Abstract
Background. It has been shown that azithromycin improves cyclosporine-induced gingival hyperplasia (GH), but its efficacy was never compared against an efficient oral hygiene program (OHP). The aim of this study was to analyze the effects of azithromycin plus OHP versus OHP alone in patients with cyclosporine-induced GH. Methods. After periodontal evaluation, 20 renal transplant recipients received detailed oral hygiene instructions and a complete OHP, and were randomized to control (OHP) or azithromycin groups (OHP plus azithromycin). Patients were re-evaluated after 15 and 30 days. Both groups were similar in time after transplant, age, gender, cyclosporine dose, and cyclosporine through level and serum creatinine. The control group had fewer patients using calcium cannel blockers (10% vs. 70%, p = 0.02). Results. All patients improved in pain, halitosis, and gum bleeding after OHP. The control group did not improve plaque index (PI) or GH. In contrast, baseline PI decreased from 1.52 ± 0.28 to 0.50 ± 0.16 on day 15 (p < 0.01) and to 0.46 ± 0.14 on day 30 (p < 0.01) in the azithromycin group, and the GH score decreased from 1.9 ± 0.27 to 0.90 ± 0.27 on day 15 (p < 0.05) and to 0.70 ± 0.21 on day 30 (p < 0.01). Conclusion. Azithromycin associated to efficient OHP induced a striking reduction in cyclosporine-induced GH, while efficient OHP alone improved oral symptoms but did not decrease cyclosporine-induced GH.
INTRODUCTION
Since its initial use in renal transplant recipients in 1978, cyclosporine has been used almost universally in the prevention of organ transplant rejection, either alone or in combination with other immunosuppressive drugs. The number of patients receiving cyclosporine has increased even further with its use in the treatment of autoimmune diseases. However, cyclosporine causes several adverse effects. Many of them are dose-dependent and potentially reversible with a decrease or discontinuation of the drug, which is not always possible due to the risk of graft loss.Citation[1]
The most noteworthy odontological side effect of CSA is the development of gingival hyperplasia (GH).Citation[2] The occurrence of GH may interfere with speech, mastication, tooth eruption, and nutrition, and it may be esthetically unpleasant, resulting in emotional and social problems for the patient, often underestimated by the health professionals.Citation[3] Moreover, it seems to impair the health-related quality of life after transplantation.Citation[4]
There is evidence that dental plaque induces gingival inflammation and exacerbates CSA-induced gingival growth. Some studies suggest that periodontal therapy with the effective control of plaque and removal of local irritants minimizes intensity and has a positive effect on the symptomatology of CSA-induced gingival growth, but this maneuver has shown controversy results in decreasing gingival growth.Citation[5–8] The treatment and prevention of drug-induced gingival growth remains unsatisfactory, and repeated gingival surgeries are frequently necessary as second therapeutic option.Citation[5],Citation[8]
In 1995, the efficacy of the macrolide antibiotic azithromycin in the resolution of CSA-induced gingival hyperplasia was casually observed in two renal transplant recipients treated for respiratory infection.Citation[9] Subsequent studies confirmed these results.Citation[10–13] The cost of this treatment is low and has lower morbidity than gingivectomy.Citation[10] However, there are no prospective studies comparing the efficacy of an efficient conservative oral treatment with the use of azithromycin in CSA-induced GH.
The objective of this study was to compare the efficacy of azithromycin combined to a gold standard oral hygiene program with the gold standard oral hygiene program alone in the treatment of gingival hyperplasia induced by the continuous use of cyclosporine in renal transplant patients.
SUBJECTS AND METHODS
This study was approved by the Ethics and Research Committee of the Sao Jose do Rio Preto Medical School and the Urology and Nephrology Institute of São José do Rio Preto. Informed consent was obtained for all patients prior to inclusion in the study.
Study Population
Patients transplanted at the University Hospital of the São José do Rio Preto Medical School and at the Urology and Nephrology Institute of São José do Rio Preto were screened. Twenty renal transplant recipients with gingival hyperplasia caused by the continuous use of cyclosporine were included. Age was 32 ± 3 years and post-transplant time was 49 ± 10 months (mean ± SE for both). The use of other drugs causing GH was not considered an exclusion criterion. Cyclosporine dosage remained stable throughout the study. All patients were able to complete the protocol.
Medical History
The following parameters were collected in the medical history: gender, age, transplant date, time receiving CSA, daily CSA dose, cyclosporine blood level at the time of the study, mean cyclosporine blood level in the last year, serum creatinine at the time of the study, mean blood pressure values in the last year, presence of hypertension defined as systolic blood pressure >139 mm Hg and/or diastolic blood pressure >89 mm Hg or the need of anti-hypertensive drugs for blood pressure control, and use of calcium channel blockers.
Odontological Assessment
A questionnaire was applied to assess the degree of oral hygiene, previous gingival surgeries, or treatment, as well as the presence of symptoms such as bleeding, pain, and bad breath. The quality of oral hygiene was evaluated by a subjective score into poor, regular, and good hygiene. This evaluation was based on the amount of bacterial plaque present and whether there was calculus.
Patients were examined for oral hygiene assessment by means of a protocol based on the Simplified Oral Hygiene Index proposed by Greene and Vermillion.Citation[14] This protocol consists in mouthwashes with plaque-disclosing solution (Replak, Herpo Produtos Dentários Ltda, Rio de Janeiro, Brazil) followed by examination of tooth surfaces, giving scores from 0 to 3 according to the presence of bacterial plaque and calculus. The Simplified Oral Hygiene Index is a combination of the bacterial plaque and the calculus indices. The plaque and calculus indices were calculated separately by adding the degrees obtained for each examined surface and dividing it by the respective number of surfaces.
The degree of gingival hyperplasia was evaluated by the index described by Angeolopoulos and Goaz.Citation[15] In this index, the degree of hyperplasia was measured from the cement-enamel junction to the free marginal gum, using scores from 0 to 3.
After clinical-odontological examination, patients were randomized according to entry order. Patients with odd baseline numbers were allocated to the azithromycin (AZI) group and those with even baseline numbers were allocated to the control group (C).
Group C received a gold standard oral hygiene program, and group AZI was offered the same program combined to azithromycin 500 mg for three days, a dosage that has been previously showed as efficient by other authors.Citation[9],Citation[10]
The gold standard oral hygiene program pursued the following therapeutic model: educational and motivational speech on oral hygiene, oral hygiene guidelines, and professional dental prophylaxis for plaque and calculus removal and home oral hygiene for 30 days.
All patients were reexamined 15 and 30 days after starting therapy. Five patients in each group were evaluated 60 days after starting therapy. On the first follow-up visit (day 15), patients were examined for the reevaluation of oral hygiene conditions by the Simplified Oral Hygiene Index and Gingival Hyperplasia Index. A questionnaire was used to record symptoms. Home oral hygiene guidelines were reinforced. On the second (day 30) and third (day 60) follow-up visits, patients were examined by repeating the first follow-up visit procedures.
All of the procedures were carried out by the same odontologist, using odontological material for routine clinical examination (i.e., mirror, explorer probe, periodontal probe, conventional curette for calculus removal, plaque disclosing solution, dental floss, brush, tooth paste, and a Robinson brush for odontological prophylaxis mounted on low rotation odontological piece).
Statistical Analysis
Results are shown as percentage or mean ± standard error. The chi-squared test or the Student's bicaudal non-paired test with Welch's correction was used to compare the two groups, as indicated. ANOVA followed by Tukey-Kramer test was used to make comparisons among baseline period, day 15, and day 30. Significance level was defined as p < 0.05.
RESULTS
Demographic Data and Medical History
The control and azithromycin groups were similar regarding gender, age, transplant time, daily CSA dose, cyclosporine blood level at the time of the study, mean cyclosporine blood levels in the last year, serum creatinine at the time of the study, mean blood pressure values in the last year, and presence of hypertension. The only difference between groups was that a significantly higher percentage of patients in the AZI group was receiving calcium channel blockers (70% versus 10%, p = 0.02; ).
Table 1 Demographic data and clinical history of patients treated with gold standard oral hygiene (control) and patients treated with gold standard oral hygiene plus azithromycin 500 mg for three days (AZI)
Odontological Assessment
Oral Hygiene (Subjective Assessment)
Most of the patients showed regular oral hygiene at the initial evaluation. When groups are compared, a similar percentage of good oral hygiene (AZI 20% and C 30%), regular (AZI 70% and C 60%), and poor (AZI 10% and C 10%) was observed.
All of the patients had unpleasant symptoms such as pain, bleeding, bad breath, and compromised esthetics at baseline.
In group AZI, five patients reported not having had prior gingival treatment, whereas five had already been submitted to plaque removal in an odontological office. Only one reported prior gingival surgery. In group C, none of the patients reported prior gingival treatment or surgery.
At the end of the study, patients in both groups reported improvement of symptoms, especially pain and bleeding.
Simplified Oral Hygiene Index (SOHI)
Plaque index (PI) in Group C was 1.50 ± 0.34 at baseline, 1.02 ± 0.28 at the first follow-up visit (day 15), and 1.54 ± 0.29 at the second follow-up visit (day 30). These differences were not statistically significant. Calculus index (CI) in Group C was 0.65 ± 0.14 at baseline, decreased at the first follow-up visit (day 15) to 0.42 ± 0.15, and decreased even further at the second follow-up visit (day 30) to 0.35 ± 0.13. These differences were not statistically significant as well. The simplified oral hygiene index was 2.15 ± 0.33 at baseline, decreasing to 1.44 ± 0.28 on day 15 (p > 0.05 vs. baseline) and maintaining 1.80 ± 0.31 on day 30 (p > 0.05 vs. baseline). On the day 60, the five evaluated patients presented results similar to the all group at day 30: PI of 0.90 ± 0.33, CI of 0.36 ± 0.22 and simplified oral hygiene index of 1.13 ± 0.38.
In Group AZI, baseline PI was 1.52 ± 0.28, significantly decreasing on day 15 to 0.50 ± 0.16 (p < 0.01 vs. baseline) and to 0.46 ± 0.14 on day 30 (p < 0.01 vs. baseline). CI was 0.62 ± 0.14 at baseline, decreasing to 0.28 ± 0.12 on day 15 and to 0.33 ± 0.12 on day 30. These differences were not statistically significant when compared to baseline. The baseline simplified oral hygiene index was 2.20 ± 0.36, decreasing to 0.73 ± 0.25 on day 15 (p < 0.01 vs. baseline), and was 0.80 ± 0.24 on day 30 (p < 0.01 vs. baseline. The values obtained on day 60 in five patients were similar to those for all patients on day 30: PI of 0.33 ± 0.15, CI of 0.26 ± 0.19, and simplified oral hygiene index of 0.59 ± 0.17.
There were no statistically significant differences between Groups C and AZI regarding baseline PI, CI, and simplified oral hygiene indices.
Gingival Hyperplasia
Gingival hyperplasia (GH) in group C decreased in only one patient and remained unchanged in the remaining patients,Citation[9] after 15 and 30 days. GH index in Group C was 1.50 ± 0.22 at baseline, 1.40 ± 0.22 at the first follow-up visit (day 15), and 1.40 ± 0.22 at the second follow-up visit (day 30). These differences were not statistically significant. In the five patients followed until day 60, GH was 1.20 ± 0.20.
In contrast, GH index decreased after 15 days of therapy in all patients receiving AZI, and decreased even further on day 30 in two of them. Thus, in Group AZI, GH index was 1.90 ± 0.27 at baseline, significantly decreasing to 0.90 ± 0.27 on day 15 (p < 0.05 vs. baseline), and decreased even further on day 30 to 0.70 ± 0.21 (p < 0.01 vs. baseline; see and ). On day 60, the GH was 0.40 ± 0.24 in the five followed patients.
Figure 1. Evaluation of GH index at baseline and after 15 and 30 days in patients treated only with gold standard oral hygiene.
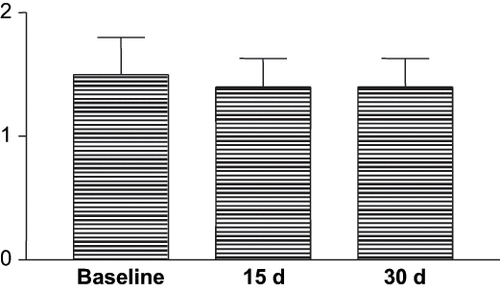
Figure 2. Evaluation of GH index in patients treated with gold standard oral hygiene plus azithromycin 500 mg for three days.
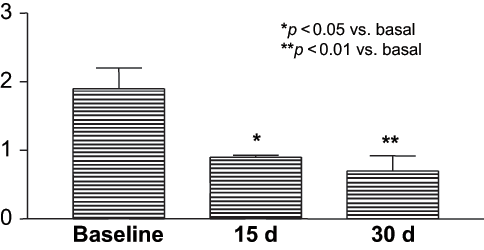
Baseline GH index was similar between the AZI and control groups.
DISCUSSION
The gold standard oral hygiene program used in this study effectively alleviated symptoms such as pain, bleeding, and bad breath; however, when used alone without azithromycin, it did not significantly improve SOHI and gingival hyperplasia. On the other hand, the gold standard oral hygiene program combined to azithromycin 500 mg for three days, alleviated symptoms, and significantly improved SOHI and gingival hyperplasia. Even more important, gingival hyperplasia completely disappeared in patients with grade 1 injury (data not shown). This result may not be attributed to baseline differences between groups, as the PI, CI, SOHI, and GH index were similar for all patients at baseline. In fact, Group AZI had a higher baseline GH index, although this difference was not statistically significant. Likewise, the characteristics of both groups were similar for age, gender, time after transplant, daily CSA dose, CSA blood levels, serum creatinine, blood pressure, and presence of hypertension. The only difference was the higher prevalence of the use of calcium channel blockers in Group AZI, which would theoretically make GH regression more difficult to occur.Citation[16] A possible bias is the fact that the study was not blinded, but the striking differences found between the two groups after treatment and the objectivity of the GH index used make this event improbable.
This study reinforces previous findings, showing clear, positive results for the use of azithromycin in CSA-induced GH.Citation[9–13] It should be emphasized again that these preceding studies did not compare the effects of efficient perioral therapy with azithromycin treatment. All of our patients receiving the antibiotic showed GH reduction, regardless of the GH degree observed at baseline. It is noteworthy that patients with lower GH grade showed total regression of the injury, which is in accordance with the findings of Gomez et al.Citation[11]
GH improvement with the use of azithromycin might be related to the antibiotic effect of this drug, eliminating oral bacteria, reducing local inflammation, and decreasing the extracellular matrix by fibroblasts. In fact, some authors observed a decrease in the growth of aerobes and spirochetes with the use of azithromycin.Citation[17] On the other hand, attempts to isolate pathogenic agents in gingival fragments with CSA-induced GH were not consistent.Citation[18],Citation[19] Moreover, the beneficial effect of azithromycin on GH was not clearly demonstrated with other antibiotics. Whereas Wong et al.Citation[20] were successful in controlling CSA-induced GH using metronidazole for seven days, Aufricht et al.Citation[21] did not obtain protection using a similar antibiotic schedule. A study testing the efficacy of metronidazole and azithromycin in controlling CSA-induced GH has shown that the latter was significantly more effective.Citation[12]
Likewise, it is not adequate to correlate GH with immunosuppression, as other immunosuppressive drugs, including calcineurin inhibitors as tacrolimus, do not produce GH,Citation[22] and drugs with no significant immunosuppressive action, such as phenytoin and nifedipine, may induce gingival hyperplasia.Citation[16]
Other mechanisms that have been suggested to explain the genesis of CSA-induced GH are the direct action of the drug on gingival cells, vasoconstriction leading to tissue ischemia, activation of factors favoring extracellular matrix deposition, and the inhibition of extracellular matrix degradation and apoptosis inhibition.Citation[23–26]
The predominant histological characteristic of gingival tissue hyperplasia is the proliferation of collagen fibers. TGFß1, the most important cytokine involved in fibrogenesis, has been related to drug-induced gingival hyperplasia.Citation[27] CSA increases TGFβ1 expression in several tissues, including the kidney.Citation[28] Recently, Yoshida et al. disclosed results suggesting that in vitro proliferation of rats' gingival cells might be TGFß1-mediated.Citation[29] Thus, another possible mechanism of the protection provided by azithromycin would be a possible antiproliferative effect of this drug. Wirnsberger et al.Citation[30] compared the culture of fibroblasts from a patient with gingival hyperplasia with fibroblast cultures of human skin. Using TGFβ1 as a stimulating factor, they observed that the gingival tissue cells produced more IL-6 than the normal skin cells when exposed to CSA. However, the addition of azithromycin in pharmacological doses did not change this response, therefore not confirming the occurrence of antiproliferative activity of the antibiotic in this experiment.
Others have suggested that azithromycin may have an antiinflammatory effect in pathological situations. Ianaro et al.Citation[31] studied the anti-inflammatory activity of four macrolide antibiotics, roxithromycin, clarithromycin, erythromycin, and azithromycin in vivo and in vitro. They have concluded that these drugs have antiinflammatory activity, which is probably dependent of the ability to prevent the production of pro-inflammatory mediators and cytokines. Prolonged treatment with azithromycin has resulted in an improvement of forced vital capacity, forced expiratory volume, and oxygen saturation in the treatment of pulmonary cystic fibrosis. The authors suggest that the observed effect must be related to the antiinflammatory activity of azithromycin and must be independent of its bactericidal action, as the blood levels were below the minimum inhibitory concentration.Citation[32] Equi et al.,Citation[33] in a randomized, double-blind, prospective study, confirmed that treatment with azithromycin for 4–6 months in patients who do not adequately respond to the conventional therapy of cystic fibrosis was extremely beneficial for the pulmonary function, suggesting a possible anti-inflammatory action of the drug.
In conclusion, this prospective, randomized, controlled study clearly showed that the administration of azithromycin combined to gold standard oral hygiene was extremely effective in reducing CSA-induced gingival hyperplasia, whereas the use of gold standard oral hygiene alone did not have a similarly significant effect.
Dr. Emmanuel A. Burdmann is partially supported by a grant from CNPq, National Council for Scientific and Technological Development, Brazil.
The authors are grateful to the Urology and Nephrology Institute of São José do Rio Preto for allowing the inclusion of their patients in the study. In addition, we thank Livia C. Burdmann for the excellent grammar review of the manuscript.
Notes
*Part of this study was presented at the 34th Annual Meeting of the American Society of Nephrology, San Francisco, California, USA, and was published in abstract form (J Am Soc Nephrol. 2001;12:944A).
REFERENCES
- De Mattos AM, Olyaei AJ, Bennett WM. Pharmacology of immunosuppressive medications used in renal diseases and transplantation. Am J Kidney Dis. 1999; 28: 631–637
- Boltchi FE, Rees Terry D, Iacopino AM. Cyclosporine A induced gingival overgrowth: A comprehensive review. Quintessence Int. 1999; 30: 775–783
- Peters TG, Spinola KN, West JC, Aeder MI, Danovitch GM, Klintma, et al. Differences in patient and transplant professional perception immunosuppression-induced cosmetic side effects. Transplantation. 2004; 78: 537–543
- Fiebiger W, Mitterbauer C, Oberbauer R. Health-related quality of life outcomes after kidney transplantation. Health Qual Life Outcomes. 2004; 2: 2
- Meraw SJ, Sheridan PJ. Medically induced gingival hyperplasia. Mayo Clin Proc. 1998; 73: 1196–1199
- Seymour RA, Smith DG. The effect of a plaque control program on the incidence and severity of cyclosporine-induced gingival changes. J Clin Periodontol. 1991; 18: 107–110
- Somacarrera ML, Hernandez G, Acero J, Moskow BS. Factors related to the incidence and severity of cyclosporine-induced gingival overgrowth in transplant patients: A longitudinal study. J Periodontol. 1994; 65: 671–675
- Kantarci A, Cebeci I, Tuncer O, Carin M, Firatli E. Clinical effects of periodontal therapy on the severity of cyclosporin A-induced gingival hyperplasia. J Periodontol. 1999; 70: 587–593
- Wahlstrom E, Zamora JU, Teichman S. Improvement in cyclosporine associated gingival hyperplasia with azithromycin therapy. N Engl J Med. 1995; 332: 753–754
- Nash MN, Zaltzman JS. Efficacy of azithromycin in the treatment of cyclosporine-induced gingival hyperplasia in renal transplant recipients. Transplantation. 1998; 65: 1611–1615
- Gómez ME, Sánchez-Nuñes JE, Sánchez C, Corte S, Aguado C, Baltar PJ, et al. Treatment of cyclosporin-induced gingival hyperplasia with azithromycin. Nephrol Dial Transplant. 1997; 12: 2694–2697
- Chand DH, Quattrocchi J, Poe SA, Terezhalmy GT, Strife CF, Cunn RJ. Trial of metronidazole vs. azithromycin for treatment of cyclosporine-induced gingival overgrowth. Pediatr Transplant. 2004; 8: 60–64
- Tokgoz B, Hi Sari, Yildiz O, Aslan S, Sipahioglu M, Okten T, et al. Effects of azithromycin on cyclosporine-induced gingival hyperplasia in renal transplant patients. Transplant Proc. 2004; 36: 2699–2702
- Greene JC, Vermillion JR. The simplified oral hygiene index. J Am Dent Assoc. 1964; 68: 7–13
- Angeolopoulos AP, Goaz PW. Incidence of diphenylhydantoin gingival hyperplasia. Oral Surg. 1972; 34: 898–906
- Bokenkamp A, Bohnhorst B, Beier C, Albers N, Offner G, Brodehl J. Nifedipine aggravates cyclosporine A-induced gingival hyperplasia. Pediatr Nephrol. 1994; 8: 181–185
- Sefton AM, Maskell JP, Beighton D, Whiley A, Shain H, Foyle D, et al. Azithromycin in the treatment of periodontal disease. Effect on microbial flora. J Clin Periodontol. 1996; 23: 998–1003
- Romito GA, Lotufo RF, Saraiva L, Pustiglioni NA, Pustiglioni FE, Stolf NA. Superinfecting microorganisms in patients under treatment with cyclosporin-A and its correlation to gingival overgrowth. Pesqui Odontol Bras. 2003; 17: 35–40
- Worm HC, Wirnsberger GH, Mauric A, Holzer H. High prevalence of Chlamydia pneumoniae infection in cyclosporin A-induced post-transplant gingival overgrowth tissue and evidence for the possibility of persistent infection despite short-term treatment with azithromycin. Nephrol Dial Transplant. 2004; 19: 1890–1894
- Wong W, Hodge MG, Lewis A, Sharpstone P, Kingswood JC. Resolution of cyclosporin-induced gingival hypertrophy with metronidazole. Lancet. 1994; 343: 986
- Aufricht C, Hogan EL, Ettenger RB. Oral metronidazole does not improve cyclosporine A-induced gingival hyperplasia. Pediatr Nephrol. 1997; 11: 552–555
- Harikrishnan P, Harden PN. Tacrolimus can resolve cyclosporin-induced gingival hyperplasia. Nephrol Dial Transplant. 1999; 14: 1805–1806
- Yamada H, Nishimura F, Naruishi K, Chou H-H, Takashiba S, Albright GM, et al. Phenytoin and cyclosporin A suppress the expression of MMP-1, TIMP-1, and cathepsin L, but not cathepsin B in cultured gingival fibroblasts. J Periodontol. 2000; 71: 955–960
- Bonnaure-Mallet M, Tricot-Doleux S, Godeau GJ. Changes in extracellular matrix macromolecules in human gingival after treatment with drugs inducing gingival overgrowth. Arch Oral Biol. 1995; 40: 393–400
- Arora PD, Silvestri L, Ganss B, Sodek J, McCulloch CA. Mechanism of cyclosporin-induced inhibition of extracellular collagen degradation. J Biol Chem. 2001; 276: 14100–14109
- Bulut S, Ozdemir BH, Alaaddinoglu EE, Oduncuoglu FB, Bulut OE, Demirhan B. Effect of cyclosporin A on apoptosis and expression of p53 and bcl-2 proteins in the gingiva of renal transplant patients. J Periodontol. 2005; 76: 691–695
- Saito K, Mori S, Iwakura M, Sakamoto S. Immunohistochemical localization of transforming growth factor B, basic fibroblast growth factor and heparan sulphate glycosaminoglycan in gingival hyperplasia induced by nifedipine and phenytoin. J Periodontol. 1996; 31: 545–555
- Vieira JM, Jr, Noronha IL, Malheiros DMAC, Burdmann EA. Cyclosporine-induced interstitial fibrosis and arteriolar TGF-β expression with preserved renal blood flow. Transplantation. 1999; 68: 1746–1752
- Yoshida T, Nagata J, Yamane A. Growth factors and proliferation of cultured rat gingival cells in response to cyclosporin A. J Periodontal Res. 2005; 40: 11–19
- Wirnsberger GH, Pfragner R. Comment on “Efficacy of azithromycin in the treatment of cyclosporine-induced gingival hyperplasia in renal transplant recipients” by Nash and Zaltzman. Transplantation. 1999; 67: 1289–1291
- Ianaro A, Ialenti A, Maffia P, Sautebin L, Rombola L, Carnuccio R, et al. Anti-inflammatory activity of macrolide antibiotics. J Pharmacol Exp Therap. 2000; 292: 156–163
- Jaffe A, Francis J, Rosenthal M, Bush A. Long term azithromycin may improve lung function in children with cystic fibrosis. Lancet. 1998; 351: 420
- Equi A, Balfour-Lynn IM, Bush A, Rosenthal M. Long term azithromycin in children with cystic fibrosis: a randomized, placebo-controlled crossover trial. Lancet. 2002; 360: 978–984