of heparin alternatives to improve system performance and prolong filter life have been investigated in both experimental and clinical settings. Regional anticoagulation with citrate is currently the best alternative to heparin; however, its use entails the frequent monitoring of liver function, acid-base status, plasma electrolytes, and total calcium levels.Citation[1] Hirudin, a specific thrombin inhibitor, is another option for which its use has been limited by a number of difficulties. Hirudin activity can only be monitored using the Ecarin Clotting Time, which is not routinely available in most laboratories; its elimination is purely renal, making its use in CRRT problematic, and it has been shown to be associated with more bleeding when compared to heparin. Danaparoid sodium, a low-molecular-weight heparinoid alternative, though providing improved filter patency, is associated with increased bleeding complications in patients with pre-existing coagulopathies. A thorough review of this work has been published by Vargas Hein and coworkers.Citation[2]
Prostacyclin and its analog iloprost are a further option in the anticoagulation of extracorporeal circulation. Prostacyclin has previously been used in patients undergoing cardiopulmonary bypassCitation[3],Citation[4] and hemodialysis.Citation[5] Prostacyclin is an endogenous prostaglandin (also known as prostaglandin I2 or PGX), and iloprost is its synthetic analog. The effects of prostacyclin and iloprost are nearly identical. The greater chemical stability of iloprost confers it with a longer half-life of 20–30 minutes, as compared to prostacyclin, which has a half-life of 2–3 minutes.Citation[6] Both have an antiaggregatory effect on platelets that is mediated by specific receptors on the platelet surface.Citation[7] Prostacyclin has been used as an anticoagulant during CVVH, which is the standard CRRT method in intensive care units.Citation[8] One significant advantage of iloprost is that, due to its longer half-life, short infusion interruptions (for example, change of syringes, transport, etc.) are possible without a significant loss of anti-coagulation.Citation[1] This seems to be the first study investigating iloprost as an additional anticoagulant in patients undergoing CVVH. In addition to its platelet inhibitory effect, iloprost has some other properties of potential benefit in critically ill patients requiring CRRT for renal failure. First, there is evidence that iloprost improves regional perfusion in septic patients. In a clinical study in septic patients, it was shown that low dose iloprost improves plasma clearance of indocyanine green, a marker of hepatosplanchnic perfusion and liver function.Citation[9] Second, it is known that iloprost improves rheological properties of red blood cells,Citation[10] increases micro-vascular blood flow, and inhibits leukocyte adherence to the vascular endothelium (an important process in inflammation).Citation[11] In a rat sepsis model, an attenuation of leukocyte adherence and an improvement in intestinal micro vascular blood flow were shown to be associated with iloprost.Citation[12] In comparison to other anticoagulants used in critically ill patients, iloprost also has the added advantages in that it is not contra-indicated in hepatic failure and monitoring is relatively straightforward. This study's objective was to compare the effect of heparin anticoagulation with and without iloprost on filter run time during CRRT in critically ill patients.
MATERIAL AND METHODS
Patients
This study was performed with prior ethical approval. Written informed consent from the patients themselves or their legal representatives was obtained in all cases. Twenty critically ill patients with acute renal failure were enrolled in this prospective, randomized, controlled clinical pilot study. Exclusion criteria were age <18 years, pregnancy, acute bleeding, hereditary coagulopathy, HIT II, platelet count <30/nl, seizure disorder, and pre-existing chronic renal failure. Break off criteria were a drop in platelet count (<30/nl), a clinically relevant drop in arterial oxygen tension, or bleeding. Acute renal failure was defined as urine output <500 mL/24h despite adequate fluid resuscitation or an increase in creatinine (normal: <1.1 mg/dL) and urea (normal: 14 – 46 mg/dL) to three times the normal values.
Groups
Enrolled patients were randomized into one of two groups. The patients in both groups received unfractionated heparin (Liquemin® N, Roche, Grenzach-Wyhlen, Germany) titrated to achieve an activated partial thromboplastin time (aPTT) of 40–50 s. The iloprost group received 1 ng/kg/min iloprost (Ilomedin®, Schering, Berlin, Germany) in addition to heparin.
Hemofiltration
A double-lumen venous catheter (2×16G, Arrow International, Reading, Pennsylvania, USA) positioned in the jugular, subclavian, or femoral vein was used for vascular access. Continuous pump driven veno-venous hemofiltration was performed with a Polyflux 11S, 1.1m2 hemofilter (Gambro Dialysatoren, Hechingen, Germany) and BM 11 + BM 14 equipment (Baxter, McGaw Park, Illinois, USA). Pump-driven blood flow in the extracorporeal circuit was maintained at 150 mL/min. Ultrafiltration dose was 1 l/h; ultrafiltrate was replaced by post-filter infusion (post-dilution mode) of hemofiltration solution (bicarbonate-buffered replacement fluid HFC-Bic 35‐210, Fresenius Medical Care, Bad Homburg, Germany). Anticoagulants were administered pre-filter into the extracorporeal system. The filter run time was defined as the time between the beginning of hemofiltration therapy and failure of CRRT system due to filter clotting (determined by visual evidence of clotting in the drip chamber or steady increase of the transmembrane pressure up to 200 cm H2O). The number of CRRT system changes due to filter clotting was recorded for each patient.
Protocol
The maximum observation time was seven days. The run time of each filter for each patient was recorded, and the median filter run time for each patient was then calculated. Blood was collected every six hours from an unheparinized arterial line in order to measure thromboplastin time (Quick), activated partial thromboplastin time (aPTT), fibrinogen, antithrombin III (AT III), platelet count, D-dimer, platelet function analysis (PFA), activated clotting time (ACT), and white blood cell count (WBC). Drawn blood samples were immediately centrifuged at 4000 rpm for 10 minutes. The blood plasma supernatant was stored at −84°C until interleukin 6 (IL-6) and soluble cluster differentiation marker 14 (sCD-14) assays were performed. Concentrations of IL-6 and sCD-14 were measured by enzyme-linked immunosorbent assay according to the manufacturer's instructions (Biosource International, Camarillo, California, USA). Creatinine, urea, and c-reactive protein (CRP) were determined as part of routine clinical practice.
Statistical Analysis
Data are expressed as median and quartiles. “Overall values” represent the medians of all observed values over the study period. Mann-Whitney U test and Fisher's exact test were used for comparisons of quantitative and qualitative measurements between groups. Kaplan-Meier curves were plotted for filter run time in both groups, and filter run times were compared by log-rank test. To compare filter patency, coagulation, and retention parameters over time, a two-factorial nonparametric analysis of variance was performed using the SAS System software (SAS Institute Inc., Cary, North Carolina, USA). A p value of < 0.05 was considered to be statistically significant.
RESULTS
Basic patient characteristics did not differ significantly between the groups (see ). Reasons for ICU admission in the heparin group were as follows: planned postoperative ICU admissions after cardiopulmonary bypass (7 patients), peritonitis (2) and brain injury (1); in the iloprost group, the reasons for admission were: planned postoperative ICU admission after cardiopulmonary bypass (8 patients) and urosepsis (2).
Table 1 Basic patient characteristics
In one patient, the iloprost infusion was stopped as a precaution because of a decrease in arterial oxygen saturation during the first CRRT run (minimum paO2 65 mmHg, paO2/FiO2 ratio 162). In another patient, the iloprost infusion was stopped because of a decrease in platelet count to 16/nl due to sepsis. One enrolled patient in the heparin group was excluded from the study due to brain stem death.
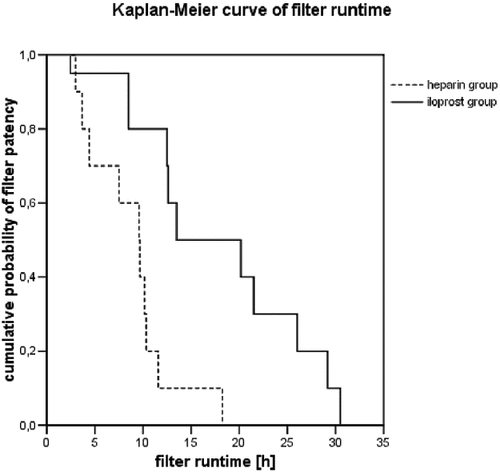
Three patients died after the study had ended, one patient in the heparin group and two patients in the iloprost group. All patients were observed until CRRT was no longer indicated or until they reached the end of the planned seven-day observation period. CVVH was not replaced by another form of renal replacement therapy in any patient. The median observation times were 44 hours (11–82 hours) in the heparin group and 44 (24–72) hours in the iloprost group. Two patients in the heparin group and one patient in the iloprost group reached the end of the planned seven-day observation period. The median filter run time was prolonged significantly in the iloprost group at 14 (13–26, n = 10) hours compared to 10 (4–12, n = 10) hours in the heparin group (see , p = 0.004). The median run time of the first filter in patients in the iloprost group was 22 (14–29, n = 10) hours and 7 (4–8, n = 10) hours in the heparin group (p = 0.014).
Data for 34 filters in the heparin group and 28 filters in the iloprost group were used in the calculation. In the heparin group, three (2–5) filters per patient were used, while in the iloprost group, two (1–3) filters were used. Filter runs were terminated for reasons other than clotting on three occasions in the heparin group (due to brain death, catheter occlusion, or CT scan) and nine occasions in the iloprost group (due to catheter occlusions, CT scan, drop in platelet count, or decrease in arterial oxygen tension). Nevertheless, the data for each of these filters were included in the calculation of median filter run times.
All CVVH catheters were placed immediately before entry into the study. In one patient in the iloprost group, the catheter was changed during replacement of the CVVH system due to catheter occlusion.
There was no significant difference in heparin dosage between the groups (p = 0.218). In the heparin group 7.4 (4.1–7.9) IU/kg/h heparin was infused, while in the iloprost group, patients received 8.2 (6.4–10.4) IU/kg/h heparin. Over the course of the study, there was no significant difference in urea (p = 0.880) or creatinine levels (p = 0.889) between the groups (see ).
Table 2 Coagulation parameters and plasma urea and creatinine levels
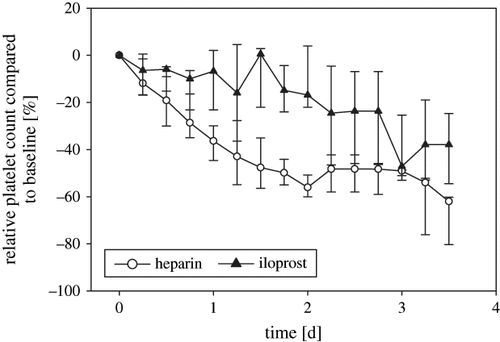
The platelet count as a percentage of the initial value showed a significantly lower decrease over the course of the study in the iloprost group compared to the control group (p = 0.012; see ). Plasma coagulation parameters and the results of platelet function analysis (PFA) did not differ significantly between the groups (see ). During the observation period, no patient received either a platelet transfusion or fresh frozen plasma. The number of administered packed red blood cells (PRBC) did not differ significantly between the groups: in the heparin group, 2 (min. 0, max. 6) units of PRBC were transfused per patient, and in the iloprost group, there was 1 (min. 0, max. 5) unit of PRBC per patient (p = 0.36). In the heparin group, the mean minimum hemoglobin level was 9.1 (8.5–9.4) mg/dL, which did not significantly differ (p = 0.684) from that of the iloprost group (9.1; 8.6–9.5) mg/dL). There was no significant difference in relative white blood cell count (WBC) over time between the groups. Maximum CRP levels during the observation time did not differ significantly between the groups, 16.6 (11.5–27.6) mg/dL in the heparin group and 11.4 (6.5–17.9) mg/dL in the iloprost group (p = 0.61), neither was there a significant difference in baseline or maximum IL-6 and sCD-14 values between the groups (see ).
Table 3 Plasma IL-6 and sCD-14 values
DISCUSSION
A significantly prolonged filter run time and a lower decrease in platelet count in was found in this study's iloprost group compared to patients receiving a standard heparin anticoagulation protocol for CRRT. However, this study has some limitations. First, surprisingly short filter run times were found. Despite comparable aPTT values, the filter run times in the heparin group were shorter than those reported in previous studies, where filter lives of between 14 and 24 hours were described.Citation[13], Citation14–Citation[15] It could be argued that the dimension of the beneficial effect of iloprost may not be of wider clinical relevance. One explanation for the shorter run times is that the run time of each filter for each patient was included in the calculation of filter patency. Notably, run times for filters that did not reach the planned end point were also included (for instance, because of the transfer to CT scan). This was the case for 3 filters in the heparin group and 10 filters in the iloprost group. The median filter run time in these truncated cases was 10 (7–13) hours in the heparin group and 5 (2–11) hours in the iloprost group. As a result, the overall filter patency in the iloprost group in particular was shortened.
When this study was designed, a rather conservative target aPTT range of 40–50 seconds was selected, as it was anticipated that this study population would include a significant number of postoperative patients at an increased risk of bleeding. In a study by Vargas Hein and coworkers, heparin therapy was guided by ACT values, and longer ACT values and filter run times were reported in comparison to our study.Citation[13] Another possible explanation for the short circuit duration in this study is a different patient population compared to other studies. 70% and 80% of the patient population in the heparin alone and iloprost groups, respectively, were post-pump cardiosurgical patients. Indeed, there is experimental evidence to suggest that the inflammatory response after cardiopulmonary bypass may result in more frequent filter clotting and in shorter filter run times.Citation[16] The aPTT values of these patients are comparable to both studies by Vargas Hein et al.Citation[13],Citation[17] Nevertheless, in this study, iloprost was able to increase filter runtime significantly.
There was no difference in the coagulation parameters between the groups. In particular, ACT and aPTT values did not significantly differ. Thus, a significant influence of varying heparin effect between groups seems unlikely.
Although it has been hypothesized that an anti-aggregatory effect on platelets is the underlying mechanism by which iloprost prolongs filter run time, the authors were unable to demonstrate a significant difference in PFA between the groups. Iloprost is known to improve rheological properties (ex vivo deformability) of red blood cells.Citation[10] Independent of the activation of the coagulation cascade, the rheological properties of blood cells seem to be an important factor in filter patency. Reduced deformability results in the formation of cell conglomerates and filter occlusion, as opposed to occlusion due to clot formation. This clogging can be reduced by improving rheological properties of blood using colloids for instance.Citation[18]
Prostacyclin as prostaglandin I2 has previously been used for anticoagulation in connection with CVVH. Kozek-Langenecker provides a review of studies on the use of short-acting antiplatelet prostaglandins during extracorporeal circulation.Citation[19] There is evidence that low-dose iloprost can be effective in prolonging hemodialysis filter life. Brierley and coworkers reported a series of four patients with reduced filter life in continuous arterio-venous hemodialysis (CAVHD). They found that the administration of 2 ng/kg/min iloprost to be successful in prolonging filter life by a minimum of 100% and up to five days.Citation[20]
This study used a low dosage of iloprost of 1 ng/kg/min. It is possible that the effect of iloprost may be more pronounced when a higher dosage of 2 ng/kg/min or higher is used. Despite the vasodilatory effect of iloprost, the dosage of 1 ng/kg/min did not seem to have a clinically relevant effect on blood pressure in the patients in this study, which is consistent with the findings in a previous study of critically ill patients.Citation[9] Iloprost causes slightly less vasodilation compared to equipotent doses of prostacyclin,Citation[6] which makes it a more attractive agent for routine clinical use. It is worth bearing in mind that when used for the complete prevention of platelet aggregation in HIT patients, a much higher dosage of iloprost, of up to 48 ng/kg/min, is required.Citation[21] In both groups in this study, no bleeding complications were observed, suggesting that the administered dosage of iloprost is safe. To prove this assumption, a larger number of patients have to be investigated.
In severe thrombocytopenia, iloprost should be used with caution. To date, no exact threshold of platelet count has been defined. In practice, iloprost is only given to patients with a platelet count >30/nl and without clinical signs of bleeding. In this study, the iloprost infusion was stopped because of a decrease in platelet count in only one patient (16/nl). Conversely, iloprost may attenuate or possibly prevent further decreases in platelet count in cases of thrombocytopenia. A lower decrease in platelet count was found in the iloprost group compared to patients treated with heparin alone. This suggests that the protective effect of iloprost on platelets is maintained, even at the low dosage administered in this study.
In one patient, the iloprost infusion was stopped because of a decrease of paO2. The correlation between the reduced paO2 and iloprost infusion was unproven. Iloprost administered intravenously can increase pulmonary shunt fraction.Citation[22] When low doses comparable to those in our regimen are used, iloprost infusion does not necessarily lead to a decrease in arterial oxygen saturation because mixed venous oxygen saturation is concurrently increased.Citation[23] Although no significant difference was found in the minimal oxygenation index (paO2/FiO2 min) between the groups retrospectively (see ), a possible detrimental influence of iloprost on oxygenation cannot be excluded, as this was an a priori defined endpoint.
The administration of iloprost did not influence CVVH efficiency. No difference was found in urea and creatinine values between the groups.
There is published experimental evidence of the effects of iloprost on leukocytes. In an experimental study, an attenuation of leukocyte activation by iloprost was shown in a rat sepsis model.Citation[12] Cytokine release, especially of IL-6, can be influenced by iloprost.Citation[24],Citation[25] Thus, it is conceivable that iloprost may reduce leukocyte count via an influence on migration into the perivascular space. In this study, no difference in WBC was found between the groups. Antibiotic therapy was comparable in both groups. In the other parameters of inflammation, such as CRP, IL-6, and sCD-14, no significant difference was found between both groups.
In conclusion, in the setting of this study, the additional administration of iloprost significantly prolongs filter run time and attenuates the fall in platelet count compared to heparin infusion alone. Therefore, in patients with activated coagulation, ongoing inflammation, and organ dysfunction, low-dose iloprost in addition to low-dose heparin may be favorable. Taking into account the good results in the prolongation of filter life using regional citrate anticoagulation, it seems reasonable to test iloprost in a cohort of patients who are at high-risk of bleeding and who have contraindications to citrate therapy (e.g., patients suffering from severe liver failure). To prove this assumption, another pilot study using a more suitable control group (using a higher aPTT target range) and a higher iloprost dosage may be required.
REFERENCES
- Bagshaw SM, Laupland KB, Boiteau PJ, Godinez-Luna T. Is regional citrate superior to systemic heparin anticoagulation for continuous renal replacement therapy?. A prospective observational study in an adult regional critical care system. J Crit Care. 2005; 20(2)155–161
- Vargas Hein O, Kox WJ, Spies C. Anticoagulation in continuous renal replacement therapy. Contrib Nephrol. 2004; 144: 308–316
- Watson HR, Belcher G, Brierley JK, Dole WP. Iloprost in cardiopulmonary bypass procedures. Agents Actions Suppl. 1992; 37: 346–353
- Antoniou T, Kapetanakis EI, Theodoraki K, et al. Cardiac surgery in patients with heparin-induced thrombocytopenia using preoperatively determined dosages of iloprost. Heart Surg Forum. 2002; 5(4)354–357
- Davenport A, Will EJ, Davison AM. Comparison of the use of standard heparin and prostacyclin anticoagulation in spontaneous and pump-driven extracorporeal circuits in patients with combined acute renal and hepatic failure. Nephron. 1994; 66(4)431–437
- Krause W, Krais T. Pharmacokinetics and pharmacodynamics of the prostacyclin analogue iloprost in man. Eur J Clin Pharmacol. 1986; 30(1)61–68
- Kennedy I, Coleman RA, Humphrey PP, Levy GP, Lumley P. Studies on the characterization of prostanoid receptors: a proposed classification. Prostaglandins. 1982; 24(5)667–689
- Fiaccadori E, Maggiore U, Rotelli C, et al. Continuous hemofiltration in acute renal failure with prostacyclin as the sole anti-hemostatic agent. Intensive Care Med. 2002; 28(5)586–593
- Lehmann C, Taymoorian K, Wauer H, Krausch D, Birnbaum J, Kox WJ. Effects of the stable prostacyclin analogue iloprost on the plasma disappearance rate of indocyanine green in human septic shock. 2000; 26(10)1557–1560
- Korbut RA, Adamek-Guzik T, Madej J, Korbut R. Endothelial secretogogues and deformability of erythrocytes. J Physiol Pharmacol. 2002; 53(4 Pt 1)655–665
- Muller B, Schmidtke M, Witt W. Adherence of leucocytes to electrically damaged venules in vivo. Effects of iloprost, PGE1, indomethacin, forskolin, BW 755 C, sulotroban, hirudin, and thrombocytopenia. Eicosanoids. 1988; 1(1)13–17
- Lehmann C, Konig JP, Dettmann J, Birnbaum J, Kox WJ. Effects of iloprost, a stable prostacyclin analog, on intestinal leukocyte adherence and microvascular blood flow in rat experimental endotoxemia. 2001; 29(7)1412–1416
- Vargas-Hein O, von-Heymann C, Lipps M, et al. Hirudin versus heparin for anticoagulation in continuous renal replacement therapy. Intensive Care Med. 2001; 27(4)673–679
- Tan HK, Baldwin I, Bellomo R. Continuous veno-venous hemofiltration without anticoagulation in high-risk patients. Intensive Care Med. 2000; 26(11)1652–1657
- Baldwin I, Tan HK, Bridge N, Bellomo R. Possible strategies to prolong circuit life during hemofiltration: three controlled studies. Ren Fail. 2002; 24(6)839–848
- Asimakopoulos G. Systemic inflammation and cardiac surgery: an update. Perfusion. 2001; 16(5)353–360
- Vargas Hein O, von Heymann C, Diehl T, et al. Intermittent hirudin versus continuous heparin for anticoagulation in continuous renal replacement therapy. Ren Fail. 2004; 26(3)297–303
- Unger JK, Haltern C, Dohmen B, et al. Albumin and hydroxyethyl starch 130 kDa/0.4 improve filter clearance and hemocompatibility in hemo- and plasmafiltration—an in vitro study. Nephrol Dial Transplant. 2005; 20(9)1922–1931
- Kozek-Langenecker SA. Anticoagulation with prostaglandins during extracorporeal circulation. Wien Klin Wochenschr. 1999; 111(4)129–140
- Brierley JK, Hutchinson A. Prolongation of filter life in continuous arterio-venous hemodialysis. Intensive Care Med. 1991; 17(3)187–188
- Kappa JR, Fisher CA, Todd B, et al. Intraoperative management of patients with heparin-induced thrombocytopenia. Ann Thorac Surg. 1990; 49(5)714–722, discussion, 723
- Honkonen EL, Kaukinen L, Kaukinen S, Pehkonen EJ, Laippala P. Dopexamine unloads the impaired right ventricle better than iloprost, a prostacyclin analog, after coronary artery surgery. J Cardiothorac Vasc Anesth. 1998; 12(6)647–653
- Opitz CF, Wensel R, Bettmann M, et al. Assessment of the vasodilator response in primary pulmonary hypertension. Comparing prostacyclin and iloprost administered by either infusion or inhalation. Eur Heart J. 2003; 24(4)356–365
- Drinda S, Neumann T, Pohlmann G, et al. The response of skin perfusion and of rheological and immunological variables to intravenous prostanoid administration in Raynaud's phenomenon secondary to collagenosis. Vasa. 2005; 34(4)243–249
- Czeslick EG, Simm A, Grond S, Silber RE, Sablotzki A. Inhibition of intracellular tumour necrosis factor (TNF)-alpha and interleukin (IL)-6 production in human monocytes by iloprost. Eur J Clin Invest. 2003; 33(11)1013–1017