Abstract
Cisplatin, an effective antineoplastic agent, frequently induces acute renal failure in animals and humans. Hyperbaric oxygen (HBO) has been shown to prevent cisplatin-induced nephrotoxicity in rats. This study investigated the effect of two different HBO regimes on renal functions, oxidative stress, and histopathological changes in rat kidneys after cisplatin treatment. Wistar rats were divided into five groups: control, HBO, cisplatin, cisplatin plus once daily HBO, and cisplatin plus twice daily HBO. Cisplatin was given as a single intraperitoneal dose of 6 mg/kg, and HBO was applied for 60 min at 2.5 atm for six days. HBO alone did not alter any biochemical parameters or histopathological findings compared with the control group. Cisplatin increased serum urea and creatinine levels and caused severe histopathological injury. In addition, cisplatin increased lipid peroxidation and impaired superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activities in kidney tissue. Once daily HBO after cisplatin treatment slightly reduced serum urea and creatinine levels and attenuated histopathological injury. HBO also reduced lipid peroxidation and increased SOD and GSH-Px activities significantly. Although twice daily HBO was determined to be more effective than once daily HBO on oxidative stress parameters, it increased serum creatinine levels and histopathological injury compared with the cisplatin group. It was concluded that HBO alone does not induce nephrotoxicity and oxidative stress in rat kidneys; once daily HBO may prevent cisplatin-induced nephrotoxicity, an effect that is partially mediated by the modification of oxidant/antioxidant systems in the kidneys; and twice daily HBO potentiates cisplatin nephrotoxicity by a ROS-independent mechanism.
INTRODUCTION
Cisplatin is an antineoplastic agent that is widely prescribed for a variety of solid tumors, such as lung, testis, ovary, bladder, head, and neck cancer.Citation[1] There are serious side effects associated with cisplatin use, including nephrotoxicity, neurotoxicity, and bone marrow suppression. Nephrotoxicity is seen in about 20% of patients and frequently limits the clinical use of cisplatin.Citation[2] Although the underlying mechanisms are not clear, recent evidence suggests that reactive oxygen species (ROS) and the renal antioxidant defense system are involved in cisplatin-induced nephrotoxicity.Citation[3],Citation[4] Cisplatin is able to induce the formation of ROS and reduce the activity of antioxidant enzymes in renal tissue.Citation[5–9]. The formation of excessive levels of ROS can lead to lethal or sub-lethal cellular damage.Citation[10]
Hyperbaric oxygen (HBO) therapy involves the inspiration of 100% oxygen at a pressure higher than normal atmospheric pressure (atm).Citation[11] HBO increases the amount of oxygen dissolved in arterial blood and leads to hyperoxia even in poorly perfused tissues. HBO therapy is used as a primary therapy in arterial gas embolism, severe carbon monoxide poisoning, gas gangrene, and decompression sickness.Citation[11] It is also employed as an adjunctive treatment in problem wounds, necrotizing soft tissue infections, refractory osteomyelitis, osteoradionecrosis, crush injury, compromised skin grafts and flaps, and thermal burns.Citation[11] It was recently shown that once daily administration of HBO (60 min at 2.5 atm for seven days) protects rat kidneys against cisplatin-induced nephrotoxicity.Citation[12] However, the mechanism of the beneficial effect of HBO is not known. Although it seems controversial, HBO has been shown to attenuate oxidative stress in different experimental models, including acute pancreatitis, acute distal colitis, and liver regeneration.Citation[13–15] It was hypothesized that HBO prevents cisplatin-induced nephrotoxicity by inducing antioxidant enzyme activities and inhibiting lipid peroxidation.
The present study investigated the effect of HBO on renal functions, oxidative stress, and histopathological changes in rat kidneys after cisplatin treatment. In addition, the efficacy of once daily versus twice daily administration of HBO was compared in the same experimental model to determine the optimal treatment protocol.
MATERIALS AND METHODS
Experimental Design
A total of 40 female Wistar rats (160–210 g), purchased from the Istanbul University Medical Faculty Experimental Research and Diagnosis Center, were housed at room temperature in a natural day/night cycle and were permitted to eat standard rat chow and drink tap water ad libitum during the experimental period. Approval for the experiments was secured from the Gulhane Military Medical Academy Ethical Committee. The rats were randomly divided into five groups, each consisting of eight animals. The control group was given a placebo (saline 2 mL, intraperitoneally) on the first day of the study. Animals in the HBO group received only HBO therapy for 60 min at 2.5 atm every day for six days. The cisplatin group (CP) was given an intraperitoneal dose of 6 mg/kg of cisplatin (Cisplatin DBL solution, 50 mg/50 mL, Orna, Istanbul, Turkey) on the first day of the study. The cisplatin plus once daily HBO (CP+HBO) group was given an intraperitoneal dose of 6 mg/kg of cisplatin and immediately followed up with 60 min of HBO at 2.5 atm daily for six days. The cisplatin plus twice daily HBO (CP+2HBO) group was given an intraperitoneal dose of 6 mg/kg of cisplatin and immediately followed up with 60 min of HBO at 2.5 atm twice a day for six days. The interval between the two HBO treatments in the CP+2HBO group was 8 hours. Body weight of all animals was recorded daily during the experimental period. At the end of the study, the rats were sacrificed by cervical dislocation, and left nephrectomy for histopathological analysis and right nephrectomy for biochemical analysis were performed. Blood samples were collected using intracardiac puncture.
Renal Function Assessment
The renal functions of rats were determined by means of serum urea and creatinine levels. Blood samples were centrifuged at 5000 rpm for 10 min, and sera were separated. Serum urea and creatinine levels were analyzed using an autoanalyzer (Olympus® AU800).
Determination of Oxidative Stress
The right kidneys of rats were removed immediately after cervical dislocation, put into tubes, frozen with liquid nitrogen, and stored at −70°C. The frozen kidney tissues were homogenized in a phosphate buffer (pH 7.4) by means of a homogenizator (Heidolph Diax 900; Heidolph Elektro GmbH, Kelhaim, Germany). The supernatant was divided into two to three parts, put in separate tubes, and stored at −70°C again. The protein content of kidney homogenates was measured using Lowry's method, with bovine serum albumin as the standard.Citation[16]
Lipid peroxidation was determined by measuring the thiobarbituric acid reactive substances (TBARS) level in kidneys, as described by Ohkawa.Citation[17] This method was used to obtain a spectrophotometric measurement of the color produced during the reaction to thiobarbituric acid (TBA) with malondialdehyde at 535 nm. For this purpose, 2.5 ml of 100 g/L trichloroacetic acid solution was added to 0.5 ml of homogenate in each centrifuge tube and placed in a boiling water bath for 15 min. The mixture was cooled and centrifuged at 1000 g for 10 min. Next, 2 ml of the supernatant was added to 1 ml of 6.7 g/L TBA solution in a test tube and placed in a boiling water bath for 15 min. The solution was then cooled and its absorbance measured using a spectrophotometer (Shimadzu UV-1601; Kyoto, Japan). TBARS levels were expressed as nmol/g-protein.
Superoxide dismutase (SOD) activity was assayed using the nitroblue tetrazolium (NBT) method described by Sun.Citation[18] The stock solution contained 10 mg of Cu,Zn-SOD from bovine liver dissolved in 10 ml of isotonic saline and was diluted to 600 μg/L with distilled water before it was used in the assay. The SOD assay reagent consisted of a combination of the following reagents: 80 ml of 0.3 mmol/L xanthine solution, 40 ml of 0.6 mmol/L ethylenediaminetetraacetic acid (EDTA) solution, 40 ml of 150 μmol/L NBT solution, 24 ml of 400 mmol/L Na2CO3 solution, and 12 ml of bovine serum albumin. The samples were subjected to ethanol-chloroform (62.5%/37.5%) extraction prior to the assay of enzyme activity. Briefly, 400 μl of ice-cold ethanol/chloroform mixture was mixed thoroughly with 250 μl of sample. After vortexing for 30 seconds and centrifugation at 3000g at 4°C for 5 min, the upper aqueous layer was collected. The collected supernatant was diluted by a factor of 100, and 0.5 ml of the diluted solution was used for the assay by adding it to 2.5 ml of SOD assay reagent. NBT was reduced to blue formazan by O2-., which has a strong absorbance at 560 nm. One unit (U) of SOD is defined as the amount of protein that inhibits the rate of NBT reduction by 50%. The calculated SOD activity was expressed as U/mg-protein.
Glutathione peroxidase (GSH-Px) activity was measured using the method described by Paglia and Valentine, in which GSH-Px activity was coupled with the oxidation of NADPH by glutathione reductase.Citation[19] The oxidation of NADPH was measured spectrophotometrically at 340 nm at 37°C. The reaction mixture consisted of 50 mmol potassium phosphate buffer (pH 7), 1 mmol EDTA, 1 mmol NaN3, 0.2 mmol NADPH, 1 mmol glutathione, and 1 U/mL of glutathione reductase. The absorbance at 340 nm was recorded for 5 min. The activity was the slope of the lines as mmol of NADPH oxidized per minute. GSH-Px activity was presented as U/g-protein.
Histopathological Evaluation
Left kidney tissues were fixed in buffered 10% formalin for 12 hours and then embedded in paraffin wax. Four micron-thick sections were stained with periodic acid-Shiff (PAS) and hematoxylin and eosin (H&E). Histopathological evaluation was performed by a pathologist in a blind manner. Tubular injury was assessed in PAS stained sections using a semi-quantitative scale in which the percentage of distal tubules as epithelial necrosis and apoptosis was assigned a score: 0 = normal; 1 = <10%; 2 = 10−25%; 3 = 26−75%; 4 = >75%.Citation[20] The percentage of hyaline cast formation in distal tubules was calculated in 30 medullary areas in each case under 400 high-power multiplication using a light microscope and scored using a semi-quantitative scale: 0 = normal; 1 = <10%; 2 = 10−25%; 3 = 26−75%; 4 = >75%.
Statistical Analysis
Data were expressed as mean ± SD. The biochemical and histopathological data and percentage of weight loss were analyzed using one-way analysis of variance (ANOVA) followed by the LSD test. All statistical analyses were performed using SPSS 11.0 statistical software. A value of p < 0.05 was considered statistically significant.
RESULTS
Renal Functions
Serum urea and creatinine levels of all groups are shown in . HBO alone did not alter serum urea and creatinine levels compared to the control group (p < 0.05). Cisplatin treatment increased serum urea and creatinine levels significantly, indicating renal failure development (p < 0.05, compared to the control group). Compared to the CP group, serum urea and creatinine levels in the CP+HBO group decreased; however, the difference between the groups was not statistically significant (p > 0.05). In addition, the difference between the CP+HBO group and control group in terms of creatinine levels was not significant (p > 0.05). The serum creatinine level was higher in the CP+2HBO group compared to the CP and CP+HBO groups (p < 0.05). Serum urea levels were similar in the CP and CP+2HBO groups (p > 0.05).
Table 1 Serum urea and creatinine levels and body weights of all experimental groups
Body Weight Loss
The body weights of the animals at the beginning of the study were similar (see ) and increased slightly in the control and HBO groups. However, in the CP, CP+HBO, and CP+2HBO groups, body weight losses were observed after six days. Compared to the CP group (25.05 ± 5.22%), both once daily (CP+HBO group; 10.40 ± 6.27%) and twice daily (CP+2HBO group; 11.45 ± 4.79%) HBO treatments significantly reduced body weight loss (p < 0.05). The difference between the CP+HBO and CP+2HBO groups was not statistically significant (p > 0.05).
Oxidative Stress
Oxidative stress parameters of the animals are presented in . HBO therapy alone did not alter kidney MDA levels, SOD, or GSH-Px activities compared to the control group (p > 0.05). Cisplatin treatment significantly increased MDA levels and reduced SOD and GSH-Px activities compared to the control and HBO groups (p < 0.05). Compared to the CP group, MDA levels increased and SOD and GSH-Px activities decreased significantly in the CP+HBO and CP+2HBO groups (p < 0.05). The differences between the CP+HBO and CP+2HBO groups regarding all oxidative stress parameters were statistically significant (p < 0.05).
Table 2 Renal thiobarbituric acid reactive substances (TBARS) levels, superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px) activities of all experimental groups
Histopathological Findings
Tubular injury and hyaline cast formation scores are presented in . HBO alone did not induce histopathological injury. Cisplatin treatment increased both tubular injury and hyalin cast formation compared to the control and HBO groups (p < 0.05). Tubular injury and hyalin cast formation scores decreased in the CP+HBO group compared to the CP group, although this difference was not significant (p > 0.05). Compared to the CP and CP+HBO groups, tubular injury and hyaline cast formation scores were significantly higher in the CP+2HBO group (p < 0.05). Sample histopathological sections are shown in .
Figure 1. Epithelial necrosis (short black arrow), cast formation, (long white arrow), and apoptotic cells (arrowhead) in the distal tubulus due to cisplatin nephrotoxicity (×200 HE; cisplatin group).
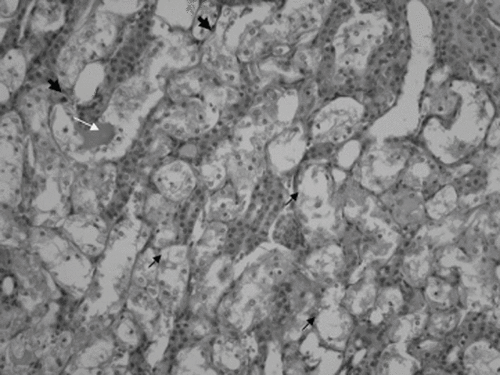
Figure 2. Minimal epithelial necrosis (short black arrow) and apoptotic cells (arrowhead) in the distal tubulus. Some of the distal tubulus shows dilatation (×200 HE; cisplatin plus once daily hyperbaric oxygen group).
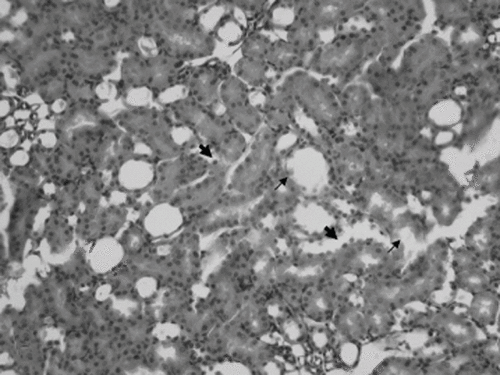
Figure 3. Epithelial necrosis (short black arrow), cast formation (long white arrow), and apoptotic cells (arrowhead) in the distal tubulus (×200 HE; cisplatin plus twice daily hyperbaric oxygen group).
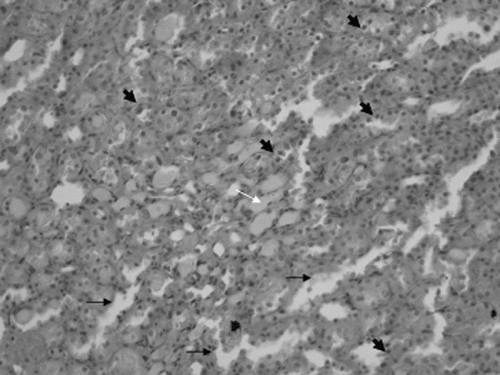
Table 3 Tubular injury and hyalin cast formation scores of all experimental groups
DISCUSSION
Using an experimental model, the effect of different doses of HBO on cisplatin-induced nephrotoxicity in rats was evaluated. Single dose cisplatin injection impaired renal functions and caused severe histopathological injury, indicating the development of acute renal failure. While once daily HBO slightly attenuated cisplatin-induced acute renal failure, twice daily HBO aggravated cisplatin nephrotoxicity.
ROS are continuously formed in vivo in healthy subjects under physiological conditions. Various enzymes and non-enzymatic compounds in the cell prevent ROS production and scavenge formed radicals to maintain a balance between ROS and the antioxidant defense system. When the amount of ROS exceeds the capacity of the cell or organ antioxidant defense system, the normal function is disrupted and tissue damage develops.Citation[21] In this study, cisplatin treatment increased lipid peroxidation and significantly impaired antioxidant enzymes' SOD and GSH-Px activities in rat kidneys. These results support the hypothesis that excessive formation of ROS and lipid peroxidation play an important role in cisplatin-induced nephrotoxicity.Citation[3],Citation[4]
A number of antioxidants have been reported to reduce oxidative stress and prevent cisplatin nephrotoxicity.Citation[5–9] Similarly, the improved renal functions, reduced kidney injury, and reduced body weight loss with once daily HBO are accompanied by a significant reduction in lipid peroxidation and by an increase in SOD and GSH-Px activities in the kidneys. The beneficial effect of HBO on cisplatin-induced nephrotoxicity seems to be partially mediated by modification of the oxidant/antioxidant system in the kidney.
It has been postulated that exposure to HBO can result in increased ROS formation, which may induce oxidative stress in tissues.Citation[22] However, whether HBO is an oxidant or an antioxidant agent is a controversial issue. In some studies, HBO has been shown to induce oxidative injury,Citation[23],Citation[24] while others have reported the opposite.Citation[13–15] Hink and Jansen suggested that some beneficial effects of HBO, such as vasoconstriction, impairment of leukocyte adhesion, inhibition of lipid peroxidation, neovascularization, and antibacterial effects, are mediated by ROS.Citation[25] HBO reduces lipid peroxidation by increasing antioxidant enzyme activitiesCitation[13–15] and by the oxygen mediated termination mechanism.Citation[26] In this study, although HBO alone did not alter any oxidative stress parameters, both once daily and twice daily HBO regimes significantly reduced cisplatin-induced oxidative stress in kidneys. The effect of HBO on oxidant/antioxidant systems may depend on the specific environment and cellular state.
Twice daily HBO sessions increased SOD and GSH-Px activities and reduced lipid peroxidation significantly compared to a single HBO session. Surprisingly, however, twice daily sessions of HBO led to the impairment of renal functions and an increase in tuberal damage. It may be speculated that the pressure, duration, and frequency of HBO determine the cellular and subcellular effects of the treatment. Barth et al. showed that while once daily HBO accelerates bone repair and vessel ingrowth, twice daily HBO retards these processes.Citation[27] Conconi et al. investigated the effects of different HBO regimes on the growth of the 3T3/J2 fibroblast cell line and determined that exposure to HBO at 2.5 atm for 15 min was ineffective, 30- and 60-min exposures raised the proliferation rate, and 120-min exposure significantly reduced these parameters and raised the apoptotic rate of cultured fibroblasts.Citation[28] There are mechanisms other than ROS in the pathogenesis of cisplatin-induced nephrotoxicity; Kruidering et al. showed that although ROS formation occurs during cisplatin-induced toxicity, it is not the direct cause of cell death.Citation[29] Twice daily HBO may have induced a ROS-independent pathway and aggravated the toxic effects of cisplatin. This warrants further investigation.
In conclusion, these findings suggest that HBO alone does not cause nephrotoxicity and oxidative stress in rat kidneys; once daily HBO may attenuate cisplatin nephrotoxicity, and this effect is partially mediated by modification of oxidant/antioxidant systems in kidneys; and twice daily HBO potentiates cisplatin nephrotoxicity by a ROS-independent mechanism.
Notes
*This work was partially supported by a research grant from Gulhane Military Medical Academy.
REFERENCES
- Boulikas T, Vougiouka M. Cisplatin and platinum drugs at the molecular level. Oncol Rep. 2003; 10: 1663–1682
- Garnick MB, Mayer RJ, Abelson HT. Acute renal failure associated with cancer treatment. Acute renal failure, BM Brenner, JM Lazarus. Churchill Livingstone, New York 1988; 621–657
- Kuhlmann MK, Burkhardt G, Kohler H. Insights into potential cellular mechanisms of cisplatin nephrotoxicity and their clinical application. Nephrol Dial Transplant. 1997; 12: 2478–2480
- Conklin KA. Dietary antioxidants during cancer chemotherapy: impact on chemotherapeutic effectiveness and development of side effects. Nutr Cancer. 2000; 37: 1–18
- Appenroth D, Frob S, Kersten L, et al. Protective effects of vitamin E and C on cisplatin nephrotoxicity in developing rats. Arch Toxicol. 1997; 71: 677–683
- Davis CA, Nick HS, Agarwal A. Manganese superoxide dismutase attenuates cisplatin-induced renal injury: importance of superoxide. J Am Soc Nephrol. 2001; 12: 2683–2690
- Ulubas B, Cimen MY, Apa DD, et al. The protective effects of acetylsalicylic acid on free radical production in cisplatin induced nephrotoxicity: an experimental rat model. Drug Chem Toxicol. 2003; 26: 259–270
- Ozen S, Akyol O, Iraz M, et al. Role of caffeic acid phenethyl ester, an active component of propolis, against cisplatin-induced nephrotoxicity in rats. J Appl Toxicol. 2004; 24: 27–35
- Cetin R, Devrim E, Kilicoglu B, et al. Cisplatin impairs antioxidant system and causes oxidation in rat kidney tissues: possible protective roles of natural antioxidant foods. J Appl Toxicol. 2006; 26: 42–46
- Nath KA, Norby SM. Reactive oxygen species and acute renal failure. Am J Med. 2000; 109: 665–678
- Hyperbaric Oxygen 2003: Indications and results. The Hyperbaric Oxygen Committee Report, JJ Feldmeier, MD Kensington. Undersea and Hyperbaric Medical Society, Kensington, Md. 2003
- Atasoyu EM, Yildiz S, Bilgi O, et al. Investigation of the role of hyperbaric oxygen therapy in cisplatin-induced nephrotoxicity in rats. Arch Toxicol. 2005; 79: 289–293
- Yasar M, Yildiz S, Mas R. The effect of hyperbaric oxygen treatment on oxidative stress in experimental acute necrotizing pancreatitis. Physiol Res. 2003; 52: 111–116
- Gulec B, Yasar M, Yildiz S, et al. Effect of hyperbaric oxygen on experimental acute distal colitis. Physiol Res. 2004; 53(5)493–499
- Ozden TA, Uzun H, Bohloli M. The effects of hyperbaric oxygen treatment on oxidant and antioxidants levels during liver regeneration in rats. Tohoku J Exp Med. 2004; 203: 253–265
- Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193: 265–275
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95: 351–358
- Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988; 34: 497–500
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967; 70: 158–169
- Ramesh G, Reeves WB. TNFR2-mediated apoptosis and necrosis in cisplatin-induced acute renal failure. Am J Physiol Renal Physiol. 2003; 285: F610–F618
- Satoh M, Kashihara N, Fujimoto S. A novel free radical scavenger, edarabone, protects against cisplatin-induced acute renal damage in vitro and in vivo. J Pharmacol Exp Therap. 2003; 305: 1183–1190
- Jamieson D. Oxygen toxicity and reactive oxygen metabolites in mammals. Free Radic Biol Med. 1989; 7: 87–108
- Benedetti S, Lamorgese A, Piersantelli M, et al. Oxidative stress and antioxidant status in patients undergoing prolonged exposure to hyperbaric oxygen. Clin Biochem. Apr, 2004; 37(4)312–317
- Oter S, Korkmaz A, Topal T, et al. Correlation between hyperbaric oxygen exposure pressures and oxidative parameters in rat lung, brain, and erythrocytes. Clin Biochem. Aug, 2005; 38(8)706–711
- Hink J, Jansen E. Are superoxide and/or hydrogen peroxide responsible for some of the beneficial effects of hyperbaric oxygen therapy?. Med Hypotheses. Dec, 2001; 57(6)764–769
- Barth E, Sullivan T, Berg E. Animal model for evaluating bone repair with and without adjunctive hyperbaric oxygen therapy (HBO): comparing dose schedules. J Invest Surg. 1990; 3(4)387–392
- Conconi MT, Baiguera S, Guidolin D, et al. Effects of hyperbaric oxygen on proliferative and apoptotic activities and reactive oxygen species generation in mouse fibroblast 3T3/J2 cell line. J Investig Med. Jul, 2003; 51(4)227–232
- Kruidering M, Van de Water B, de Heer E, et al. Cisplatin-induced nephrotoxicity in porcine proximal tubular cells: mitochondrial dysfunction by inhibition of complexes I to IV of the respiratory chain. J Pharmacol Exp Ther. 1997; 280(2)638–649
- Thom SR, Elbuken ME. Oxygen-dependent antagonism of lipid peroxidation. Free Radic Biol Med. 1991; 10(6)413–426