Abstract
Insulin resistance is commonly observed in uremic patients. Glucose-based peritoneal dialysis solutions have long-term metabolic complications like hyperinsulinemia, hyperlipidemia, and obesity. The purpose of this study was to examine the insulin resistance in patients undergoing continuous ambulatory peritoneal dialysis (CAPD) with standard glucose and icodextrin containing solutions. The entire non diabetic CAPD patients of our center were studied: forty-four patients in all who were on CAPD treatment for 36.2 ± 23.7 months. Twenty-seven of them (11 male and 16 female) with a mean age of 46 ± 16 years were treated with standard glucose solutions (glucose group). The other 17 patients (10 male and 7 female) with a mean age of 49 ± 16 years were treated with standard glucose solutions during the day and icodextrin dwell during the night, for a median of 12 ± 6.3 months (icodextrin group). Morning fasting serum insulin levels were 20.59 ± 17.86 in the glucose group and 10.15 ± 6.87 in the icodextrin group (p = 0.0001).
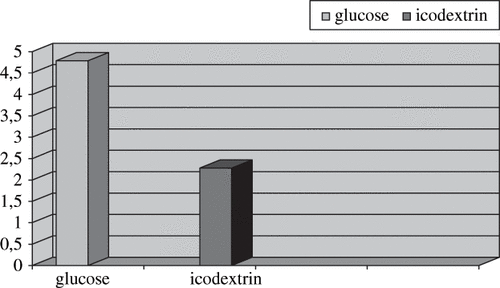
Homeostasis Model Assessment Method scores of the glucose group were significantly higher (4.8±4.1 vs 2.3± 1.7; p = 0.025) than the icodextrin group. A significant positive correlation of HOMA score with insulin, fasting plasma glucose, and triglyceride levels were found in HOMA (IR+) patients. Twenty patients of the icodextrin group (74%) and 15 patients of the glucose group (88%) were hypertensive, but there was no statistically significant difference between the two groups (p = 0.13). The groups showed no significant differences for body mass index and serum levels of glucose, total cholesterol, LDL cholesterol, VLDL cholesterol, HDL cholesterol, triglyceride, intact parathyroid hormone (iPTH), and fibrinogen. In conclusion, the use of icodextrin in the long nighttime dwell can reduce serum insulin levels and increase insulin sensitivity in CAPD patients.
INTRODUCTION
Many patients with chronic renal insufficiency have defects in glucose metabolism, causing carbohydrate intolerance. These defects are characterized by slightly elevated serum glucose and significantly elevated serum insulin levels.Citation[1] Hyperinsulinemia has also been implicated as a direct causative factor in the pathogenesis of atherosclerosis.Citation[2] It is widely known that hypertension and hyperlipidemia play important roles in the progression of renal disease and that insulin resistance may be involved in the pathogenesis of hypertension.Citation[3],Citation[4] Nutritional, metabolic, and cardiovascular complications of renal disease may be consequences of abnormal insulin action.Citation[5] The etiology of insulin resistance in uremia is not clearly known. There are deficiencies in insulin secretion and degradation as well as tissue resistance for insulin at both receptor and post receptor levels. DeFronzo et al. described that there is a decrease in insulin-dependent glucose uptake by skeletal muscles of uremic patients, and this is correlated with total body glucose intake.Citation[6] Uremic toxins, hyperparathyroidism, anemia,Citation[7],Citation[8] malnutrition, products of protein catabolism,Citation[9] metabolic acidosis,Citation[10] iron toxicity,Citation[11] and HCV infectionsCitation[12] are thought to be factors causing this tissue resistance. The choice and duration of therapies are also effective on insulin resistance.Citation[13] Peritoneal dialysis is different from hemodialysis, such that the solutions used cause an additional glucose load reaching 100–300 g/day.
Different kinds of solutions are available for peritoneal dialysis. The difference between these solutions is the type of osmotic agents in the solutions and their concentrations. Standard solutions contain different concentrations of glucose as their osmotic agents. Although these solutions have various advantages, the metabolic side effects such as hyperglycemia and hyperinsulinemia as well as a deficiency of ultrafiltration due to the rapid absorption of glucose through peritoneal membranesCitation[14] brought newly developed peritoneal solutions into consideration.
Icodextrin, a starch-derived, high molecular weight glucose polymer (dextrin), differs from glucose-containing solutions in terms of both ultrafiltration kinetics and its metabolic effects. It is iso-osmolar to uremic serum and exerts its osmotic effect in a sustainable way for up to 12 hours through the mechanism of colloidal osmosis. Thus, the potential damage due to hyperosmolarity is removed, and relatively little of the substance is absorbed, unlike low molecular weight hyperosmolar solutions. Hyperinsulinemia associated with glucose does not occur with icodextrin, and thus glycosylation may be prevented.Citation[15]
The aim of this study is to compare the effect of icodextrin and glucose-containing solutions on insulin resistance in non-diabetic CAPD patients.
SUBJECTS AND METHODS
The study included 44 patients who were on CAPD therapy for 36.2 ± 23.7 months. Twenty-seven of them (11 males and 16 females with a mean age of 46 ± 16 years) were using glucose-containing solutions, and they made up the glucose group. The ten patients of the glucose group (37%) were using two exchanges of 1.36% glucose, one exchange of 2.27% glucose and one exchange of 3.86% glucose bags (Dianeal, Baxter) per day. The rest of them were using three exchanges of 1.36% glucose and one exchange of 3.86% glucose bags. The other 17 patients (10 males and 7 females with a mean age of 49 ± 16 years) were using standard glucose solutions during the day and icodextrin dwell during the night for a median of 12.06 ± 5.47 months, and they made up the icodextrin group. The six patients of the icodextrin group (35%) were using two exchanges of 1.36% glucose, one exchange of 2.27% glucose, and one icodextrin containing exchange (Extraneal, Baxter) per day. The rest of them were using three exchanges of 1.36% glucose and one icodextrin exchange. All of the patients were using four 2 L exchanges per day. Patients with repeated fasting blood glucose levels below 110 mg/dL without known diabetes mellitus were included in the study. Patients with acute infectious conditions such as peritonitis were excluded. Body mass index (BMI) of all patients was below 30. İn both groups, venous serum samples were taken after 12 hours of fasting and the first two hours standard glucose exchange. Serum concentrations of insulin; glucose; total cholesterol; LDL-, VLDL-, and HDL-cholesterol; triglycerides; intact parathyroid hormone (iPTH); ferritin; iron; transferrin saturation; hemoglobin; hematocrit; and fibrinogen were measured. The insulin level was measured by electro-chemiluminescence immunoassay with Roche Modular Analytics (E 170) insulin kit, and glucose was measured with hexokinase spectrophotometrically.
Insulin resistance was characterized by the Homeostasis Model Assessment Method (HOMA), a simple measure of insulin resistance, which is calculated using the following formula:
The patients were called HOMA-IR (+) if their HOMA score were ≥2.5.
All analyses were performed using the Statistical Package SPSS for Windows, version 10.0. The differences between groups were analyzed using the Student's t-test. Comparison of the values between the two groups was performed using the Mann-Whitney test because it was not normally distributed. The chi-square test was used to compare the differences in demographic values between the groups. A p value of <0.05 was considered statistically significant.
RESULTS
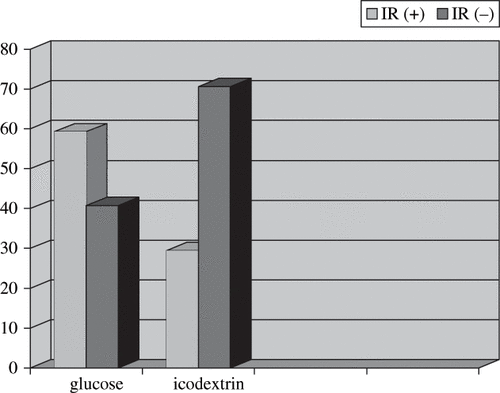
The following characteristics of the groups were similar: age, body mass index, duration of CAPD treatment, serum levels of glucose, total cholesterol, LDL cholesterol, VLDL cholesterol, HDL cholesterol, triglyceride, ferritin, iron, transferrin saturation, hemoglobin, hematocrit, intact parathyroid hormone (iPTH), and fibrinogen (see ). HOMA scores were >2.5 in 16 of 27 patients in the glucose group (59.25%) and 5 of 17 patients in the icodextrin group (29.41%; p < 0.05) . HOMA scores and insulin levels of the glucose group were significantly higher than the icodextrin group (see ). A significant positive correlation of HOMA score with insulin (r = 0.990; p = 0.000), fasting glucose (r = 0.369; p = 0.016), BMI (r = 0.451; p = 0.003), and triglyceride (r = 0.360; p = 0.019) levels were found in HOMA-IR (+) patients. Fibrinogen, ferritin, and intact parathyroid hormone levels were elevated in both groups without any correlation to hyperinsulinemia. There was a positive correlation between CAPD duration and hemoglobin (r = 0.350; p = 0.021) level. There were no significant correlations between HOMA score and several other individual variables, including ferritin, intact parathyroid hormone, and magnesium in HOMA-IR(+) patients. Twenty patients of the icodextrin group (74.07%) and 15 patients of the glucose group (88.23%) were hypertensive, but there was no statistically significant difference between the groups (p = 0.132).
Table 1 Demographic and biochemical parameters of both groups
DISCUSSION
Increasing numbers of patients are being treated with dialysis therapy, and atherosclerotic cardiovascular disorders have been found to have a great impact on mortality in these patients.Citation[16] Insulin resistance contributes to the pathogenesis of atherosclerotic cardiovascular disease and thus has an important impact on the mortality of uremic patients. Insulin resistance in uremic patients has a negative impact on prognosis.Citation[17] There are little data available regarding the effect of CAPD therapy on insulin sensitivity. This is important because of the possibility that glucose loading may worsen insulin sensitivity. Kobayashi et al. showed that CAPD therapy with standard glucose containing solutions for 5.4 weeks normalized insulin resistance similar to hemodialysis therapy.Citation[18] In contrast, Delarue reported that CAPD patients display an insulin resistance not explained by increased lipid oxidation. The maintenance of intracellular glucose utilization at the expense of higher glycemic and insulinemic responses suggests a defective glucose transport.Citation[19] Using icodextrin, which is an alternative osmotic agent, as a peritoneal solution decreased daily glucose load by 30–35%. It is presumed that icodextrin doesn't stimulate insulin secretion due to its complex carbohydrate structure and slow metabolism, and in this way decreases metabolic complications.
In this study, the serum insulin level and the HOMA score of the glucose group were significantly higher than the icodextrin group. This low level of insulin secretion stimulation and increased insulin sensitivity with icodextrin solutions has also been reported by Amici et al.Citation[20] The insulin resistance of the glucose group (59.25%) was significantly higher than the icodextrin group (29.41%), and there was a positive correlation of HOMA score with insulin, fasting glucose level, BMI, and serum triglyceride level (see ). There was no significant difference in the glucose and icodextrin groups in which one of the 1.36% glucose bag was replaced with 2.27% glucose bag per day (37% vs 35%). Serum triglyceride level of the glucose group was higher than the icodextrin group, but without a statistically significant difference. The other serum lipid parameters like total cholesterol and HDL-, LDL-, and VLDL-cholesterol levels were similar in both groups. Sisca reported that the use of icodextrin instead of glucose for the nocturnal exchange was associated with a marked reduction of the elevated triglyceride levels induced by conventional CAPD treatment, while serum total cholesterol level decreased by a small and not significant degree.Citation[21] At variance with our study, Sisca studied patients with elevated triglyceride levels on standard CAPD therapy and switched to icodextrin because they manifested inadequate fluid removal after the nocturnal exchange with hypertonic glucose solution. These selection criteria might explain the not significant triglyceride reduction in these patients.
Mak et al.,Citation[7],Citation[22] among others, demonstrated that the correction of anemia by erythropoietin or treatment with 1,25 dihydroxycholecalciferol reversed insulin resistance and hyperinsulinemia.Citation[23],Citation[24] Fadda et al. suggested that insulin resistance and hyperinsulinemia of uremia may be related to secondary hyperparathyroidism.Citation[25] In this study, all of the patients with hyperphosphatemia used calcium-containing phosphate binders (calcium carbonate and acetate), and the patients with elevated iPTH levels were treated with calcitriol. All patients were receiving erythropoietin so that hematocrit increased up to 30%, and the hemoglobin and hematocrit levels were not different in both groups. The serum ferritin and iPTH levels were elevated in both groups, and there was no difference in the groups and no correlation with HOMA score. The serum iron and transferrin saturations were similar in the groups. The ferritin level might be higher as an acute phase reactant, but another acute phase reactant fibrinogen was also high, and there was no difference in both groups. In the icodextrin group, there were fewer hypertensive patients (74.07%) than in the glucose group (88.23%), but the difference was not significant.
Glucose-based CAPD solutions have long-term metabolic complications, including hyperinsulinemia, hyperlipidemia, and obesity. In addition, there has been growing concern that the hyperosmolality and low pH of these solutions may damage the peritoneum and thereby threaten its viability as a dialyzing membrane.Citation[26] When the residual renal function declines, there may be a need for an increase in the number of the hypertonic glucose exchanges.Citation[27] The use of peritoneal dialysis solutions not using glucose as their osmotic agent has been suggested as a strategy to reduce glucose exposure in peritoneal dialysis patients.Citation[28] The hyperinsulinemia associated with glucose-containing solutions does not occur with icodextrin, and glycosylation may be prevented.
In conclusion the use of icodextrin in the long dwell exchanges is associated with reduced insulin resistance, with fewer metabolic and cardiovascular complications.
REFERENCES
- Fliser D, Pacini G, Engelleiter R, et al. Insulin resistance and hyperinsulinemia are already present in patients with incipient renal disease. Kidney Int. 1998; 53: 1343–1347
- Stout RW. Diabetes and atherosclerosis—the role of insulin. Diabetologia. 1979; 16: 141–148
- Moorhead JF, Chan MK, Varghase Z. The role of abnormalities of lipid metabolism in the progression of renal disease. The Progressive Nature of Renal Disease, BM Brenner, JH Stein. Churchill Livingstone, New York, NY 1986; 133–149
- Ikeda T, Gomi T, Hirawa N, Sakurai J, Yoshikawa N. Improvement of insulin sensitivity contributes to blood pressure reduction after weight loss in hypertensive subjects with obesity. Hypertension. 1996; 27: 1180–1186
- Mak RHK, De Fronzo RA. Glucose and insulin metabolism in uremia. Nephron. 1992; 61: 377–382
- DeFronzo RA, Alvestrand A, Smith D, Hendler R, Hendler E, Wahren J. Insulin resistance in uremia. J Clin Invest. 1981; 67: 563–570
- Mak RHK. Correction of anemia by erythropoietin reverses insulin resistance and hyperinsulinemia in uremia. Am J Physiol. 1996; 270: F839–844
- Mak RHK. Metabolic effects of erythropoietin in patients on peritoneal dialysis. Pediatr Nephrol. 1998; 12: 660–665
- Mak RHK, Turner C, Thompson T, Haycock GB, Chantler C. The effect of dietary protein restriction and amino acid / keto acid suplements on glucose metabolism in uremic patients. J Clin Endocrinol Metab. 1986; 63: 985–989
- DeFronzo RA, Beckles AD. Glucose intolerance following chronic metabolic acidosis in man. Am J Physiol. 1979; 236: E328–E334
- Hernandez C, Genesca J, Ignasi Esteban J, Garcia L, Simo R. Relationship between iron stores infected by hepatitis C virus: A case-control study. Med Clin (Barc). Jun 3, 2000; 115(1)21–22
- Yıldız A, Tutuncu Y, Yazıcı H, Akkaya V, Kayacan SM, Sever MS, et al. Association between hepatitis C virus infection and development of posttransplantation diabetes mellutus in renal transplant recipient. Transplantation. Oct 27, 2000; 74(8)1109–1113
- DeFronzo RA, Tobin JD, Rowe JW, Anders R. Glucose intolerance in uremia: quantification of pancreatic beta cell to glucose and tissue sensitivity to insulin. J Clin Invest. 1978; 62: 425–435
- Mistry CD, Gokal R, Peers E. A randomized multicenter clinical trial comparing isoosmolar icodextrin with hyper osmolar glucose solutions in CAPD. Midas study group. Multicenter investigation of icodextrin in ambulatory peritoneal dialysis. Kidney Int. 1994; 46: 496–503
- Brown CB, Gokal R, Mistry CD. Scientific Symposium on Icodextrın in ID, December 1993 London UK. Perit Dial Int. 1994; 14(Suppl 2)
- Feldman HA, Singer I. Endocrinology and metabolism in uremia and dialysis: a clinical rewiev. Medicine. 1974; 54: 345–376
- Linder A, Charra B, Sherpard DJ, Scribner BH. Accelerated atherosclerosis in prolonged maintenance hemodialysis. N Engl J Med. 1974; 290: 697–701
- Kobayashi S, Maejima S, Ikeda T, Nagase M. Impact of dialysis therapy on insulin resistance in end-stage renal disease: comparison of haemodialysis and continous ambulatory peritoneal dialysis. Nephrol Dial Transplant. 2000; 15: 65–70
- Delarue J, Maingourd C, Couet C, Vidal S, Bagros P, Lamisse F. Effects of oral glucose on intermediary metabolism in continous ambulatory peritoneal dialysis patients versus healthy subjects. Peritoneal Dial Int. 1998; 18: 505–511
- Amici G, Orrasch M, Da Rin G, Bocci C. Hyperinsulinsm reduction associated with icodextrin treatment in continous ambulatory peritoneal dialysis patients. Adv Perit Dial. 2001; 17: 80–83
- Sisca S, Maggiore U. Benefical effect of icodextrin on the hypertriglyceridemia of CAPD patients. Perit Dial Int. 2002; 22: 727–729
- Mak RHK. 1,25 dihydroxycholecalciferol corrects glucose intolerance in hemodialysis patients. Kidney Int. 1992; 41: 1048–1054
- Borissova AM, Djambazova A, Todorov K, Dakovska L, Tankova T, Kirilov G. Effect of erythropoietin on the metabolic state and peripheral insulin sensitivity in diabetic patients on hemodialysis. Nephrol Dial Transplant. 1993; 8: 93–95
- Kokot F, Wiecek A, Grzeszczak W. Influence of erythropoietin treatment on endocrine abnormalities in hemodialyzed patients. Erythropoietin: From Molecular Structure to Clinical Application, CA Baldamus, KM Koch, P Scigalla, L Wieczorek. Karger, Basel 1990; 257–272
- Fadda GZ, Tanakitcharu P, Smogorzewski M, Massry SG. Parathyroid hormone raises citosolic calcium in pancreatic islets: study on mechanisms. Kidney Int. 1993; 43: 554–560
- Mistry CD, Gokal R, Peers E. A randomized multicenter clinical trial comparing isosmolar icodextrin with hyperosmolar glucose solutions in CAPD. Kidney Int. 1994; 46: 496–503
- Lindholm B, Bergstrom J. Nutritional aspects of CAPD. Continous Ambulatory Peritoneal Dialysis, R Gokal. Churchill Livingstone, Edinburgh 1985; 228–264
- Holmes C, Shockley T. Strategies to reduce glucose exposure in peritoneal dialysis patients. Perit Dial Int. 2000; 20(Suppl. 2)37–41